Abstract
Purpose:
To explore a new therapeutic option for patients with hepatocellular carcinoma (HCC), the efficacy and safety of a group of traditional Chinese medicines (Banxia XieXin recipe) as monotherapy for patients with advanced HCC was studied.
Materials and Methods:
The study included 68 patients with advanced HCC from August 16,2016 to August 15,2019 for analysis. These eligible patients received treatment with Banxia XieXin recipe for at least 1 month. The primary endpoints were progression-free survival (PFS) and overall survival (OS). The secondary efficacy endpoints included objective response rate (ORR) and disease control rate (DCR). In addition, safety was also assessed.
Results:
The median treatment duration of these 68 patients was 10.3 months (range = 1.6-33.5 months), and follow-up is still ongoing. The median PFS was 6.07 months (95% confidence interval [CI] = 3.748-8.392 months), and the median OS was 12.60 months (95% CI = 8.019-17.181 months). The ORR was 10.3% and the DCR was 41.2%. In the subgroup analysis, the median OS in the transcatheter arterial chemoembolization (TACE) group was not reached, and the median OS in the NO TACE group was 11.30 months (95% CI = 3.219-19.381 months). In addition, no drug-related serious adverse events were observed during the study.
Conclusion:
This is the first clinical analysis of traditional Chinese medicine as a single treatment for advanced HCC. The obtained results are encouraging as they suggest that this panel of Chinese herbs is safe and it may be effective for patients with advanced HCC in a real-world clinical setting.
Keywords: hepatocellular carcinoma, herbs, TACE, Banxia XieXin recipe, clinical research
Introduction
Liver cancer is one of the most frequent malignant tumors and the third leading cause of cancer-related deaths worldwide. Both the incidence and mortality rates for liver cancer are increasing, while the overall incidence and mortality for all cancers combined tend to decline.1,2 Hepatocellular carcinoma (HCC) is the most common and deadly form of liver cancer. In China, chronic hepatitis B virus (HBV) infection and subsequent liver fibrosis and cirrhosis are the leading causes of liver cancer. Due to the lack of effective treatment options as well as the high recurrence and metastasis rates, HCC is associated with an extremely poor prognosis. At present, surgical resection and liver transplantation are effective treatments for liver cancer, but only a few patients are surgical candidates due to tumor extension, poor hepatic functional reserve, or underlying liver cirrhosis.3,4 Even for the few lucky patients who have completed the surgery, the recurrence rates at 5 years following liver resection have been reported to exceed 70%.5 For patients with advanced liver cancer, it is essential to improve general well-being, promote quality of life, and prolong survival time. It is well known that chemotherapy effects in patients with advanced liver cancer are not outstanding.6,7 Some patients can consider local treatment, such as transarterial chemoembolization (TACE). The progression-free survival (PFS) of patients receiving TACE treatment is reported to be 4.3 months.8 Molecular targeted drugs are also one of the options for patients with advanced liver cancer, such as sorafenib and lenvatinib. However, according to reports on sorafenib, the only approved molecular targeted therapy for advanced HCC, the median PFS of patients treated with sorafenib was only 2.8 months in the Asia-Pacific region.9 In recent years, immunotherapy, which has been attracting worldwide attention, is also becoming an important treatment method. For example, nivolumab and pabolizumab, which have been approved by the Food and Drug Administration for second-line treatment of advanced HCC, can bring certain benefits to patients with advanced liver cancer, but are expensive and difficult to afford for many patients.
In China, traditional Chinese medicine (TCM) therapies, which can date back more than 2000 years, are widely used in cancer treatment.10 Banxia XieXin decoction was created by the ancient doctor Zhang Zhongjing. It is mainly used to treat digestive diseases such as bloating and abdominal pain. It has a history of more than 1800 years. In a previous retrospective study, it was found that Banxia XieXin decoction can bring survival benefits to patients with advanced liver cancer, and one of them achieved complete response (CR).11 As a TCM hospital, many patients with advanced liver cancer come to our hospital to undergo Chinese medicine for treatment. Therefore, we designed a prospective study of Banxia XieXin decoction as monotherapy for advanced liver cancer and analyzed its treatment effect and safety.
Methods
Study Design and Patient Enrollment
In this prospective open-label study, patients from the Oncology Department of the Affiliated Hospital of the Chengdu University of Traditional Chinese Medicine, Chengdu, China, were recruited.
According to the American Association for the Study of Liver Diseases criteria, eligible patients were adults with advanced HCC confirmed by pathological assessment or noninvasive assessment.12 These patients had not received any cancer-related treatment within 4 weeks before the start of study treatment, and the disease had progressed after the prior treatment. In addition, these patients had to have at least one measurable lesion defined by modified Response Evaluation Criteria in Solid Tumors for HCC (mRECIST) and RECIST version 1.1.13 Inclusion criteria also included Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3; an estimated life expectancy of >2 months; Child-Pugh liver function class A or B; adequate organ function, such as white blood cell count ≥2.0 × 109 cells/L, neutrophils ≥1.0 × 109 cells/L, and platelets ≥75 × 109 cells/L; and the absence of any serious comorbidities, such as significant cardiac, cerebrovascular, hepatic, or nephritic abnormities or other important organ dysfunctions.
Key exclusion criteria included patients with low compliance or (1) use of other antitumor treatments at the same time, (2) diagnosis of other malignant tumors, and (3) HCC with brain metastasis.
The protocol was approved by the Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (No. 2015BL-003). All patients provided written informed consent before study entry.
Study Treatment and Procedures
The enrolled patients received treatment with Banxia XieXin recipe in the form of decoction. The herbal components and their doses in the Banxia XieXin recipe decoction are listed in the appendix. With the use of an automatic herb-boiling machine, the decoction volume of each prescription was about 250 mL. Patients were instructed to take 50 mL each time, 3 times daily.
Treatment was continued until disease progression, death, development of unacceptable toxicity, patient’s refusal to continue, or at the discretion of the investigator. When the disease progressed, treatment could also be continued for 1 month to assess tumor response as long as the patient agreed to do so.
Following an initial baseline assessment within 14 days of the start of study treatment, the investigator assessed tumor response by using computed tomography (CT) scan after 3 to 6 months of treatment. Subsequently, follow-up assessments were done every 2 months until the patient died. Adverse events were recorded continuously from enrollment to the end of the final study visit.
Outcomes
The primary endpoints were PFS (time from enrollment to radiological disease progression or death) and overall survival (OS; time from enrollment to death due to any cause). The secondary efficacy endpoints included the proportion of patients who had an objective response (complete or partial response [PR]) according to RECIST version 1.1,13 namely, objective response rate (ORR) and disease control rate (DCR; the proportion of patients who had a CR, PR, or stable disease). Besides, safety was also assessed by the incidence and nature of adverse events.
Statistical Analysis
The PFS and OS were estimated using the Kaplan-Meier method. All statistical analyses were performed using GraphPad Prism software (version 6, GraphPad Software Inc) and log-rank test.
Results
From August 16, 2016, to August 15, 2019, a total of 68 patients with advanced HCC were enrolled from the Oncology Department of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine. However, only the results of the medium-term analysis are presented here, since 4 patients were still on treatment and had not yet reached the assessment time point. Therefore, 68 patients were finally included in the current analysis. Meaningful results were found to report in this mid-term analysis. Of course, we will update the results according to the latest follow-up data in the future. The baseline characteristics of the included patients are shown in Table 1. Notably, 18 patients (18/68) were without any treatment in this study. All patients (50/68) who were previously treated with surgery or TACE had progressive disease, which was similar to the clinical trials of sorafenib.9 The number of patients receiving chemotherapy and molecular targeted therapy is 5 and 6, respectively. Patients who had received previous systemic therapy were excluded in the clinical trials of sorafenib, but these 11 patients had progressive disease after the prior treatment according to RECIST version 1.1 in this study.
Table 1.
The Clinical Characteristics of the 68 Patients With Advanced Hepatocellular Carcinoma.
| Variables | Number of patients | Percentage |
|---|---|---|
| Age (years) | ||
| <65 | 30 | 44.1 |
| ≥65 | 38 | 55.9 |
| Sex | ||
| Male | 56 | 82.4 |
| Female | 12 | 17.6 |
| ECOG-PS | ||
| 0 | 10 | 14.7 |
| 1 | 40 | 58.8 |
| 2 | 10 | 14.7 |
| 3 | 8 | 11.8 |
| Childs-Pugh class | ||
| A | 50 | 73.5 |
| B | 18 | 26.5 |
| Hepatitis B virus infection | ||
| Yes | 49 | 72.1 |
| No | 19 | 27.9 |
| Liver cirrhosis | ||
| Yes | 28 | 41.2 |
| No | 40 | 58.8 |
| AFP (µg/L) | ||
| ≥400 | 24 | 35.3 |
| <400 | 44 | 64.7 |
| Tumor size (cm) | ||
| <3 | 14 | 20.6 |
| 3-5 | 8 | 11.8 |
| 5-10 | 26 | 38.2 |
| ≥10 | 20 | 29.4 |
| PVTT | ||
| Yes | 20 | 29.4 |
| No | 48 | 70.6 |
| Extrahepatic spread | ||
| Yes | 54 | 79.4 |
| No | 14 | 20.6 |
| Involved disease sites per patient | ||
| 1 | 36 | 52.9 |
| 2 | 22 | 32.4 |
| ≥3 | 10 | 14.7 |
| Previous treatment | ||
| No | 18 | 26.5 |
| Sur | 22 | 32.4 |
| TACE | 23 | 33.8 |
| RF | 4 | 5.9 |
| Che | 5 | 7.4 |
| MTT | 6 | 8.8 |
| AJCC stage | ||
| IIIb | 10 | 14.7 |
| Iva | 42 | 61.8 |
| IVb | 16 | 23.5 |
Abbreviations: ECOG-PS, Eastern Cooperative Oncology Group–Performance Status; PVTT, portal vein tumor thrombus; Sur, surgical resection; TACE, transcatheter arterial chemoembolization; RF, radiofrequency; Che, chemotherapy; MTT: molecular targeted therapy; AJCC, American Joint Committee on Cancer.
The data cutoff date for the current analysis was August 15, 2019. The median treatment duration of these 68 patients was 10.3 months (range = 1.6-33.5 months), and follow-up is still ongoing since if the disease progressed, treatment could also be continued for 1 month to assess tumor response as long as the patient agreed to do so or if the-patient would like to use it as an adjuvant treatment to other treatment, such as TACE, molecular-targeted therapy, and so on.
In the primary analysis, the median PFS was 6.07 months (95% confidence interval [CI] = 3.748-8.392; Figure 1), and the median OS was 12.60 months (95% CI = 8.019-17.181; Figure 2). Thirty-six patients died, all due to disease progression. The 1-year survival rate was 51.7%.
Figure 1.
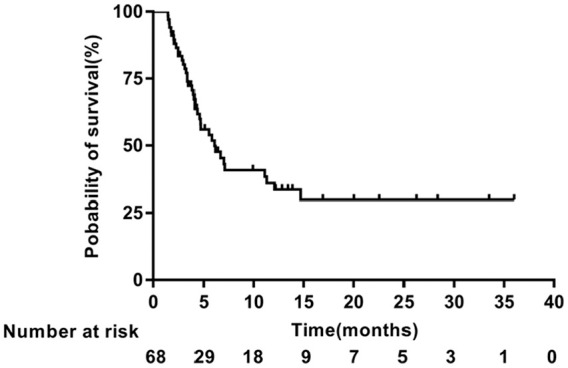
Kaplan-Meier analysis of progression-free survival.
Figure 2.
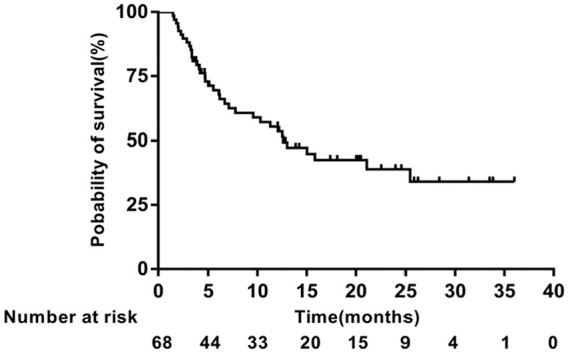
Kaplan-Meier analysis of overall survival.
Notably, most of these patients received prior treatments such as TACE and targeted molecular therapy before enrollment. In order to explore the potential effect of previous treatment, we performed a subgroup analysis. The median PFS (7.0 months, 95% CI = 3.538-10.462) was longer in the subgroup of patients who had previously received TACE compared with the median PFS (5.8 months, 95% CI = 3.756-7.844) who had not previously received TACE. Thus, the treatment effect in this type of patient seems to be better (P = .2158; Figure 3).
Figure 3.
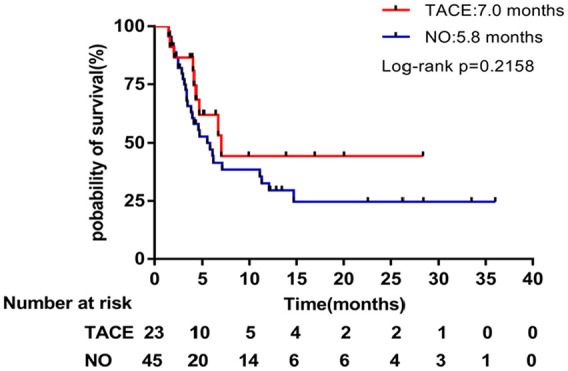
Kaplan-Meier subgroup analysis of progression-free survival stratified by prior TACE treatment.
The median OS of advanced HCC patients who had previously received TACE was not reached, and the median OS of subgroups who had not received TACE was 11.3 months (95% CI = 3.219-19.381), as shown in Figure 4.
Figure 4.
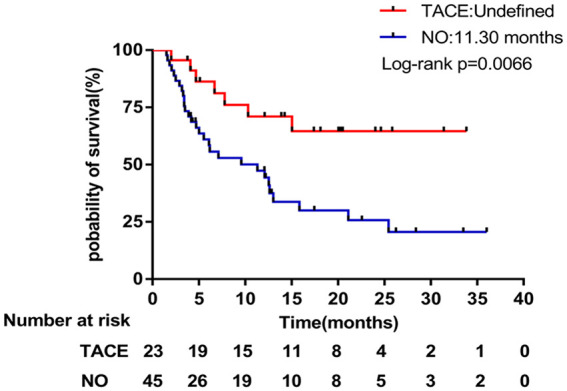
Kaplan-Meier subgroup analysis of overall survival stratified by prior TACE treatment.
In terms of tumor response after treatment with Banxia XieXin recipe, 7 patients achieved a PR, 21 had stable disease. The ORR was 10.3% and the DCR was 41.2% (Table 2).
Table 2.
Tumor Response According to RECIST Version 1.1.
| Variables | Number of patients | Percentage |
|---|---|---|
| CR | 0 | 0 |
| PR | 7 | 10.3 |
| SD | 21 | 30.9 |
| PD | 40 | 58.8 |
| ORR | 10.3% | |
| DCR | 41.2% | |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
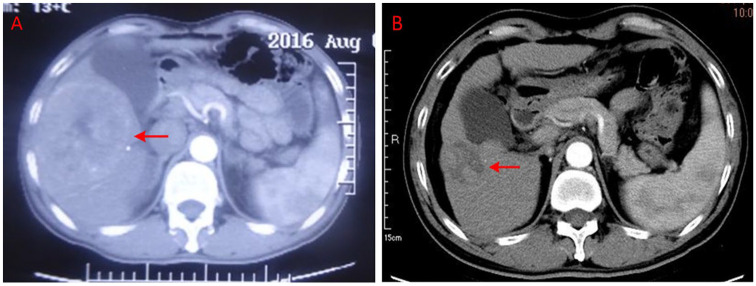
Particularly, the imaging data of 2 typical cases were presented as follows. The first patient was a 58-year-old male who was diagnosed with advanced HCC with metastasis to the right kidney on August 16, 2016. At baseline, the CT scan showed that the size of the target lesion in the liver was 12.7 cm × 9.2 cm × 9.0 cm (Figure 5A); blood tests revealed elevated α-fetoprotein (302.1 µg/mL) and the ECOG performance status score was 2. This patient had not received any anticancer treatment previously. After 7.0 months treatment with Banxia XieXin recipe, the CT scan showed that the target lesion in the liver shrank to 3.9 cm × 3.0 cm (Figure 5B) and reached a PR according to RECIST version 1.1. The ECOG performance status score improved from 2 to 0. The patient is still currently in treatment and has not yet had progressive disease.
Figure 5.
(A, B) The imaging data of one partial response patient diagnosed as advanced hepatocellular carcinoma with metastasis to the right kidney.
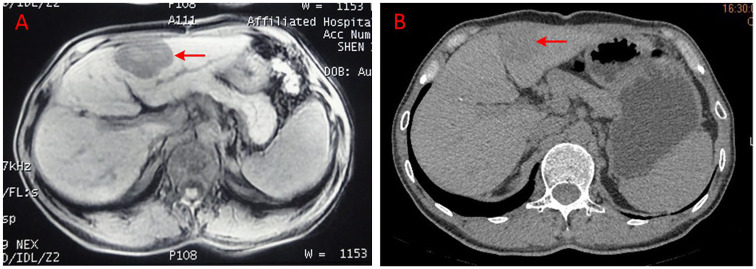
One 54-year-old male patient was first diagnosed to have HCC in December 2015. He was treated by surgical resection on January 18, 2016. No antineoplastic treatment was done thereafter. When a regular review was conducted on September 30, 2016, it showed intrahepatic recurrence and right lung metastasis and then he received treatment with Chinese herbs. At baseline, the CT scan showed that the size of the target lesion in the liver was 5.1 cm × 3.7 cm × 4.4 cm (Figure 6A); blood tests revealed elevated α-fetoprotein (>1210 µg/mL) and the ECOG performance status score was 0. After a 3.2-month treatment with modified XieXin recipe, the CT scan showed that the target lesion in the liver shrank to 3.2 × 3.0 cm (Figure 6B) and reached a PR according to RECIST version 1.1. The duration of the PR was 2.3 months. He continued to insist on treatment after the disease progressed and he died in October 2017.
Figure 6.
(A, B) The imaging data of another partial response patient diagnosed as advanced hepatocellular carcinoma with intrahepatic recurrence and right lung metastasis.
Concerning safety, no adverse events were observed except for the occurrence of mild nausea. Three patients reported mild nausea after taking pills, but the symptom was relieved spontaneously after 1 week without any treatment. In addition, no serious adverse events such as liver or kidney damage were detected during the follow-up period. Thus, the TCM treatment was safe and well-tolerated in these patients with advanced HCC.
According to the International Tumor Chemotherapy Adverse Drug Evaluation System–CTCAE v4.0,14 68 patients included in this study were evaluated for safety. During the follow-up, the adverse reactions were nausea, vomiting, diarrhea, abdominal distension, oral ulcers, the painless mass of the scalp, and so on. The overall adverse reaction rate was 29.5%, all of which were grade I to II adverse reactions, and responded to symptomatic treatment. There was no adverse reaction of grade III or above, and no drug-related lethal events occurred. Specific adverse reaction events and their incidence rates are shown in Table 3.
Table 3.
Adverse Reactions.
| Grade I | Grade II | Grade III | Grade IV | Any grade (%) | >Grade III (%) | |
|---|---|---|---|---|---|---|
| Nausea | 2 | 1 | 0 | 0 | 3 (4.4%) | 0 |
| Vomiting | 0 | 3 | 0 | 0 | 3 (4.4%) | 0 |
| Diarrhea | 2 | 3 | 0 | 0 | 5 (7.4%) | 0 |
| Bloating | 2 | 5 | 0 | 0 | 7 (10.3%) | 0 |
| Ulcer | 0 | 1 | 0 | 0 | 1 (1.5%) | 0 |
| Superficial painless mass | 0 | 1 | 0 | 0 | 1 (1.5%) | 0 |
Discussion
It is well-known that HCC is one of the leading causes of cancer-related mortality worldwide. The long-term survival rate remains unsatisfactory due to the high recurrence and metastasis rates after conventional treatment. To improve the outcome of patients with HCC, there is an urgent need for more effective therapies. At present, TCM is often used as an adjuvant therapy to conventional treatments, such as TACE in treating patients with liver cancer,15-18 to reduce the toxicities of other treatments, relieving symptoms, and improving quality of life. TCM has only been considered as a complementary or alternative treatment for cancer patients, and there is a paucity of data regarding the tumor control effect of TCM as monotherapy for patients with liver cancer. Banxia XieXin decoction has been used for treating digestive diseases for more than 1800 years. In our previous retrospective analysis, it was found that Banxia XieXin decoction can relieve the symptoms of patients with advanced liver cancer, inhibit tumor growth, and prolong survival. One of the patients’ tumors bulk was significantly reduced to reach CR status. This state has been maintained for up to 8 months.11 Therefore, it is necessary to carry out further scientific research.
To the authors’ best knowledge, this is the first prospective study of Chinese herbs alone for the treatment of advanced HCC that used the widely accepted endpoints of PFS, OS, ORR, and DCR according to RECIST version 1.1.13 In the present study, the median PFS was 6.07 months. In clinical studies of sorafenib, an oral multikinase inhibitor, which is the first molecularly targeted drug approved by multiple countries around the world for first-line treatment of advanced HCC, the median PFS was 5.5 months in Western countries19 and only 2.8 months in the Asia-Pacific region.9 In current research work, all patients were from China, and in particular, 28 of 68 patients had been diagnosed with liver cirrhosis when enrolled (Table 1). The manifestations of liver decompensation such as splenomegaly and portal hypertension had appeared. However, the Child-Pugh class was A or B. It had been reported that HCC patients with liver cirrhosis showed significantly worse OS than non-cirrhotic HCC patients (7.9 months vs 13.8 months, P = .033).20 Even so, the median PFS reached 6.07 months, and the median OS reached 12.60 months in a real-world clinical setting. These findings suggest that the efficacy of the Banxia XieXin recipe may be superior to that of sorafenib in patients with advanced HCC, although this was not a head-to-head comparison. A further study directly comparing Chinese herbs versus sorafenib for advanced HCC will be conducted in the future.
In the REFLECT trial,21 a subgroup analysis of the Chinese population found that the OS of the lenvatinib group was significantly longer than that of the sorafenib group by 4.8 months, with statistical differences. In addition, when combined with HBV infection, the survival benefit was significantly different between the lenvatinib group and the sorafenib group (OS = 14.9 months vs 9.9 months). Therefore, it was approved by the China Food and Drug Administration on September 5, 2018, and approved for domestic listing. It is important to note that the experimental conditions for entering the REFLECT trial were strict, excluding ECOG performance status patients with a score of 2 or 3 and a large liver mass. However, in patients enrolled in the present study, 29.4% (20/68) had liver masses >10 cm, and 26.4% (18/68) had ECOG scores of 2 to 3. Nevertheless, the median OS of this study was 12.60 months. Therefore, for patients with worse economic and physical conditions, Banxia XieXin decoction may be a better choice for many patients with advanced liver cancer. In addition, some other new drugs have been tried for the treatment of HCC. Unfortunately, all phase 3 trials assessing novel systemic drugs22-24 have failed to improve outcomes over sorafenib.
In immunotherapy, immune checkpoint inhibitors represented by PD-1/PD-L1 monoclonal antibodies have made rapid progress. The results of the CheckMATE-040 study25 showed that nivolumab achieved an OS of 28.6 months in newly diagnosed patients and a 15.0-month survival benefit in patients who had undergone sorafenib treatment. Pabrizumab, which is also a PD-1 immunosuppressant, used in the KEYNOTE-224 study26 for patients with advanced HCC who had previously progressed with sorafenib, achieved a PFS of 4.9 months, median OS of 12.9 months. Compared with the enrollment conditions for KEYNOTE-224, the condition of the patients enrolled in the present study are more complicated. The largest diameter of the mass in 67.6% of patients is ≥5 cm, 85.3% of patients are in stage IV, 72.1% of patients had HBV infection, and 41.2% of patients had hepatic cirrhosis; even so, this study still achieved a PFS of 6.07 months, higher than the above chemotherapy and immune drugs. It can be seen that the efficacy of Banxia XieXin decoction for the treatment of advanced liver cancer may be better than the above-mentioned immune drugs, and it has the potential to become one of the important methods for liver cancer treatment.
Further subgroup analysis revealed that previous treatment might affect the efficacy of Chinese herbs. In the current analysis, 23 patients had been treated with TACE previously, whose outcome was slightly better compared with those who had not been treated with TACE previously (PFS = 7.0 vs 5.8 months; P = .2158). In terms of OS, the difference is more obvious (OS = undefined vs 11.3 months; P = .0066). Subsequent treatment with Banxia XieXin decoction seems to have a greater benefit for patients with advanced liver cancer who have progressed with interventional therapy.
Overall, the ORR was 10.3% and the DCR was 41.2% in this study. In comparison with the data reported in related literature, the ORR and DCR were, respectively, 3.3% and 35.3% in the Orient trial of sorafenib.9,19 It should be noted that the ECOG-PS score of the included patients was 0 to 3 in our study, but 0 to 2 in the Orient trial, indicating that TCM treatment may have a wider scope of application and may benefit more patients compared with targeted therapy.
In terms of safety, no serious adverse events such as liver or kidney damage were observed during the follow-up period. Thus, Banxia XieXin recipe was generally safe and well-tolerated in patients with advanced HCC.
As for the antitumor mechanism of TCM, it has been reported that TCM or its extracts have direct antitumor effects, such as Pinellia,27 ginseng,28,29 Coptis,30 Glycyrrhiza,31 Atractylodes macrocephala,32 tangerine peel,33 and Scutellaria.34 The effect of inducing apoptosis is also noted, such as with berberine contained in Coptis.35-37 The effect of regulating immunity is noted, such as with Pinellia,27 ginseng,38,39 Atractylodes macrocephala,40 and milkvetch root.41 Antitumor vascular growth, such as that of scutellarin,42,43 can inhibit protein kinase B (Akt) phosphorylation, downregulate VEGF, and inhibit tumor angiogenesis, thus playing a role in inhibiting tumor growth and metastasis. Also, it has been reported that the imbalance of intestinal flora not only affects the occurrence and development of intestinal cancer and irritable bowel syndrome but also is closely related to the occurrence of breast cancer, lung cancer, liver cancer, and other malignant tumors.44-46 However, Atractylodes macrocephala, Scutellaria baicalensis, and Coptis40,47 can indirectly inhibit tumors by regulating intestinal flora. It is worth mentioning that much basic research has found that Pinellia,48 Coptis,49,50 ginseng,51 Scutellaria,52 Atractylodes macrocephala,53 and Astragalus54 have anti-hepatoma effects. For example, berberine,49,50 a constituent of the extract of Coptis in Banxia XieXin decoction, can inhibit the Pi3K/AKT pathway of liver cancer, downregulate MHcc97-H and Hep-G2 cells, phosphorylate the expression of AKT and Pi3K, and inhibit the growth of liver cancer cells in a dose-dependent manner. Besides, it can induce cell cycle arrest and promote apoptosis to treat HCC.
There are only a few patients with tumor shrinkage in the present work. It is believed that the direct antitumor effect of Banxia XieXin decoction is not outstanding, and there may be other mechanisms. After analysis, it was observed that the more prominent effect of Banxia XieXin decoction on liver cancer is reflected in OS, which is similar to the efficacy characteristics of PD-1 immune checkpoint inhibitors. Banxia XieXin decoction contains Pinellia,27 ginseng,38,39 Atractylodes,40 and Astragalus41 medicinal herbs with immunomodulatory effects. For example, ginseng38 could relieve immunosuppression by increased viability of natural killer cells, enhanced immune organ index, improved cell-mediated immune response, increased content of CD4+ and ratio of CD4+/CD8+, and recovery of macrophage function. Therefore, it was speculated that liver cancer patients in this study may benefit from indirect antitumor mechanisms, such as anti-vascular, immunomodulation, and others. In addition, some studies55,56 confirmed that some ingredients of Banxia XieXin decoction could improve liver function and regulate gastrointestinal function. These comprehensive effects can also partially explain why liver cancer patients in this study seem to obtain longer PFS and OS.
In China, most patients with HCC have liver diseases such as hepatitis and cirrhosis. Among the patients enrolled in this study, 49 patients had a history of hepatitis B (72.1%) and 28 patients had liver cirrhosis (41.2%). HCC and basal liver disease often affect each other and form a vicious circle. The scutellaria baicalensis, ginseng, licorice, tangerine peel, and astragalus in Banxia XieXin decoction all have liver-protective effects, and licorice has a direct anti-HBV and hepatitis C virus effects.57,58 From this point of view, this is also one of the reasons why Banxia XieXin decoction may be effective in treating liver cancer. Although much basic research has confirmed the antitumor effects of single herbal extracts in Banxia XieXin decoction, a single herb may not have a good antitumor effect in clinical use, even though the decoction affects liver cancer after being prescribed. Still, each herb may play its respective role whether it causes a new antitumor effect or through compatibility with the effects of other herbs. The antitumor mechanism is unclear and needs further study.
Limitations
The report is limited by its single-arm research design and cannot be directly compared with standard chemotherapy, targeted therapy, or immunotherapy. At the same time, the small number of cases in this study is also one of the disadvantages, but this study is still continuing, and there will be more data analysis in the future. Due to the willingness of patients to choose TCM for treatment in TCM hospitals, it is difficult to avoid certain limitations when we adopt the single treatment of TCM. Although oral Chinese medicine decoction is difficult to take according to scientific equal dosage and concentration, it is in line with the use habits of TCM.
Conclusions
In conclusion, this is the first prospective study of Chinese herbs as monotherapy for the treatment of advanced HCC that used the internationally accepted endpoints of PFS, OS, ORR, and DCR. The findings are encouraging as they suggest that this panel of Chinese herbs is safe and may be effective for patients with advanced HCC in a real-world clinical setting. Further studies are required to assess the comparative efficacy of TCM treatment versus other antitumor therapies in this patient population.
Acknowledgments
The authors acknowledge the contributions of the other investigators in this trial.
Appendix
Appendix.
Components and Their Doses in the Modified XieXin Recipe Decoction (Chinese, Latin, and English Names).
| Chinese name | Latin name | English name | Place of origin | Production batch | Dose (g) |
|---|---|---|---|---|---|
| Banxia | Rhizoma Pinelliae | Pinellia tuber | Sichuan | 19040204 | 10 |
| Huangqin | Radix Scutellariae | Baikal skullcap root | Shanxi | 1904115 | 10 |
| Ganjiang | Rhizoma Zingiberis | Dried ginger | Sichuan | 190301 | 5 |
| Renshen | Radix Ginseng | Ginseng | JIlin | 190601 | 20 |
| Huanglian | Rhizoma Coptidis | Coptis | Sichuan | 190501 | 3 |
| Dazao | Fructus Jujubae | Jujubae, Chinese date | Xinjiang | 190419 | 10 |
| Gancao | Radix Glycyrrhizae | Licorice root | Sichuan | 190701 | 3 |
| Baizhu | Rhizoma Atractylodis Macrocephalae | Largehead Atractylodes rhizome | Zhejiang | 190601 | 15 |
| Chenpi | Pericarpium Citri Reticulatae | Tangerine peel | Sichuan | 19010105 | 10 |
| Huangqi | Radix Astragali | Milkvetch root | Gansu | 19010105 | 100 |
Footnotes
Author Contributions: Lijuan Wang, Jianlong Ke, and Shaoquan Xiong conceived and designed the study. Lijuan Wang, Jianlong Ke, Cui Wang Yaling Li, Qiuyue Luo, Rui Cai, Qian Ding, Panpan Lv, and Tingting Song collected the data. Lijuan Wang, Jianlong Ke, and Cui Wang processed the data. Lijuan Wang and Jianlong Ke wrote the manuscript. All authors have participated in the drafting, review, and approval of the report and the decision to submit for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Natural Science Foundation of China (No. 81573968) and Sichuan Provincial Science and Technology Department Project (No.2017JY0327).
ORCID iDs: Lijuan Wang  https://orcid.org/0000-0003-1704-8847
https://orcid.org/0000-0003-1704-8847
Jianlong Ke  https://orcid.org/0000-0001-5058-7581
https://orcid.org/0000-0001-5058-7581
Panpan Lv  https://orcid.org/0000-0002-7172-7659
https://orcid.org/0000-0002-7172-7659
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3. Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets. 2005;5:131-138. doi: 10.2174/1568009053202063 [DOI] [PubMed] [Google Scholar]
- 4. Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. Published online February 16, 2020. doi: 10.1002/hep.31187 [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. doi: 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 6. Lee DW, Lee KH, Kim HJ, et al. A phase II trial of S-1 and oxaliplatin in patients with advanced hepatocellular carcinoma. BMC Cancer. 2018;18:252. doi: 10.1186/s12885-018-4039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58:81-88. doi: 10.1016/j.jhep.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Cui W. Raltitrexed based transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma: a single-center randomized controlled study. Chemotherapy, 2016;05(02). doi: 10.4172/2167-7700.1000192 [DOI] [Google Scholar]
- 9. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 10. Anonymous. Miraculous Pivot (Ling Shu Jing; originally published during Warring States Period, 475 to 221 BC). People’s Health Press; 1981. [Google Scholar]
- 11. Shao-Quan X, Li-Jian W, Qiu-Yue L, et al. Retrospective analysis of modified Xiexin recipe combined retrospective analysis of modified Xiexin recipe combined. Chin J Integr Tradit Western Med. 2018,38:936-939. [Google Scholar]
- 12. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 14. Gao WJ, Liu YY, Yuan CR. International evaluation system for adverse events of chemotherapeutic drugs in cancer treatment: CTCAE v4.0. Tumor. 2012;32:142-144. [Google Scholar]
- 15. Meng MB, Cui YL, Guan YS, et al. Traditional Chinese medicine plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma. J Altern Complement Med. 2008;14:1027-1042. doi: 10.1089/acm.2008.0060 [DOI] [PubMed] [Google Scholar]
- 16. Xu H, Deng Y, Zhou Z, Huang Y. Chinese herbal medicine (Chaihu-Huaji decoction) alleviates postembolization syndrome following transcatheter arterial chemoembolization and improves survival in unresectable hepatocellular cancer: a retrospective study. Evid Based Complement Alternat Med. 2019;2019:6269518. doi: 10.1155/2019/6269518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang CW, Zhu M, Feng WM, Bao Y, Zheng YY. Chinese herbal medicine, Jianpi Ligan decoction, improves prognosis of unresectable hepatocellular carcinoma after transarterial chemoembolization: a retrospective study. Drug Des Devel Ther. 2016;10:2461-2466. doi: 10.2147/DDDT.S113295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen QF, Wu PH, Huang T, Shen LJ, Huang ZL, Li W. Efficacy of treatment regimens for advanced hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98:e17460. doi: 10.1097/MD.0000000000017460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. doi: 10.1200/JCO.2005.01.3441 [DOI] [PubMed] [Google Scholar]
- 20. Rostom Y, Gouda MY, Al-Nabhi A, Elsaka R, Noman H. Comparison between cirrhotic HCC patients versus non-cirrhotic HCC patients. Pan Arab J Oncol. 2017;10:6-11. [Google Scholar]
- 21. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 22. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. doi: 10.1200/JCO.2012.45.8372 [DOI] [PubMed] [Google Scholar]
- 23. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. doi: 10.1200/JCO.2012.48.4410 [DOI] [PubMed] [Google Scholar]
- 24. Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172-179. doi: 10.1200/JCO.2013.54.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu AX, Finn RS, Edeline J, et al. ; KEYNOTE-224 Investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 27. Yoon JS, Seo JC, Han SW. Pinelliae rhizoma herbal-acupuncture solution induced apoptosis in human cervical cancer cells, SNU-17. Am J Chin Med. 2006;34:401-408. doi: 10.1142/S0192415X0600393X [DOI] [PubMed] [Google Scholar]
- 28. King ML, Murphy LL. Role of cyclin inhibitor protein p21 in the inhibition of HCT116 human colon cancer cell proliferation by American ginseng (Panax quinquefolius) and its constituents. Phytomedicine. 2010;17:261-268. doi: 10.1016/j.phymed.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi HS, Kim KH, Sohn E, et al. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817-1825. doi: 10.1271/bbb.80085 [DOI] [PubMed] [Google Scholar]
- 30. Letasiová S, Jantová S, Miko M, Ovádeková R, Horváthová M. Effect of berberine on proliferation, biosynthesis of macromolecules, cell cycle and induction of intercalation with DNA, dsDNA damage and apoptosis in Ehrlich ascites carcinoma cells. J Pharm Pharmacol. 2006;58:263-270. doi: 10.1211/jpp.58.2.0015 [DOI] [PubMed] [Google Scholar]
- 31. Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002;62(suppl 1):94-100. doi: 10.1159/000048283 [DOI] [PubMed] [Google Scholar]
- 32. Long F, Wang T, Jia P, et al. Anti-tumor effects of atractylenolide-I on human ovarian cancer cells. Med Sci Monit. 2017;23:571-579. doi: 10.12659/msm.902886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang DQ, Tian YP, Song SZ, Wang L. Anti-mutagenesis effects of total flavonoids of Astragalus [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2003;28:1164-1167. [PubMed] [Google Scholar]
- 34. Ye F, Xui L, Yi J, Zhang W, Zhang DY. Anticancer activity of Scutellaria baicalensis and its potential mechanism. J Altern Complement Med. 2002;8:567-572. doi: 10.1089/107555302320825075 [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Gu L, Li J, et al. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010;70:9895-9904. doi: 10.1158/0008-5472.CAN-10-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370-5379. doi: 10.1158/0008-5472.CAN-08-0511 [DOI] [PubMed] [Google Scholar]
- 37. Abdelmoneem MA, Mahmoud M, Zaky A, et al. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J Control Release. 2018;287:78-93. doi: 10.1016/j.jconrel.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 38. Chen LX, Qi YL, Qi Z, et al. A comparative study on the effects of different parts of Panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules. 2019;24:1096. doi: 10.3390/molecules24061096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lim TG, Jang M, Cho CW, et al. White ginseng extract induces immunomodulatory effects via the MKK4-JNK pathway. Food Sci Biotechnol. 2016;25:1737-1744. doi: 10.1007/s10068-016-0265-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji GQ, Chen RQ, Zheng JX. Macrophage activation by polysaccharides from Atractylodes macrocephala Koidz through the nuclear factor-κB pathway. Pharm Biol. 2015;53:512-517. doi: 10.3109/13880209.2014.929152 [DOI] [PubMed] [Google Scholar]
- 41. Zhou L, Liu Z, Wang Z, et al. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. 2017;7:44822. doi: 10.1038/srep44822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou M, Song X, Huang Y, et al. Wogonin inhibits H2O2-induced angiogenesis via suppressing PI3K/Akt/NF-κB signaling pathway. Vascul Pharmacol. 2014;60:110-119. doi: 10.1016/j.vph.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 43. Dai ZJ, Lu WF, Gao J, et al. Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don. BMC Complement Altern Med. 2013;13:150. doi: 10.1186/1472-6882-13-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: models and mechanisms. Free Radic Biol Med. 2017;105:3-15. doi: 10.1016/j.freeradbiomed.2016.10.504 [DOI] [PubMed] [Google Scholar]
- 45. Yang J, Tan Q, Fu Q, et al. Gastrointestinal microbiome and breast cancer: correlations, mechanisms and potential clinical implications. Breast Cancer. 2017;24:220-228. doi: 10.1007/s12282-016-0734-z [DOI] [PubMed] [Google Scholar]
- 46. Herreros Martínez B. Gastric microbiota and carcinogenesis—current evidence and controversy. Rev Esp Enferm Dig. 2016;108:527-529. doi: 10.17235/reed.2016.4559/2016 [DOI] [PubMed] [Google Scholar]
- 47. Garrett WS. Cancer and the microbiota. Science. 2015;348:80-86. doi: 10.1126/science.aaa4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian WT, Zhang XW, Liu HP, Wen YH, Li HR, Gao J. Structural characterization of an acid polysaccharide from Pinellia ternata and its induction effect on apoptosis of Hep G2 cells. Int J Biol Macromol. 2020;153:451-460. doi: 10.1016/j.ijbiomac.2020.02.219 [DOI] [PubMed] [Google Scholar]
- 49. Auyeung KKW, Ko JKS. Coptis chinensis inhibits hepatocellular carcinoma cell growth through nonsteroidal anti-inflammatory drug-activated gene activation. Int J Mol Med. 2009;24:571-577. doi: 10.3892/ijmm_00000267 [DOI] [PubMed] [Google Scholar]
- 50. Song L, Luo Y, Wang X, et al. Exploring the active mechanism of berberine against HCC by systematic pharmacology and experimental validation. Mol Med Rep. 2019;20:4654-4664. doi: 10.3892/mmr.2019.10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X, Zhang S, Sun Q, Jiao W, Yan Y, Zhang X. Compound K induces endoplasmic reticulum stress and apoptosis in human liver cancer cells by regulating STAT3. Molecules. 2018;23:1482. doi: 10.3390/molecules23061482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ye F, Che Y, McMillen E, et al. The effect of Scutellaria baicalensis on the signaling network in hepatocellular carcinoma cells. Nutr Cancer. 2009;61:530-537. doi: 10.1080/01635580902803719 [DOI] [PubMed] [Google Scholar]
- 53. Zhu Y, Li C, Lin X, Sun J, Cheng Y. Effect of Atractylodes macrocephala polysaccharide on proliferation and invasion of hepatocellular carcinoma cells in vitro [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1180-1185. doi: 10.12122/j.issn.1673-4254.2019.10.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lai X, Xia W, Wei J, Ding X. Therapeutic effect of Astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose Response. 2017;15:1559325816685182. doi: 10.1177/1559325816685182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Sun R, Liu R. Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol Res. 2019;144:210-226. doi: 10.1016/j.phrs.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 56. Chen G, Yang Y, Liu M, et al. Banxia Xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J Ethnopharmacol. 2015;166:149-156. doi: 10.1016/j.jep.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 57. Sato H, Goto W, Yamamura J, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171-177. doi: 10.1016/0166-3542(96)00942-4 [DOI] [PubMed] [Google Scholar]
- 58. Wang L, Yang R, Yuan B, Liu Y, Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5:310-315. doi: 10.1016/j.apsb.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]