Abstract
Central venous access devices (CVADs) have completely changed the care for patients who require long-term venous access. With the widespread use of CVADs, the incidence of catheter-related thrombus (CRT) has increased. Catheter-related thrombus is a common complication in patients who use CVADs and is mainly caused by endothelial injury, blood stasis, and hypercoagulability. In recent years, the correlations between oxidative stress (OS) and microRNA (miRNA) and CRT have become a hot topic in clinical research. When a catheter punctures the vessel wall, it causes OS damage to the vascular endothelial cells, leading to a series of CRT diseases. MicroRNAs can regulate the mechanism of thrombus and play an important role in the formation of anti-thrombus. Numerous studies have shown that resistance exercise can reduce the level of OS in vascular endothelial cells, inhibit vascular endothelial cell dysfunction, and maintain the stability of hemodynamics and biochemical state. In the current work, the recent studies on the effects of resistance exercise on OS and miRNA in vascular endothelial cells were reviewed.
Keywords: central venous catheter, resistance exercise, catheter-related thrombosis (CRT), oxidative stress, microRNAs (miRNAs)
Introduction
The central venous access device (CVAD), also known as the central venous catheter, is a small, soft, and hollow catheter whose tip is inserted percutaneously into a large vein near the heart.1 Its advantages of long indwelling time and few puncture times promote its wide use in clinical practice to provide interventional treatment for acute and chronic diseases.2,3 With the widespread application of CVADs, a series of symptoms, such as catheter blockage and thrombosis, has continuously emerged.4 Catheter-related thrombosis (CRT) is one of the most common and most serious complications.5 Catheter-related thrombus is mainly caused by injury to endothelial cells and hemodynamic changes that promote the hypercoagulability of blood.6 The oxidative stress (OS) level of endothelial cells in the cardiovascular system regulates vascular physiology, and OS injury and vascular endothelial dysfunction can promote thrombosis.7 Thrombotic diseases involve multiple factors and systems. Studies on the molecular mechanism of thrombosis have shown that OS and microRNAs (miRNAs) play an important role in the pathological process of thrombotic diseases, such as atherosclerosis, myocardial infarction, and other arterial thrombotic diseases; and pulmonary embolism, deep vein thrombosis, and other venous thrombotic diseases.8 In recent years, resistance exercise has been widely used in clinical practice to prevent CRT, and exercise methods, such as ball clenching, grip clenching, and routine fist clenching, have been adopted to reduce the occurrence of CRT.9,10 The relationship between resistance exercise and thrombus is currently a hot topic among many scholars. The current study reviews the relationship between resistance exercise and related markers and the signaling pathways of OS and miRNAs in CRT prevention.
Epidemiological Characteristics of Peripherally Inserted Central Catheter-Associated Thrombus
Peripherally inserted central catheters (PICCs) have become a conventional treatment for patients with cancer and blood diseases. Peripherally inserted central catheters are mainly used for antitumor drug infusion and adjuvant therapy, including blood transfusion. Catheter-related thrombus refers to the formation of blood clots on the inner wall of a vessel and the wall where the catheter is located; these blood clots result in the partial or complete blockage of the catheter with or without clinical symptoms after catheterization due to a direct injury to the vascular intima via puncture or the catheter itself and due to the patient’s own state.11 A prospective study of patients with cancer with PICCs revealed that 51.4% of the patients developed CRT according to ultrasonic examination; 45.6% of them were asymptomatic,12 and the incidence of symptomatic CRT was only 1% to 5%.13 Symptoms included swelling, pain or numbness in the limbs, erythema in the limbs, and venous dilatation. Severe cases can lead to recurrent deep vein thrombosis, postthrombotic syndrome, or pulmonary embolism.14,15 Catheter-related thrombus increases pain and the risk of death as it can lead to catheter dysfunction, central vein stenosis, and treatment interruption.
Thrombus Formation and Treatment
Thrombosis is a blood clot formed by red blood cells, white blood cells, fibrin, and platelets in the vein. Thrombosis formed in the axillary vein, the subclavian vein of the upper extremity, the vein at the confluent of the upper extremity deep vein, and the vein at the brachial vein is called upper extremity deep vein thrombosis (Table 1). Anticoagulant therapy is complex and often complicated by severe thrombocytopenia among patients with a high risk of bleeding. The systemic prophylactic use of anticoagulants can reduce or eliminate the high coagulation state of blood but will also lead to complications, such as bleeding and hematoma.16 Available data from a randomized controlled trial and observational study do not support the routine use of fixed-dose warfarin to prevent thrombosis.17 In 2012, the International Institute of Thrombosis and Hemostasis and Thoracic Society Clinical Practice Guideline did not recommend the routine use of anticoagulants to prevent CRT in patients with cancer with CVADs.18,19 Warfarin has attracted attention in recent years in the treatment of CRT, but its safety and efficacy are unknown.20
Table 1.
Thrombus Formation and Treatment.
| Thrombus composition | Position of venous thrombosis in upper limb | Anticoagulation | Side effects |
|---|---|---|---|
| Red blood cells | Axillary vein | Warfarin | Bleeding |
| White blood cells | Subclavian vein of upper extremity | Heparin | Hematoma |
| Fibrin | The vein at the confluent of the upper extremity deep vein | ||
| Platelet | The vein at the brachial vein |
In clinical practice, the mechanism of CRT formation is believed to be mainly caused by puncture or the catheter itself (Figure 1). The 3 major factors of classical venous thrombosis are vascular intima injury, blood stasis, and high blood coagulation. Colwell et al16 pointed out that the difference between anticoagulant drugs and physical exercise in reducing the incidence of thrombus is not statistically different and that physical exercise can effectively prevent the complications of CRT. Resistance exercise not only improves vascular endothelial function and blood flow velocity but also inhibits the adverse effects of anticoagulants, such as warfarin and heparin, to prevent CRT formation.
Figure 1.
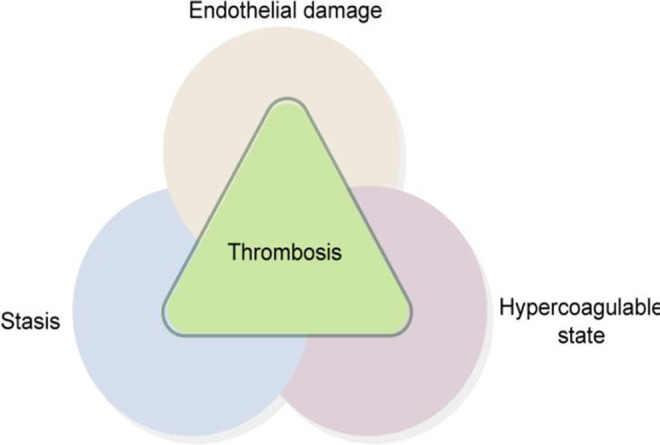
Three major factors of central vein-related thrombosis.
Resistance Exercise in Recent Years
Resistance exercise, also known as resistance training or strength training, usually refers to the process through which the body overcomes resistance to achieve muscle growth and increase strength. Effective upper limb exercise can improve blood circulation and reduce the incidence of CRT.21,22 Early aerobic exercise and resistance exercise at the side of catheterization can increase blood flow velocity, muscle strength, endurance, the pressure load of the heart, and the blood perfusion under the endocardium; such effects result in the improved balance between the supply and demand of cardiovascular oxygen and enhanced cardiovascular function.23 Furthermore, CVADs are mainly used for the infusion treatment of elderly and young patients, those in intensive care units, and those with tumors. Such patients are often sick and weak and would have difficulties in achieving the amount of exercise and intensity required for aerobic exercise. In clinical practice, low-intensity resistance exercise mediates blood flow velocity and thus prevents the occurrence of CRT. As a primary form of prevention, resistance exercise is safe, noninvasive, straightforward to learn, economical for patients, and easy to popularize; such factors provide the basis for the clinical system management of the upper limb exercise of patients after catheterization.
Factors Influencing the Effects of Resistance Exercise on Vascular Endothelial Injury
Located on the lumen of the vessel wall, the vascular endothelium is a complete structure composed of a monolayer of endothelial cells. It is in direct contact with the blood stream and plays anticoagulant and anti-inflammatory roles by keeping the blood vessels unobstructed mainly through the secretion of a series of molecular substances, such as nitric oxide, thrombin A2, prostacyclin, and endothelin-1.24 When the vascular intima is damaged, endothelial cells can quickly cover and replenish the damaged endothelium.24 According to statistics, 70% of deep vein thrombosis in the upper limb is caused by catheters, accounting for 10% of all types of venous thrombosis.25 Regular and moderate physical exercise is one of the most effective interventions to improve endothelial cell function. Souza et al26 demonstrated the positive exercise response in the hearts of rats undergoing resistance training for 12 weeks; this result suggests that resistance exercise increases the thickness of the aortic wall. Regular exercise with moderate intensity can change the shear stress of the bloodstream, regulate antioxidant protection, inhibit endothelial inflammatory signaling pathways, and improve vascular endothelial function and morphology.27,28 Thus, this form of exercise plays a role in preventing venous thrombosis to some extent.
Factors Influencing the Effects of Resistance Exercise on Hemodynamics
The catheter tip is mainly placed in the inferior vena cava whose vascular diameter is relatively narrow and blood flow is relatively minimal and slow. It prolongs the contact time between the vascular endothelium and the stimulating liquid or chemotherapy drugs, increases the degree of damage to the vascular endothelium, and causes deep vein thrombosis. In addition to the vascular damage caused by catheter implantation, when blood flow is slowed down or irregular, the catheter through the narrow venous lumen can promote platelet aggregation to release serotonin, clotting factor, and other substances. When central venous catheterization itself causes blood stasis, the patient consciously reduces the exercise of the limb; hence, the blood flow slows down, and the risk of thrombosis increases. Studies have shown that intravascular catheter placement can decrease local blood flow velocity by approximately 60%.29 The sharp decrease in blood flow shear stress may directly induce OS damage to the vascular endothelial cells and promote CRT formation. At present, clinical researchers have reported that exercise intervention can prevent venous thrombosis. Several researchers subjected patients implanted with PICC to grip strength training. After the patients squeezed the elastic ball for 10 seconds, they were tired out and allowed to relax for 10 seconds; the maximum blood flow of the axillary vein was detected by ultrasound.9 In study by Ayse and colleagues,10 patients with PICC were required to exercise for 20 minutes a day for 5 days a week and to contract and relax the forearm muscles by gripping and relaxing a ball for 4 seconds to increase blood flow to the forearm.
Factors Influencing the Effects of Resistance Exercise on the Hypercoagulable State
Numerous studies have shown that venous thrombosis and its development originate in the venous sinuses or valves.30 The hypercoagulable microenvironment caused by the blood stasis and hypoxia in the valvular sinus results in the decreased expression of thromboregulatory protein and endothelial protein C receptor; thus, thrombus formation is promoted. The change in blood composition in a high coagulation state is the decisive factor for CRT formation, which is manifested by increased fibrin degradation products and platelets, hyperfibrinogenemia, and prothrombin prolongation. In the elderly, blood viscosity increases, and erythrocyte deformability is poor. With increasing age, the fibrinolytic activity decreases, and the antifibrinolytic activity increases, thereby increasing the possibility of thrombus formation. Vascular injury caused by catheter insertion, venous stasis caused by catheter indwelling, continuous movement of the catheter in the vein, platelet adhesion, and coagulation caused by the interaction of some tumor cells and their products with host cells, and the release of prothrombin kinase can promote high blood coagulation, which in turn results in occluded mural thrombus.31 Chemotherapy drugs are prone to antigen and antibody reactions in the blood vessels, which cause vascular endothelial injury and thrombin release. The feedback causes an increase in fibrinogen, thus aggravating the hypercoagulable state of the blood, which further causes vascular endothelial injury. Resistance exercise not only makes the muscle contract and squeezes the upper limb blood vessels but also promotes the venous blood flow of the catheter’s arm, improves fibrinolytic activity, and timely dissolves platelets and fibrinogen aggregation; under these conditions, the incidence of CRT is ultimately reduced.32
Relationship Between the Molecules Associated With OS and Thrombus
Overview of Oxidation System
Oxidative stress was initially described as a pro-oxidant-antioxidant imbalance disorder that may lead to cell damage and temporary or long-term reactive oxygen species (ROS) elevation, which disrupts cell metabolism and damages cell components.33 When a catheter punctures the vessel wall or is left in the vessel for a long time, the vascular endothelial cells become damaged, and their biological function is consequently affected. A series of defense reactions then occurs. Oxidative stress refers to the excessive production of ROS and reactive nitrogen species resulting from the harmful stimulation of the body and the imbalance between oxidation and antioxidants in the body; most of these reactions are negative.34 The OS damage of the vascular intima is mainly mediated by ROS. Under normal circumstances, ROS content in the body and the antioxidants in cells are in a complex balance, but an increase or decrease in ROS may lead to an antioxidant state that causes cell damage.
In the cardiovascular system, ROS plays a role in the control of inflammation, proliferation, apoptosis, endothelial function, and angiogenesis. Reactive oxygen species is one of the important factors leading to thrombosis development.35 Aizawa et al35 showed that cellular ROS can promote the occurrence of thrombus and that the increase in ROS in endothelial cells is a major factor for endothelial dysfunction and the occurrence and development of cardiovascular diseases. Studies have shown that when intravascular blood flow is slowed down or blocked, endothelial cell OS damage is induced, and endothelial cell dysfunction may occur.36 Numerous animal studies have demonstrated that ROS overexpression has a pathogenic effect on atherosclerosis37 and thrombotic cerebral apoplexy,38 which has attracted considerable attention in many fields. When ROS increases, atherosclerosis, aging, and ischemia–reperfusion injury may occur.36,39
A high ROS content can peroxidize the polyunsaturated fatty acids on the cell membrane, produce malondialdehyde (MDA), cause further tissue OS damage, change the permeability of the cell membrane, and finally induce the rupture of the double-layer structure of the cell membrane.40 Malondialdehyde, an oxidase, is a product of lipid peroxide degradation that can cause the reaction of macromolecules to produce strong biological toxicity. Damage to the vascular endothelial cells can cause the lipid peroxidation of polyunsaturated fatty acids on the cell membrane and their degradation to MDA. In animal experiments of deep vein thrombosis in the upper limb,41 atherosclerosis, 42 and myocardial infarction, the MDA content in the experimental group greatly increased.41–43 Malondialdehyde, as a transmitter of OS, plays an important role in thrombosis. Several studies found that the MDA content in the deep vein thrombosis group was significantly higher than that in the control group (2.49 vs 1.01 μm/L; P < .05); these studies also observed changes in deep vein thrombosis.44 Therefore, OS plays a key role in thrombosis.
Effects of Resistance Exercise on Oxidation System
The imbalance between antioxidants and pro-oxidants is a major cause of vascular damage. Resistance exercise can effectively reduce OS, improve vascular health, and reduce cardiovascular risk factors.45 Regular physical activity can increase the antioxidant defense system, weaken the concentration of ROS, and confer protection against OS-related diseases; as such, it can significantly improve the cardiovascular autonomic regulation ability of myocardial infarction in rats and make the rehabilitation of myocardial infarction safe and effective.46 An hour of moderate endurance exercise can increase the body’s lipid peroxides, which are systematic markers of OS and antioxidant capacity in healthy people. The role of ROS in exercise is of considerable interest to researchers.47 High levels of MDA are used as markers of OS during acute exercise, and exercise-induced apoptosis is a regulatory process designed to eliminate damaged cells without producing a significant inflammatory response, thus ensuring optimal body function.48 Malondialdehyde production has been reported to decrease after regular resistance exercise training.49
Effects of Antioxidant System on Thrombus
The antioxidant system, which mainly includes enzyme and nonenzyme antioxidant systems, can effectively remove free radicals from living organisms to protect cells from OS damage. Oxidative stress damage is mainly related to ROS-mediated oxidases, such as superoxide dismutase (SOD), heme oxygenase (HO), and catalase. Nonenzymatic systems mainly consist of glutathione, vitamin E, vitamin C, and some trace elements.50 In the antioxidant system, SOD and HO-1, in particular, reduce ROS to achieve the effect of the vascular antioxidant system.
Superoxide dismutase is an important antioxidant enzyme that scavenges reactive oxygen free radicals and serves as an important marker of intracellular OS. Superoxide dismutase is the main antioxidant in vascular endothelial cells that can remove lipid peroxides in the body, reduce peroxidation damage to cells, bind platelet/endothelial cell adhesion molecule 1 antibodies, and specifically bind to endothelial cells so as to play an antioxidant defense role in vascular endothelial cells. Villegas et al51 reported that according to the results of a large number of animal experiments, SOD can reduce hypoxic-induced pulmonary hypertension and pulmonary vascular remodeling. Bahnson et al52 inserted catheters into the external carotid artery of rats to establish a carotid injury model and found that SOD is involved in regulating OS and inhibiting the development of new intima.
Heme oxygenase is a speed-limiting enzyme in heme catabolism, and carbonic oxide in the human body is mainly produced via HO metabolism.53 Heme oxygenase has 3 types: oxygen stress induction (HO-1), enzyme composition (HO-2), and HO-3. Heme oxygenase 3 and HO-2 have homologous expressions in multiple organs.54 Heme oxygenase 1, a member of the HO protein family, is a common inducible enzyme in mammals that protects cells from OS damage by degrading pro-oxidizing heme to produce the antioxidant bilirubin.55 Heme oxygenase 1 is expressed in the vascular endothelial cells and smooth muscle cells and can inhibit vascular smooth muscle cell proliferation and migration from the middle layer of the artery to the intima; thus, it can protect damaged blood vessels.56 Studies have shown that HO-1 induction inhibits arterial and venous thrombosis in animal models.57 Many studies have reported that the expression levels of HO-1 are related to the expression levels of atherosclerosis biopsy.58 The results indicate that increasing the expression of antioxidant enzymes and stress proteins could prevent the development of cardiovascular diseases.
Effects of Resistance Movement on Antioxidant Systems
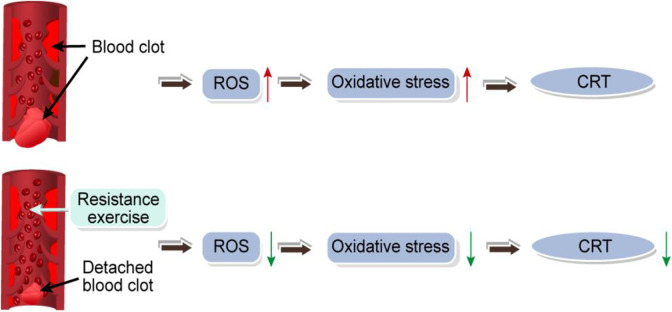
Resistance training brings the redox reaction to a balanced state, thereby reducing metabolic and immune-related diseases. After resistance training, the increase in total SOD activity is inhibited because the adaptation of endogenous antioxidants produced by high levels of exogenous antioxidants in the skeletal muscle is inhibited.59 After short-term strenuous exercise and 24 hours recovery, antioxidants exert a blocking effect on SOD activity.60 Regular physical exercise plays an important role in improving vascular function. In studies related to the reduction of pro-inflammatory cytokines, statistically differences in the effects of 12 weeks physical exercise and high-triglyceride diet on HO-1 concentration in rat aorta (P < .0001), voluntary wheel movement restored the concentration and activity of HO enzymes in the aorta and heart.61 Exercise training preserves vascular endothelial function, but its underlying molecular mechanisms are unclear. Heme oxygenase 1 induced by training mainly exists in the vascular endodermis and perivascular layer.62 Oxidative stress is likely to participate in CRT formation; resistance exercise can regulate OS and affect CRT formation (Figure 2). However, the underlying downstream targets and related signaling pathways still need to be further explored.
Figure 2.
The effect of resistance exercise on oxidative stress caused by catheter perforation of vascular endothelium.
Pathway of Resistance Exercise and Oxidative Stress-Mediated Thrombus
Oxidative stress can promote thrombotic diseases by activating the nuclear factor kappa-B (NF-κB) and the mitogen-activated protein kinase (MAPK) signaling pathways. Nuclear factor κB is a member of the transcription factor protein family that is widely found in cells in the form of various NF-κB complexes.63 When the cell is stimulated by a variety of extracellular motions, physical and biochemical interventions, and so on, the inhibitor of NF-κB kinase is activated, leading to the phosphorylation, ubiquitination, and degradation of the IκB protein and causing the release of NF-κB dipolymers. Dissociative NF-κB quickly shifts to the nucleus, combines with specific gene sequences, and induces the transcription of related genes; this series of events results in tissue damage and thus causes a variety of cardiovascular diseases.64 Onai et al65 injected a novel IκB phosphorylation inhibitor to inhibit NF-κB in a rat myocardial infarction model and observed improvements in the ventricular remodeling and left ventricular diastolic function. Reactive oxygen species can activate the NF-κB signaling pathway to accelerate vascular endothelial cell apoptosis and increase the production of tissue factors and thrombogenic molecules to promote venous thrombosis. Tabassum et al66 downregulated the NF-κB expression with Perizol to reduce OS and improve the outcome of thrombotic stroke. Therefore, many experts have proposed that inhibiting NF-κB and other signaling pathways can effectively control the state of OS, improve the antioxidant capacity of endothelial cells, and prevent the occurrence and development of thrombosis. Regulating reactive oxygen production induced by regular training can be adaptive against oxidase gene expression through the NF-κB pathway in the skeletal muscle. In other words, exercise training can improve the antioxidant system of the body and increase its resistance to OS.49 After resistance exercise training, the activation of the NF-κB signaling pathway leads to the phosphorylation of its inhibitor (IκB), leading to the release and binding of NF-κB from its position to the DNA; as such, the gene expressions of SOD, catalase, and other enzymes are stimulated, and antioxidant defense is strengthened.67 Therefore, resistance exercise training can stimulate the NF-κB signaling pathway, leading to the expression of certain antioxidants. The body’s total antioxidant capacity is enhanced, thereby inhibiting the formation of CRT.
The MAPK signaling pathway is a serine/threonine kinase family activated by the phosphorylation of various extracellular or intracellular stimuli, such as growth factors, cytokines, and OS; it regulates cell proliferation, differentiation, and apoptosis.68 , 69 The increased phosphorylation of p38, JNK, and extracellular-regulated protein kinases (ERK) caused by ROS70 suggests that ROS-induced apoptosis may be mediated by regulating the MAPK signaling pathway.71 Yang72 determined that the platelet scavenger receptor CD36 promotes thrombosis by increasing MAPK extracellular signals to activate ERK5.
The mechanism of the downregulation of P38 MAPK and NF-κB signals by low-dose and low-oxygen mesenchymal stem cells can inhibit pulmonary embolism and thrombosis.73 After resistance exercise training, the supplementation of antioxidants can block the cell signaling pathways associated with muscle hypertrophy (MAPK and p70S6 kinase) without affecting the partial synthesis rate of muscle proteins. Evidence suggests that MAPK is activated by OS.74,75
The activation of JNK is related to the regulation of cell proliferation, apoptosis, inflammation, and DNA repair. The elevated ROS in skeletal muscles caused by exercise may play an important role in exercise adaptation, and antioxidant supplementation inhibits JNK phosphorylation.48 We hypothesized that resistance movement could downregulate the P38 MAPK and NF-κB signaling pathways, induce ROS production, reduce OS damage, and improve vascular endothelial function.
Meanwhile, miRNAs and MDA produced by the peroxidation of polyunsaturated fatty acids caused by a high content of ROS can be used as indicators for the early diagnosis and prognosis assessment of venous thrombus.76 However, the study of OS and miRNAs in the context of deep vein thrombosis is still in the initial stage, and extensive research is needed to clarify their mechanisms of action.
Relation Between miRNAs and CRT Disease
MicroRNAs are a class of noncoding small RNA molecules with an endogenous length of about 22 nucleotides. They control gene expression mainly by degrading mRNAs by binding to the 3′-UTR region of the target gene mRNA and/or inhibiting the translation control gene expression of the target gene at the transcriptional level.77 More than 500 human miRNAs have been identified so far. MicroRNAs are important gene adjusting factors in the body that participate in cell proliferation, migration, angiogenesis, insulin secretion, neural differentiation, and heart embryonic organization development process. Many studies have demonstrated that miRNAs play an important role and function in the development of and changes in diseases. Comprehensive research data prove that miRNAs are the targets of effective treatments for many diseases, such as tumor- and thrombosis-related diseases. Different miRNAs have different transport mechanisms and specific releases. They are surrounded by different extracellular vesicles that prevent RNA enzymes from degrading them. As an endothelium-specific miRNA, miR-126 is only expressed in endothelial cells. Therefore, miR-126 is considered as an important factor regulating angiogenesis and inflammation in clinical practice. Moreover, miR-126 directly regulates the adhesion of endothelial monocytes to target Vascular Cell Adhesion Molecule-1 to control vascular inflammation.78 Other miRNAs can combine with proteins to form complexes. For example, miR-92a can be transported after binding with lipid proteins. MicroRNAs can form complexes that are not degraded by RNA enzymes to some extent.
Currently, most studies on miRNAs focus on their effects on cancer, and their role in the cardiovascular system has been gradually confirmed. MicroRNAs released from cells can be stable in the circulation of peripheral blood and are highly sensitive and easy to detect. Therefore, many experts agree that miRNAs may be a new marker for the diagnosis of thrombotic diseases. Qin et al79 confirmed that miRNAs play a crucial role in deep vein thrombosis and that the detection of peripheral blood miRNAs may replace d-dimers as markers for the diagnosis of thrombotic diseases. Meng et al78 reported that miRNAs play an important role in thrombolytic recanalization and that the expression levels of miR-199a and miR-483 in patients with venous thrombosis were significantly changed relative to those in healthy controls. Further studies have confirmed the involvement of miR-199a and miR-483 in thrombolytic recanalization and angiogenesis. Moreover, miR-92a has been found to be highly expressed in human vascular endothelial cells. Studies have shown that plasma miR-92a mainly comes from Annexin V + / CD31 + microparticles (MPs) released by endothelial cells and platelets. Its main mechanism may be as follows. After vascular endothelial cells and platelets are activated during thrombosis, miR-92a is released in the form of MPs78 to inhibit angiogenesis. In summary, the catheter puncture injury of the vascular wall and long-term catheterization lead to the dysfunction of endothelial cells in patients and thereby causes a series of CRT diseases. MicroRNAs play an important role in the formation of antithrombotic agents and may become a new marker for the prevention of CRT. MicroRNAs may also be involved in the regulation of certain signaling pathways, such as miR-10a, which mediates thrombotic diseases by regulating the NF-κB pathway. In the endothelial cells mediated by OS, the expression of miR-200c is upregulated.80 Therefore, miRNAs may be involved in the occurrence and development of thrombotic diseases in different ways.
Regulation of miRNA by Resistance Exercise to Prevent CRT
A few studies have explored the hemodynamic effects of resistance exercise training on central venous catheters. As resistance exercise training may also affect the target genes of OS and cardiovascular autonomic function, the current research status of the correlation between miRNAs and CRT diseases should be further verified. The latest research results of our team81 have confirmed that miR-92a-3p and OS are related to venous thrombosis. The expression levels of venous miR-92a-3p, HO-1, p38 MAPK, and NF-κB p65 mRNA in the CRT group were significantly upregulated, and miR-92a-3p was positively correlated with HO-1 (r = 0.770, P = .015). The relationship between specific signaling pathways that are activated during resistance exercise is changing considerably. After acute endurance exercise, the increased gene expressions of biogenic mechanisms and members of the output pathways of miRNAs have been observed in skeletal muscles.82 The studies on differential miRNA expression and the potential role of miRNAs in specific pathways have revealed that increased resistance exercise is associated with changes in circulating miRNA levels.83,84
Conclusion
The implantation of central venous catheters leads to the OS injury of vascular endothelial cells. Moreover, miRNA-mediated endothelial cell apoptosis and vascular inflammation lead to thrombosis. These conditions lead to different degrees of thrombotic diseases involving multiple systems and organs, seriously endangering the life of patients. Resistance exercise is a popular strategy for promoting health and fitness because of its beneficial effects on the cardiovascular system. Early resistance exercise interventions can change blood flow velocity, reduce the occurrence of CRT, and thereby minimize related complications and improve cardiovascular function.85 At present, a few clinical studies have proved that OS and miRNA-related markers are closely related to thrombosis. Further studies are needed to provide a strong basis and direction for the prevention and treatment of CRT.
Acknowledgments
The authors would like to express gratitude to Huihan Zhao for her support and advice.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Science Foundation of China (Grant No. 81860032).
ORCID iD: Cui Wen  https://orcid.org/0000-0001-9368-9799
https://orcid.org/0000-0001-9368-9799
Jianpeng Zhou  https://orcid.org/0000-0002-0333-6111
https://orcid.org/0000-0002-0333-6111
References
- 1. Gavin NC, Webster J, Chan RJ, Rickard CM. Frequency of dressing changes for central venous access devices on catheter-related infections. Cochrane Database Syst Rev. 2016;2:CD009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ullman AJ, Mrash G, Mihala C, Rickard CJ. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331–1344. [DOI] [PubMed] [Google Scholar]
- 3. Balsorano P, Virgili G, Villa G, et al. Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: a systematic review and meta-analysis. J Vasc Access. 2019:21(5):1129729819852203. [DOI] [PubMed] [Google Scholar]
- 4. Gunther SC, Schwebel C, Roy RH, et al. Complications of intravascular catheters in ICU: definitions, incidence and severity. A randomized controlled trial comparing usual transparent dressings versus new-generation dressings (the ADVANCED study). Intensive Care Med. 2016;42(11):1753–1765. [DOI] [PubMed] [Google Scholar]
- 5. Gunawansa N, Sudusinghe DH, Wijayaratne DR. Hemodialysis catheter-related central venous thrombosis: clinical approach to evaluation and management. Ann Vasc Surg. 2018;51:298–305. [DOI] [PubMed] [Google Scholar]
- 6. Wall C, Moore J, Thachil J. Catheter-related thrombosis: a practical approach. J Intensive Care Soc. 2016;17(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Gorlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol. 2017;174(12):1533–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li G, Zhou R, Zhao X, Liu R, Ye C. Correlation between the expression of IL18 and deep venous thrombosis. Int J Mol Med. 2018;42(2):2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu K, Zhou Y, Xie W, et al. Handgrip exercise reduces peripherally-inserted central catheter-related venous thrombosis in patients with solid cancers: a randomized controlled trial. Int J Nurs Stud. 2018;86:99–106. [DOI] [PubMed] [Google Scholar]
- 10. Ozkaraman A, Yesilbalkan OU. Effect of isometric hand grip exercises on blood flow and placement of IV catheters for administration of chemotherapy. Clin J Oncol Nurs. 2016;20(2):E55–E59. [DOI] [PubMed] [Google Scholar]
- 11. Joks M, Czyz A, Poplawski D, Komarnicki M. Incidence and risk factors for central venous catheter-related thrombosis in hematological patients. Med Oncol. 2014;31(1):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Gao Y, Wei L, Chen W, Ma X, Song L. Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Therap Clin Risk Manag. 2015;11:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014(1):306–311. [DOI] [PubMed] [Google Scholar]
- 14. Joks M, Czyż A, Popławski D, Komarnicki M. Incidence and risk factors for central venous catheter-related thrombosis in hematological patients. Med Oncol. 2013;31(1):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant JD, Stevens SM, Woller SC, et al. Diagnosis and management of upper extremity deep-vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108. [DOI] [PubMed] [Google Scholar]
- 16. Colwell CW, Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527–535. [DOI] [PubMed] [Google Scholar]
- 17. Mokrzycki MH, Jerome KJ, Rush H, Zdunek MP, Rosenberg SO. A randomized trial of minidose warfarin for the prevention of late malfunction in tunneled, cuffed hemodialysis catheters. Kidney Int. 2001;59(5):1935–1942. [DOI] [PubMed] [Google Scholar]
- 18. Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11(1):71–80. [DOI] [PubMed] [Google Scholar]
- 19. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl): e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41(1):206–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cunha PM, Ribeiro AS, Tomeleri CM, et al. The effects of resistance training volume on osteosarcopenic obesity in older women. J Sports Sci. 2018;36(14):1564–1571. [DOI] [PubMed] [Google Scholar]
- 22. Gorski LA. The 2016 infusion therapy standards of practice. Home Healthc Now. 2017;35(1):10–18. [DOI] [PubMed] [Google Scholar]
- 23. Metsios GS, Moe RH, van der Esch M, et al. The effects of exercise on cardiovascular disease risk factors and cardiovascular physiology in rheumatoid arthritis. Rheumatol Int. 2020;40(3):347–357. [DOI] [PubMed] [Google Scholar]
- 24. Cui Y, Zhou F, Wei L, et al. In situ endothelialization promoted by SEMA4D and CXCL12 for titanium-based biomaterials. Semin Thromb Hemost. 2018;44(1):70–80. [DOI] [PubMed] [Google Scholar]
- 25. Kreuziger LB, Jaffray J, Carrier M. Epidemiology, diagnosis, prevention and treatment of catheter-related thrombosis in children and adults. Thromb Res. 2017;157:64–71. [DOI] [PubMed] [Google Scholar]
- 26. Souza RR, de Franca E, Madureira D, Pontes CCR, Santana JO, Caperuto EC. Resistance training improves aortic structure in Wistar rats. Braz J Phys Ther. 2017;21(4):244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatzizisis YS, Baker AB, Sukhova GK, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011;123(6):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Theodoris CV, Li M, White MP, et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160(6):1072–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest. 2011;140(1):48–53. [DOI] [PubMed] [Google Scholar]
- 30. Behravesh S, Hoang P, Nanda A, et al. Pathogenesis of thromboembolism and endovascular management. Thrombosis. 2017;2017:3039713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forauer AR, Theoharis CG, Dasika NL. Jugular vein catheter placement: histologic features and development of catheter-related (fibrin) sheaths in a swine model. Radiology. 2006;240(2):427–434. [DOI] [PubMed] [Google Scholar]
- 32. Zhang M, Fang X-x, L-e, Zheng C-h, Xi huan Z, Xiao-qin L. The effect of hand grip exercise on the hemodynamic parameters in upper limbs in healthy adult. Chin J Nurs. 2014;49(11):1325–1329. [Google Scholar]
- 33. Pesta D, Roden M. The Janus head of oxidative stress in metabolic diseases and during physical exercise. Curr Diab Rep. 2017;17(6):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6(8):662–680. [DOI] [PubMed] [Google Scholar]
- 35. Aizawa K, Takahari Y, Higashijima N, et al. Nicorandil prevents sirolimus-induced production of reactive oxygen species, endothelial dysfunction, and thrombus formation. J Pharmacol Sci. 2015;127(3):284–291. [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Wen L, Martin M, et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation. 2015;131(9):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Awad MA, Aldosari SR, Abid MR. Genetic alterations in oxidant and anti-oxidant enzymes in the vascular system. Front Cardiovasc Med. 2018;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feng Y, Liao S, Wei C, et al. Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models. J Neuroinflammation. 2017;14(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hort MA, Straliotto MR, Oliveria JD, et al. Diphenyl diselenide protects endothelial cells against oxidized low density lipoprotein-induced injury: involvement of mitochondrial function. Biochimie. 2014;105:172–181. [DOI] [PubMed] [Google Scholar]
- 40. Milicevic NP, Busch CJ, Binder CJ. Malondialdehyde epitopes as targets of immunity and the implications for atherosclerosis. Adv Immunol. 2016;131:1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Y, Chu X, Pang XB, Wang SH, Du GH. Antithrombotic effects of the effective components group of Xiaoshuantongluo formula in vivo and in vitro. Chin J Nat Med. 2015;13(2):99–107. [DOI] [PubMed] [Google Scholar]
- 42. Chen L, Jiang JQ, Zhang Y, Feng H. Experimental study on oral sulfhydryl as an adjuvant for improving nitrate ester tolerance in an animal model. Eur Rev Med Pharmacol Sci. 2018;22(5):1469–1477. [DOI] [PubMed] [Google Scholar]
- 43. Yang J, Yin HS, Cao YJ, et al. Arctigenin attenuates ischemia/reperfusion induced ventricular arrhythmias by decreasing oxidative stress in rats. Cell Physiol Biochem. 2018;49(2):728–742. [DOI] [PubMed] [Google Scholar]
- 44. Ferrante M, Fiore M, Conti GO, et al. Transition and heavy metals compared to oxidative parameter balance in patients with deep vein thrombosis: a case-control study. Mol Med Rep. 2017;15(5):3438–3444. [DOI] [PubMed] [Google Scholar]
- 45. Cook MD, Heffernan KS, Ranadive S, Woods JA, Fernhall B. Effect of resistance training on biomarkers of vascular function and oxidative stress in young African-American and Caucasian men. Jo Hum Hypertens. 2013;27(6):388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quinteiro H, Buzin M, Conti FF, et al. Aerobic exercise training promotes additional cardiac benefits better than resistance exercise training in postmenopausal rats with diabetes. Menopause. 2015;22(5):534–541. [DOI] [PubMed] [Google Scholar]
- 47. Dantas FF, Santos Mdo SB, Batista RM, et al. Effect of strength training on oxidative stress and the correlation of the same with forearm vasodilatation and blood pressure of hypertensive elderly women: a randomized clinical trial. PLoS One. 2016;11(8):e0161178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pastor R, Tur JA. Antioxidant supplementation and adaptive response to training: a systematic review. Curr Pharm Des. 2019;25(16):1889–1912. [DOI] [PubMed] [Google Scholar]
- 49. Karabulut AB, Kafkas ME, Kafkas AS, Onal Y, Kiran TR. The effect of regular exercise and massage on oxidant and antioxidant parameters. Indian J Physiol Pharmacol. 2013;57(4):378–383. [PubMed] [Google Scholar]
- 50. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villegas LR, Kluck D, Field C, et al. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal. 2013;18(14):1753–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bahnson ES, Koo N, Medellin NC, et al. Nitric oxide inhibits neointimal hyperplasia following vascular injury via differential, cell-specific modulation of SOD-1 in the arterial wall. Nitric Oxide. 2015;44:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu ML, Ho YC, Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal. 2011;15(7):1835–1846. [DOI] [PubMed] [Google Scholar]
- 54. Toprak EK, Erkek OK, Mete GA, et al. Contribution of heme oxygenase 2 to blood pressure regulation in response to swimming exercise and detraining in spontaneously hypertensive rats. Med Sci Monit. 2018;24:5851–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Przeczek AG, Dulak J, Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond). 2012;122(3):93–103. [DOI] [PubMed] [Google Scholar]
- 56. Choi YK. Role of carbon monoxide in neurovascular repair processing. Biomol Therap. 2018;26(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fei D, Meng X, Zhao M, et al. Enhanced induction of heme oxygenase-1 suppresses thrombus formation and affects the protein C system in sepsis. Transl Res. 2012;159(2):99–109. [DOI] [PubMed] [Google Scholar]
- 58. Cheng C, Noordeloos AM, Jeney V, et al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119(23):3017–3027. [DOI] [PubMed] [Google Scholar]
- 59. Morrison D, Hughes J, Della Gatta PA, et al. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radical Biol Med. 2015;89:852–862. [DOI] [PubMed] [Google Scholar]
- 60. Jowko E, Dlugolecka B, Makaruk B, Cieslinski I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur J Nutr. 2015;54(5):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varga C, Veszelka M, Kupai K, et al. The effects of exercise training and high triglyceride diet in an estrogen depleted rat model: the role of the heme oxygenase system and inflammatory processes in cardiovascular risk. J Sports Sci Med. 2018;17(4):580–588. [PMC free article] [PubMed] [Google Scholar]
- 62. Sun MW, Zhong MF, Gu J, Qian FL, Gu JZ, Chen H. Effects of different levels of exercise volume on endothelium-dependent vasodilation: roles of nitric oxide synthase and heme oxygenase. Hypertens Res. 2008;31(4):805–816. [DOI] [PubMed] [Google Scholar]
- 63. Chaudhari N, Talwar P, Parimisetty A, d’Hellencourt CL, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang WC, Hung MC. Beyond NF-kappaB activation: nuclear functions of IkappaB kinase alpha. J Biomed Sci. 2013;20(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Onai Y, Suzuki J, Maejima Y, et al. Inhibition of NF-{kappa}B improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292(1):H530–H538. [DOI] [PubMed] [Google Scholar]
- 66. Tabassum R, Vaibhav K, Shrivastava P, et al. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-kappaB in middle cerebral artery occlusion rats. Eur J Pharmacol. 2015;747:190–199. [DOI] [PubMed] [Google Scholar]
- 67. Alikhani S, Vatani DS. Oxidative stress and anti-oxidant responses to regular resistance training in young and older adult women. Geriatr Gerontol Int. 2019;19(5):419–422. [DOI] [PubMed] [Google Scholar]
- 68. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. [DOI] [PubMed] [Google Scholar]
- 69. Sun J, Nan G. The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. J Mol Neurosci. 2016;59(1):90–98. [DOI] [PubMed] [Google Scholar]
- 70. Hao W, Yuan X, Yu L, et al. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci Rep. 2015;5:10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li SW, Wang CY, Jou YJ, et al. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-beta1 via ROS/p38 MAPK/STAT3 pathway. Sci Rep. 2016;6:25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang M, Cooley BC, Li W, et al. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood. 2017;129(21):2917–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu YY, Chiang CH, Hung SC, et al. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS One. 2017;12(11):e0187637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paulsen G, Cumming KT, Hamarsland H, Borsheim E, Berntsen S, Raastad T. Can supplementation with vitamin C and E alter physiological adaptations to strength training? BMC Sports Sci Med Rehabil. 2014;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paulsen G, Hamarsland H, Cumming KT, et al. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol. 2014;592(24):5391–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kong L, Du X, Hu N, et al. Downregulation of let-7e-5p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis via targeting FASLG. Thromb Res. 2016;138:30–36. [DOI] [PubMed] [Google Scholar]
- 77. Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6(11):8474–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Meng Q, Wang W, Yu X, et al. Upregulation of microRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem. 2015;116(8):1613–1623. [DOI] [PubMed] [Google Scholar]
- 79. Qin J, Liang H, Shi D, et al. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J Thromb Thrombolysis. 2015;39(2):215–221. [DOI] [PubMed] [Google Scholar]
- 80. Magenta A, Cencioni C, Fasanaro P, et al. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18(10):1628–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gan X, Zhao H, Wei Y, Jiang Q, Wen C, Ying Y. Role of miR-92a-3p, oxidative stress, and p38MAPK/NF-kappaB pathway in rats with central venous catheter related thrombosis. BMC Cardiovasc Disord. 2020;20(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hinkley JM, Konopka AR, Suer MK, Harber MP. Short-term intense exercise training reduces stress markers and alters the transcriptional response to exercise in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2017;312(3):R426–R433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nielsen S, Akerstrom T, Rinnov A, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One. 2014;9(2):e87308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Polakovicova M, Musil P, Laczo E, Hamar D, Kyselovic J. Circulating microRNAs as potential biomarkers of exercise response. Int J Mol Sci 2016;17(10):1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ziemba AW, Moneta JC, Uscilko HK, et al. Early effects of short-term aerobic training. Physiological responses to graded exercise. J Sports Med Phys Fitness. 2003;43(1):57–63. [PubMed] [Google Scholar]