Abstract
Nowadays, the development of factor VIII and IX inhibitors in patients with hemophilia is considered as the most challenging in the treatment of hemophilia. Immune tolerance induction (ITI) therapy is an approach for eradication of inhibitors. Some ITI protocols are routinely in use for the eradication of inhibitors in patients with hemophilia. Moreover, such a therapeutic regimen may facilitate the tendency to reduced bone density in patients with inhibitor. This study scheduled to investigate whether that predisposing role of ITI protocols with an immunosuppressive agent has considered or not. By a literature review, published ITI protocols in hemophilia with inhibitors were evaluated. Among them, 51 papers found and studied thoroughly. None of them had performed the bone mineral examination in patients with hemophilia and inhibitor under treatment. Since there are 2 coexisting facilitating factors in these protocols, considering the bone mineral density study for patients with inhibitor who are undergoing ITI protocols with an immunosuppressive agent is recommended.
Keywords: hemophilia A, hemophilia B, immunosuppressive agents, immune tolerance induction (ITI), blood coagulation factor inhibitors, osteopenia, decreased bone mass, osteoporosis, bone mineral densitometry
Introduction
Hemophilia, the most common bleeding disorder, has a prevalence of around 400 000 individuals worldwide.1,2 Different genetic abnormalities in factor VIII and IX genes terminate to the absence or reduced plasma levels of factor VIII and IX in the blood.3 These diverse defects translate to various types of bleeding manifestations in the clinical aspect. The standard therapeutic approach is replacement therapy using plasma-derived or recombinant factor VIII and IX concentrate. The main adverse events of replacement therapy include the development of an inhibitor, the risk of thrombosis, and allergic reaction. The development of inhibitors is considered the most challenging topics in hemophilia care in the current decade.4 Inhibitors neutralize infused coagulation factor concentrates and makes it an extremely challenging complication in the management of hemorrhagic episodes.5 It causes treatment of hemorrhagic episodes in hemophilia more exigent topics and also the economically more expensive treatment for the health providers’ system.4,6 Improving health and safety surveillance are noticeable topics in a lifelong disease such as hemophilia.7 Hence, recognizing, evaluating, and communicating treatment-related adversarial complications are particularly important and should be monitored as early as possible.
Inhibitors in Hemophilia
Development of inhibitors in hemophilia is a multifactorial process that dynamically comprises inherited factors (ABO blood group,8 ethnicity,9 human leukocyte antigen,10 haplotype,11 type of factor VIII or factor IX mutations,12,13 and family history of developing inhibitors14) and environmental factors (infection and vaccination,15–17 type of infused coagulation factor,18 and age at start of treatment).8,4,19 About 30% of patients with severe hemophilia A, 0.9% to 7% of patients with moderate and mild hemophilia A,20 and around 3% to 4% of individuals with severe hemophilia B develop inhibitors.21–23 The development of inhibitors significantly complicates the control of hemorrhagic episodes in patients with hemophilia and makes challenges for hematologists because bleeds may not respond to conventional replacement therapy.24,25 Therefore, the development of inhibitors is associated with poor quality of life, higher morbidity rate, and a higher cost of care for health provider systems.26 Patients with a high dose of inhibitors (>5 Bethesda unit) do not respond to standard replacement therapy and need to be managed by a bypassing therapy.27 The current treatment strategy for high-titer inhibitors comprises utilizing a bypassing agent, including activated prothrombin complex concentrates (APCC) FEIBA (Takeda Company) or recombinant activated factor VII (rFVIIa; NovoSeven; Novo Nordisk).28–30 In practice, the failure rate of treatment with bypassing agents ranges from 10% to 30%.31 Another widely used therapeutic approach is immune tolerance induction (ITI), which leads to an eradication rate of about 70% to 85% of inhibitors.32 Hence, ITI is the first choice option, especially in children with inhibitors.33–35 The hallmark protocols for ITI regimens include the Bonn, van Creveld, and Malmö protocols.36–38
Susceptibility of Patients With Severe Hemophilia to Reduced Bone Density
Several surveys have demonstrated reduced bone density in patients with severe hemophilia compared to age- and sex-matched control group.39–47 It seems osteopenia and osteoporosis in hemophilia is a multifactorial process. It needs to be cleared pathophysiologically.48 Because patients with hemophilia usually experience hemophilia arthropathy secondary to bleeding into joints, they have low physical activity. This phenomenon may cause reduced peak of bone mass during childhood. It can translate to reduced bone density in later stages of life.49 The influence of some blood-borne viruses, including hepatitis C virus and HIV, has brought up.45 The development of osteoporosis in hemophilia increases the risk of bone fracture during normal daily activities when an occurrence of fracture seems to be an illogical event.50 Despite these truths, hemophilia does not considered as a secondary cause of osteoporosis.48
Clinical Question
What are the defects of ITI protocol with an immunosuppressive agent that are in employing for the eradication of inhibitors in patients with hemophilia regarding predisposing patients to reduced bone density? How can these defects be improved?
Method
The Strategy of Search
Assessment of the published articles was carried out to unravel whether the facilitator effect of ITI therapies with an immunosuppressive agent to reduced bone density has considered and paid attention or not. Hence, medical search engines of PubMed and Scopus were searched without any time limitation. The literature review was done on English language papers from the past till March 15, 2019. The following keywords were used for searching: “hemophilia A + hemophilia B + immune tolerance induction + ITI + immunosuppressive + inhibitor.” The authors screened the retrieved articles, relevance separately. All types of published articles or e-print of ahead papers, including original article, case series, case report, brief report, and letter to the editor that contains data on patient with hemophilia and inhibitors who underwent ITI protocol, entered to the study. The review articles that discussed ITI protocol and patients with hemophilia and inhibitors were not included in the study. All discrepancies and cross-checking of identifying papers were resolved in a debriefing session. From the target articles, some data were extracted, such as the type of hemophilia, number of treated patients, year of publishing data, type of administrated immunosuppressive agent, the country in which protocol was done, and whether bone density has done or not. And moreover, it was investigated whether the supplementary regimen for protection against reduced bone density has administrated or not?
Results
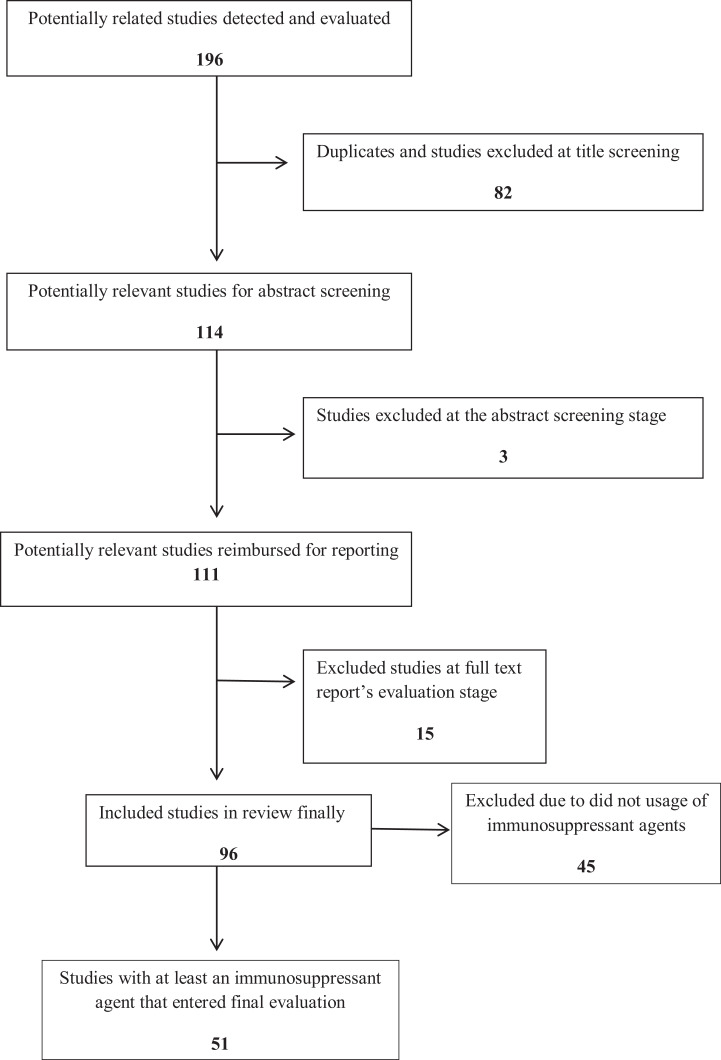
Overall, 196 related abstracts were identified through the search strategy. After deleting repeated titles, 114 abstracts were selected. Among them, 111 full texts were selected. All 111 full texts read to clear administration of an immunosuppressive agent as part of the ITI protocol and also evaluation of patients for reduced bone density. Among them, 12 review articles and 3 abstracts were excluded from the study. The whole 96 original articles were read. As reflected in the flow diagram of the study, 51 surveys had used at least one of the immunosuppressive agents in their ITI protocols,37,51-99 while 45 studies had used a version of ITI without any immunosuppressive agent (Figure 1). Among the 51 reported studies on the eradication of inhibitors in hemophilia A and B using an immunosuppressive agent or combination of them, the used immunosuppressive agents included mycophenolate mofetil, corticosteroid, rituximab, rapamycin, Solumedrol, dexamethasone, hydrocortisone, prednisolone, prednisone, cyclophosphamide, methylprednisolone, fluprednisolone, and cyclosporine A (Table 1). At the reviewing of articles, about 284 patients with hemophilia A, B, or acquired hemophilia had undergone one of the ITI protocols with at least an immunosuppressive agent in various countries across the world. There was no evidence of the detection status of bone density among studied patients. Moreover, no evidence found about prescription for supplementary calcium for the patients. Among the founded articles in this survey on published articles about the eradication of inhibitors, 29 studies had published between 2000 and 2018 and 22 articles published before 2000. Moreover, Sweden and the United States had reported the most studies (9 articles by each country).
Figure 1.
The inclusion and exclusion flow diagram of studies.
Table 1.
The List of Published Original Articles on Eradication of Factor VIII or IX Inhibitors by ITI Protocols, Including Immunosuppressant Agents Before November 2015.a
| Authors | Year of publication | Hemophilia | Sample size | Country | Immunosuppressant agent | BMD ref. |
|---|---|---|---|---|---|---|
| Freiburghaus et al | 1999 | A and B | 16 and 7 | Germany | Cyclophosphamide | 38 |
| Yuste et al | 2016 | A | 5 | M | NC | 51 |
| Antun et al | 2015 | A | 5 | M | NC | 52 |
| Kobayashi et al | 2015 | B | 1 | Japan | Prednisolone and hydrocortisone | 53 |
| Cos and Martorell | 2014 | A | 1 | Spain | IV prednisolone | 54 |
| Batorova et al | 2013 | B | 1 | Slovakia | Dexamethasone | 55 |
| Zhang et al | 2010 | A | 1 | China | NC | 56 |
| Unuvar et al | 2008 | A | Total 21 treated NC | Turkey | Cyclophosphamide | 57 |
| Astermark et al | 2006 | A and B | 29 and 5 | M | NC | 58 |
| Nemes and Pitlik | 2000 | AHA | 14 | Hungary | Cyclophosphamide and methylprednisolone | 59 |
| Carlborg et al | 2000 | A | 4 | Sweden | Cyclophosphamide | 60 |
| Nilsson et al | 1988 | A | 11 | Sweden | Cyclophosphamide | 61 |
| bBatlle J et al | 1999 | A | 1/11c | M | Cyclophosphamide and prednisone | 62 |
| Aznar et al | 1984 | A | 5 | Spain | Fluprednisolone | 63 |
| Kucharski et al | 1996 | A | 15 | Poland | Cyclophosphamide | 64 |
| Nilsson et al | 1981 | B | 1 | Sweden | Cyclophosphamide | 65 |
| Nilsson et al | 1995 | B | 3 | Sweden | Cyclophosphamide | 66 |
| Green et al | 1993 | AHA | 31 | United States | Cyclophosphamide and prednisone | 67 |
| Shibata et al | 2003 | B | 3 | Japan | Hydrocortisone | 68 |
| Pfliegler et al | 1989 | AHA | 1 | Hungary | Cyclosporin and Prednisone | 69 |
| Hedner and Tengborn | 1985 | A | 1 | Sweden | Hydrocortisone and cyclophosphamide | 70 |
| Dormandy and Sultan | 1975 | A | 3 | United Kingdom | Cyclophosphamide | 71 |
| Robbins et al | 2001 | A | 2 | United States | Dexamethasone and prednisone | 72 |
| White et al | 2000 | A | 2 | Ireland | Cyclophosphamide and prednisone | 73 |
| Gruppo et al | 1992 | A | 8 | United States | Cyclophosphamide and prednisone | 74 |
| Mauser Bunschoten et al | 1995 | A | 2 | The=Netherlands | Cyclophosphamide | 37 |
| Beutel et al | 2009 | B | 1 | Germany | Dexamethasone, MMF, and rituximab | 75 |
| Thornburg and Ducore | 2018 | A | 1 | United States | Solumedrol, rituximab |
76 |
| Nilsson et al | 1976 | A and B | 9 and 3 | Sweden | Cyclophosphamide |
77 |
| Nilsson et al | 1974 | A | 4 | Sweden | Cyclophosphamide |
78 |
| Ruggeri et al | 1975 | A | 8 | Italy and France | Cyclophosphamide | 79 |
| Kobrinsky et al | 2004 | A | 4 | United States | Cyclophosphamide |
80 |
| Lian et al | 1989 | AHA and A | 12 and 5 | United States | Prednisone, vincristine, and cyclophosphamide |
81 |
| Vlot et al | 2002 | A | 1 | The Netherlands | Cyclophosphamide | 82 |
| Zettervall et al | 1985 | A | 1 | Sweden | Cyclophosphamide | 83 |
| Nilsson et al | 1973 | B | 1 | Sweden | Cyclophosphamide | 84 |
| Edson et al | 1973 | A | 1 | United States | Cyclophosphamide and dexamethasone | 85 |
| Streif et al | 2009 | A | 2 | Germany | Rituximab and cyclosporine A | 86 |
| Carcao et al | 2006 | A | 5 | Canada | Rituximab | 87 |
| Collins et al | 2009 | A | 15 | United Kingdom | Rituximab | 88 |
| Ranta et al | 2011 | A | 1 | Finland | Rituximab | 89 |
| Barnes et al | 2010 | B | 1 | Australia | Rituximab | 90 |
| Aleem et al | 2009 | A | 3 | Saudi Arabia | Rituximab | 91 |
| Chowdhury et al | 2006 | A | 1 | United Kingdom | Rituximab | 92 |
| Wiestner et al | 2002 | A | 4 | United States | Rituximab | 93 |
| Dunkley et al | 2006 | A | 3 | Australia | Rituximab | 94 |
| Klarmann et al | 2008 | B | 2 | Germany | MMF | 95 |
| Beck et al | 1969 | A | 2 | United Kingdom | Prednisone | 96 |
| Lin et al | 2011 | A | 1 | Taiwan | Prednisone, azathioprine, and methotrexate | 97 |
| Cross et al | 2007 | B | 1 | The Netherlands | Cyclosporin A | 98 |
| Shaffer et al | 1997 | AHA | 9 | United States | Cyclophosphamide and prednisone | 99 |
Abbreviations: AHA, acquired hemophilia A; BMD, bone mineral density evaluation; IV, intravenous; M, multicenter; MMF, mycophenolate mofetil; NC, not cited.
a In the current study, 11 cases have been reported, but only 1 case has received cyclophosphamide and prednisone.
bThe first author of this manuscript is Batlle J.
cOne family members exists in this study.
What Does the Reviewing of the Evidence Conclude?
The landscape of the treatment of patients with hemophilia has undergone very speedy and significant advances during recent decades. The various available therapeutic regimens span from plasma-derived coagulation factor concentrate to bypassing agents (rFVII and APCC).100,101 The coagulation factor concentrates are switching from plasma-derived form to recombinant coagulation factors, and half-life extended coagulation factor.102 Now bispecific monoclonal antibody that mimics the function of factor VIII (emicizumab) is on trial and usage in developed countries.103 There are also multiple therapeutic regimens for eradication of inhibitors too. The ITI protocols consider as the mainstay of eradication of inhibitors in hemophilia.104 Suppression of T cells, induction of T-cell anergy, inhibition of B cell, and usage of anti-idiopathic antibodies comprise the mechanism action of ITI protocol.105 Hence, ITI protocols are in use with a 60% to 80% success rate in hemophilia A now.106,107 On the other hand, there is no therapeutic agent that can provide 100% protection against all hemorrhagic episodes in all patients without concern about the development of inhibitors.
Discussion
Osteoporosis, a disease that involves reduced bone density, results in the impaired and abnormal texture of bone mass that may lead to unforeseen fractures during normal daily activities. Osteoporosis is a significant cause of mortality and morbidity in adults. Indeed, the balance between bone development during childhood and the subsequent bone loss during adulthood comprises the bone mass. The factors, such as smoking, alcoholism, thalassemia, hypogonadism, hematological malignancies, vitamin D deficiency, and some drugs such as exogenous glucocorticoid excess, anticoagulants of warfarin, and heparin, consider to be the secondary predisposing factors toward reduced bone density (RBD). Moreover, the risk of bone fracture correlates with bone architecture in various races, which is evaluated by fracture risk assessment tool (Frax).
Hemophilia presents with a bleeding tendency and reduced plasma levels of coagulation factors VIII and IX in the blood.1 Some researchers have shown that there is reduced bone density both in the lumbar spine and the femoral bone of males who have hemophilia via several case–control trials.41,48,108 Osteoporosis in various races’ groups of patients with hemophilia has adequately studied in evidence of the literature. Despite this, hemophilia has not been considered a cause of secondary osteoporosis yet.109
Apart from this, the development of inhibitors of relevant transfused coagulation factors is a major challenge that complicates the treatments for hemophilia bleeding. There are various types of neutralizing antibodies that bind to relevant coagulation factors and may impair functions of coagulation proteins in the coagulation system. The frequencies of developing inhibitors have generally ranged from approximately 18% to 28% in hemophilia A. The predisposing factors for developing inhibitors determine the severity of hemophilia, the underlying genetic defects, the age of patients at the time of treatment onset, the type of infused coagulation factor concentrates (plasma derived or recombinant factors), and the ethnic background of patients.
Immune tolerance induction is a therapeutic protocol for the eradication of inhibitors in hemophilia. The specific function by which ITI works is not completely clear. Among the suggested targets, it seems that factor VIII-specific T and B cells are more significant.110 The use of ITI therapy may require 12 months of treatment to observe improvements. It may require 2 years or longer period in more complicated patients with hemophilia and inhibitors.111,112 Some ITI protocols comprise the use of one or more immune suppressant agents (corticosteroids, cyclophosphamide, etc). Additionally, glucocorticoid-induced osteoporosis has addressed entirely in the literature.113,114 It seems safety, pharmacoeconomic, and efficacy aspects of ITI therapy need to be designated.115 Hence, for patients with hemophilia and inhibitor who tend to have reduced bone density, when they undergo ITI protocol (with at least an immunosuppressive agent) for the eradication of inhibitors, it is expected that they will have a higher risk for reduced bone density.
The limitation of the current study includes restricting the search to English papers. Although English is the universal language for scientific reports of studies, there may be other published papers in this field and this topic in local languages has been missed in our literature review.
Conclusion
Various ITI protocols are in use for the eradication of factor VIII and IX inhibitors in patients with hemophilia A and B. Some of ITI protocols include one or more immunosuppressive agent that has known to be associated with the side effect of reduced bone density. On the other hand, it has demonstrated that patients with hemophilia tend to have reduced bone density secondary to various factors. These 2 ameliorating factors push patients with hemophilia and inhibitors who are receiving ITI protocols with an immunosuppressive agent toward reduced bone density. This gap has not considered in the treatment of the current patients and needs to pay attention as a part of the ITI protocols for the patients with hemophilia and inhibitors. The ultimate goal of this study is to follow patients with hemophilia and inhibitor about bone mineral status before beginning of any ITI protocol with an immunosuppressive agent. Apart from this, in future versions of ITI protocols, evaluation of bone mineral density would be taken account. According to the current literature review, there is a body of evidence that implies bone mineral density in patients with inhibitors has not been considered. It is, thus, clearly accepted that well-planned randomized trials will shed more light on this issue.
Future Prospects
At the moment, the bone mineral examination is not routinely performed for patients with hemophilia. At present, tendency to reduced bone density has demonstrated in patients with hemophilia. And moreover, immunosuppressive agents pave the way toward osteopenia, and it would be expected that bone mineral examination should to be done for them. In addition, for patients with hemophilia and inhibitors who want to eradicate inhibitors by an immunosuppressive agent, a supplementary regimen of calcium and vitamin D would be considered. The prevention of osteopenia in patients with hemophilia is very cheaper than treatment of broken bones in hemophilia. Hence, implementation of bone density examination as part of the ITI protocols will be beneficial for both patients and health provider systems.
On the other hand, it would be expected that hemophilia to be considered as a risk factor in Frax criteria assessment tool. As a part of the ITI protocols, it is a good idea and essential to perform bone mineral density scans and provide calcium, vitamin D supplements, or bisphosphonate and anti-RANKL as protective drugs to the patients. Finally, assessment of BMD before and after ITI therapy for patients with hemophilia and inhibitor is recommended.
Acknowledgments
The authors would like to thank the Mashhad University of Medical Sciences for financial support.
Authors’ Note: Z.R. gave idea; extracted and reviewed the piece of literatures, concluded of the findings, revised, and approved final draft of the manuscript. H.M. searched the medical search engines, extracted, and reviewed the literature, concluded of the findings, and wrote the primary version of draft.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Mashhad University of Medical Sciences [grant number of 951533].
ORCID iD: Hassan Mansouritorghabeh  https://orcid.org/0000-0002-4904-0156
https://orcid.org/0000-0002-4904-0156
References
- 1. Mansouritorghabeh H. Clinical and laboratory approaches to hemophilia A. Iran J Med Sci. 2015;40(3):194. [PMC free article] [PubMed] [Google Scholar]
- 2. Dorgalaleh A, Dadashizadeh G, Bamedi T. Hemophilia in Iran. Hematol. 2016;21(5):300–310. [DOI] [PubMed] [Google Scholar]
- 3. Gholami MS, Valikhani M, Dorgalaleh A, Mousavi SH, Pezeshkpoor B. Hemophilia A. In: Congenital Bleeding Disorders. Springer; 2018:103–137. [Google Scholar]
- 4. Tabrizi S, Gholampour M, Mansouritorghabeh H. A close insight to factor VIII inhibitor in the congenital hemophilia A. Expert Rev Hematol. 2016;9(9):903–913. [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi T, Kudo N, Endo S, Usui T, Imashuku S. Management of acquired hemophilia A in elderly patients. Case Rep Hematol. 2018;2018:6757345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modaresi AR, Torghabeh HM, Pourfathollah AA, Shooshtari MM, Yazdi ZR. Pattern of factor VIII inhibitors in patients with hemophilia A in the north east of Iran. Hematology. 2006;11(3):215–217. [DOI] [PubMed] [Google Scholar]
- 7. Lassila R, Makris M. Safety surveillance in haemophilia and allied disorders. J Intern Med. 2016;279(6):515–523. [DOI] [PubMed] [Google Scholar]
- 8. Carcao M, Re W, Ewenstein B. The role of previously untreated patient studies in understanding the development of FVIII inhibitors. Haemophilia. 2016;22(1):22–31. [DOI] [PubMed] [Google Scholar]
- 9. Viel KR, Ameri A, Abshire TC, et al. Inhibitors of factor VIII in black patients with hemophilia. N Eng J Med. 2009;360(16):1618–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moise L, Song C, Martin WD, Tassone R, De Groot AS, Scott DW. Effect of HLA DR epitope de-immunization of Factor VIII in vitro and in vivo. Clin Immunol. 2012;142(3):320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarres RK. Current controversies in the formation and treatment of alloantibodies to factor VIII in congenital hemophilia A. ASH Educ Program. 2011;2011(1):407–412. [DOI] [PubMed] [Google Scholar]
- 12. Oldenburg J, Pavlova A. Genetic risk factors for inhibitors to factors VIII and IX. Haemophilia. 2006;12(suppl 6):15–22. [DOI] [PubMed] [Google Scholar]
- 13. Astermark J. Why do inhibitors develop? Principles of and factors influencing the risk for inhibitor development in haemophilia. Haemophilia. 2006;12(suppl 3):52–60. [DOI] [PubMed] [Google Scholar]
- 14. Chalmers E, Brown S, Keeling D, et al. Early factor VIII exposure and subsequent inhibitor development in children with severe haemophilia A. Haemophilia. 2007;13(2):149–155. [DOI] [PubMed] [Google Scholar]
- 15. Astermark J. Prevention and prediction of inhibitor risk. Haemophilia. 2012;18(suppl 4):38–42. [DOI] [PubMed] [Google Scholar]
- 16. Astermark J. FVIII inhibitors: pathogenesis and avoidance. Blood. 2015;125(13):2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Astermark J, Altisent C, Batorova A, et al. Non-genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report. Haemophilia. 2010;16(5):747–766. [DOI] [PubMed] [Google Scholar]
- 18. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Eng J Med. 2016;374(21):2054–2064. [DOI] [PubMed] [Google Scholar]
- 19. Franchini M, Lippi G. Prevention of inhibitor development in hemophilia A in 2016. A glimpse into the future? Thromb Res. 2016;148:96–100. [DOI] [PubMed] [Google Scholar]
- 20. Darby SC, Keeling DM, Spooner RJ, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2(7):1047–1054. [DOI] [PubMed] [Google Scholar]
- 21. Lovgren K, Sondergaard H, Skov S, Wiinberg B. Non-genetic risk factors in haemophilia A inhibitor management–the danger theory and the use of animal models. Haemophilia. 2016;22(5):657–666. [DOI] [PubMed] [Google Scholar]
- 22. Castaman G, Bonetti E, Messina M, et al. Inhibitors in haemophilia B: the Italian experience. Haemophilia. 2013;19(5):686–690. [DOI] [PubMed] [Google Scholar]
- 23. Gouw SC, Van Der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Eng J Med. 2013;368(3):231–239. [DOI] [PubMed] [Google Scholar]
- 24. Mahlangu J, Andreeva T, Macfarlane D, Walsh C, Key N. Recombinant B-domain-deleted porcine sequence factor VIII (r-pFVIII) for the treatment of bleeding in patients with congenital haemophilia A and inhibitors. Haemophilia. 2016;23(1):33–41. [DOI] [PubMed] [Google Scholar]
- 25. Leissinger CA. Advances in the clinical management of inhibitors in hemophilia A and B. Semin hematol. 2016;53(1):20–27. [DOI] [PubMed] [Google Scholar]
- 26. Dimichele DM. Management of factor VIII inhibitors. Int J Hematol. 2006;83(2):119–125. [DOI] [PubMed] [Google Scholar]
- 27. Villarrubia R, Oyaguez I, Roman MA, Castellano MM, Parra R, Casado M. Cost analysis of prophylaxis with activated prothrombin complex concentrate vs. on-demand therapy with activated factor VII in severe haemophilia A patients with inhibitors, in Spain. Haemophilia. 2015;21(3):320–329. [DOI] [PubMed] [Google Scholar]
- 28. de Paula EV, Kavakli K, Mahlangu J, et al. Recombinant factor VIIa analog (vatreptacog alfa [activated]) for treatment of joint bleeds in hemophilia patients with inhibitors: a randomized controlled trial. J Thromb Haemost. 2012;10(1):81–89. [DOI] [PubMed] [Google Scholar]
- 29. Pasi KJ, Georgiev P, Mant T, et al. Fitusiran, an investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia: interim results from a phase 2 extension study in patients with hemophilia A or B with and without inhibitors. Res Pract Thromb Haemost. 2017;1(suppl 1):25. [Google Scholar]
- 30. Coppola A, Franchini M, Castaman G, et al. Treatment regimens with bypassing agents in patients with hemophilia A and inhibitors: a survey from the Italian Association of Hemophilia Centers (AICE). Semin Thromb Hemost. 2018;44(6):551–560. [DOI] [PubMed] [Google Scholar]
- 31. Astermark J, Donfield SM, DiMichele DM, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA Novoseven Comparative (FENOC) Study. Blood. 2007;109(2):546–551. [DOI] [PubMed] [Google Scholar]
- 32. Hay CRM, DiMichele DM. . The principal results of the international immune tolerance study: a randomized dose comparison. Blood. 2012;119(6):1335–1344. [DOI] [PubMed] [Google Scholar]
- 33. Rocino A, Coppola A, Franchini M, et al. Principles of treatment and update of recommendations for the management of haemophilia and congenital bleeding disorders in italy. Blood Transfus. 2014;12(4):575–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coppola A, Di Minno MN, Santagostino E. Optimizing management of immune tolerance induction in patients with severe haemophilia A and inhibitors: towards evidence-based approaches. Br J Haematol. 2010;150(5):515–528. [DOI] [PubMed] [Google Scholar]
- 35. Dimichele DM, Hoots W, Pipe SW, Rivard G, Santagostino E. International workshop on immune tolerance induction: consensus recommendations 1. Haemophilia. 2007;13(suppl 1):1–22. [DOI] [PubMed] [Google Scholar]
- 36. Brackmann HH, Oldenburg J, Schwaab R. Immune tolerance for the treatment of factor VIII inhibitors—twenty years’ ‘bonn protocol’. Vox Sang. 1996;70(suppl 1):30–35. [DOI] [PubMed] [Google Scholar]
- 37. Mauser Bunschoten EP, Nieuwenhuis HK, Roosendaal G, van den Berg HM. Low-dose immune tolerance induction in hemophilia A patients with inhibitors. Blood. 1995;86(3):983–988. [PubMed] [Google Scholar]
- 38. Freiburghaus C, Berntorp E, Ekman M, Gunnarsson M, Kjellberg B, Nilsson IM. Tolerance induction using the Malmo treatment model 1982-1995. Haemophilia. 1999;5(1):32–39. [DOI] [PubMed] [Google Scholar]
- 39. Rezaeifarid M, Soveid M, Ghaemi S, Karimi M. Bone mineral density in Iranian patients with haemophilia: the first experience in southern Iran. Haemophilia. 2011;17(3):552–553. [DOI] [PubMed] [Google Scholar]
- 40. Wells AJ, McLaughlin P, Simmonds JV, et al. A case-control study assessing bone mineral density in severe haemophilia A in the UK. Haemophilia. 2015;21(1):109–115. [DOI] [PubMed] [Google Scholar]
- 41. Mansouritorghabeh H, Rezaieyazdi Z, Badiei Z. Are individuals with severe haemophilia A prone to reduced bone density? Rheumatol Int. 2008;28(11):1079–1083. [DOI] [PubMed] [Google Scholar]
- 42. Khawaji M, Astermark J, Mackensen SV, Akesson K, Berntorp E. Bone density and health-related quality of life in adult patients with severe haemophilia. Haemophilia. 2011;17(2):304–311. [DOI] [PubMed] [Google Scholar]
- 43. Alioglu B, Selver B, Ozsoy H, Koca G, Ozdemir M, Dallar Y. Evaluation of bone mineral density in Turkish children with severe haemophilia A: Ankara hospital experience. Haemophilia. 2012;18(1):69–74. [DOI] [PubMed] [Google Scholar]
- 44. Linari S, Montorzi G, Bartolozzi D, et al. Hypovitaminosis D and osteopenia/osteoporosis in a haemophilia population: a study in HCV/HIV or HCV infected patients. Haemophilia. 2013;19(1):126–133. [DOI] [PubMed] [Google Scholar]
- 45. Gerstner G, Damiano ML, Tom A, et al. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia. 2009;15(2):559–565. [DOI] [PubMed] [Google Scholar]
- 46. Mansouritorghabeh H, Rezaieyazdi Z, Bagheri M. Successful use of factor VIII concentrate and fresh frozen plasma for four dental extractions in an individual with combined factor V and VIII deficiency. Transfus Med Hemother. 2009;36(2):138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anagnostis P, Vakalopoulou S, Slavakis A, et al. Reduced bone mineral density in patients with haemophilia A and B in northern Greece. Thromb Haemost. 2012;107(3):545–551. [DOI] [PubMed] [Google Scholar]
- 48. Paschou SA, Anagnostis P, Karras S, et al. Bone mineral density in men and children with haemophilia A and B: a systematic review and meta-analysis. Osteoporos Int. 2014;25(10):2399–2407. [DOI] [PubMed] [Google Scholar]
- 49. Forsyth AL, Quon DV, Konkle BA. Role of exercise and physical activity on haemophilic arthropathy, fall prevention and osteoporosis. Haemophilia. 2011;17(5):e870–e876. [DOI] [PubMed] [Google Scholar]
- 50. Kovacs CS. Hemophilia, low bone mass, and osteopenia/osteoporosis. Transfusion Apher Sci. 2008;38(1):33–40. [DOI] [PubMed] [Google Scholar]
- 51. Yuste VJ, Oldenburg J, Rangarajan S, Jordán RP, Santagostino E. Long-term outcome of haemophilia A patients after successful immune tolerance induction therapy using a single plasma-derived FVIII/VWF product: the long-term ITI study. Haemophilia. 2016;22(6):859–865. [DOI] [PubMed] [Google Scholar]
- 52. Antun A, Monahan PE, Johnson MM, et al. Inhibitor recurrence after immune tolerance induction: a multicenter retrospective cohort study. J Thromb Haemost. 2015;13(11):1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kobayashi R, Sano H, Suzuki D, et al. Successful treatment of immune tolerance induction with rituximab in a patient with severe hemophilia B and inhibitor. Blood Coagul Fibrinolys. 2015;26(5):580–582. [DOI] [PubMed] [Google Scholar]
- 54. Cos CD, Martorell JR. Successful immune tolerance induction with a plasma-derived FVIII concentrate and intravenous immunoglobulins in a pediatric patient with congenital severe hemophilia A and poor prognostic factors. Blood Coagul Fibrinolysis. 2014;25(1):77–80. [DOI] [PubMed] [Google Scholar]
- 55. Batorova A, Morongova A, Tagariello G, Jankovicova D, Prigancova T, Horakova J. Challenges in the management of hemophilia B with inhibitor. Semin Thromb Hemost. 2013;39(7):767–771. [DOI] [PubMed] [Google Scholar]
- 56. Zhang L, Xue F, Liu X, et al. Immune tolerance induction in a severe hemophilia A patient with inhibitor [in Chinese]. Zhonghua Xue Ye Xue Za Zhi. 2010;31(9):577–580. [PubMed] [Google Scholar]
- 57. Unuvar A, Kavakli K, Baytan B, et al. Low-dose immune tolerance induction for paediatric haemophilia patients with factor VIII inhibitors. Haemophilia. 2008;14(2):315–322. [DOI] [PubMed] [Google Scholar]
- 58. Astermark J, Morado M, Rocino A, et al. Current European practice in immune tolerance induction therapy in patients with haemophilia and inhibitors. Haemophilia. 2006;12(4):363–371. [DOI] [PubMed] [Google Scholar]
- 59. Nemes L, Pitlik E. New protocol for immune tolerance induction in acquired hemophilia. Haematologica. 2000;85(10 suppl):64–68. [PubMed] [Google Scholar]
- 60. Carlborg E, Astermark J, Lethagen S, Ljung R, Berntorp E. The Malmö model for immune tolerance induction: impact of previous treatment on outcome. Haemophilia. 2000;6(6):639–642. [DOI] [PubMed] [Google Scholar]
- 61. Nilsson IM, Berntorp E, Zettervall O. Induction of immune tolerance in patients with hemophilia and antibodies to factor VIII by combined treatment with intravenous IgG, cyclophosphamide, and factor VIII. N Eng J Med. 1988;318(15):947–950. [DOI] [PubMed] [Google Scholar]
- 62. Batlle J, Lopez M F, Brackmann H H, et al. Induction of immune tolerance with recombinant factor VIII in haemophilia A patients with inhibitors. Haemophilia. 1999;5(6):431–435. [DOI] [PubMed] [Google Scholar]
- 63. Aznar J, Jorquera J, Peiro A, Garcia I. The importance of corticoids added to continued treatment with factor VIII concentrates in the suppression of inhibitors in haemophilia A. Thromb Haemost. 1984;51(2):217–221. [PubMed] [Google Scholar]
- 64. Kucharski W, Scharf R, Nowak T. Immune tolerance induction in haemophiliacs with inhibitor to FVIII: high-or low-dose programme. Haemophilia. 1996;2(4):224–228. [DOI] [PubMed] [Google Scholar]
- 65. Nilsson IM, Jonsson S, Sundqvist SB, Ahlberg A, Bergentz SE. A procedure for removing high titer antibodies by extracorporeal protein-A-sepharose adsorption in hemophilia: substitution therapy and surgery in a patient with hemophilia B and antibodies. Blood. 1981;58(1):38–44. [PubMed] [Google Scholar]
- 66. Nilsson I, Berntorp E, Rickard KA. Results in three Australian haemophilia B patients with high-responding inhibitors treated with the Malmö model. Haemophilia. 1995;1(1):59–66. [DOI] [PubMed] [Google Scholar]
- 67. Green D, Rademaker A, Briet E. A prospective, randomized trial of prednisone and cyclophosphamide in the treatment of patients with factor VIII autoantibodies. Thromb Haemostasis. 1993;70(5):753–757. [PubMed] [Google Scholar]
- 68. Shibata M, Shima M, Misu H, Okimoto Y, Giddings J, Yoshioka A. Management of haemophilia B inhibitor patients with anaphylactic reactions to FIX concentrates. Haemophilia. 2003;9(3):269–271. [DOI] [PubMed] [Google Scholar]
- 69. Pfliegler G, Boda Z, Harsfalvi J, et al. Cyclosporin treatment of a woman with acquired haemophilia due to factor VIII: C inhibitor. Postgrad Med J. 1989;65(764):400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hedner U, Tengborn L. Management of haemophilia A with antibodies—the effect of combined treatment with factor VIII, hydrocortisone and cyclophosphamide. Thrombosis Haemostasis. 1985;54(4):776–779. [PubMed] [Google Scholar]
- 71. Dormandy K, Sultan Y. The suppression of factor VIII antibodies in haemophilia. Pathol Biol. 1975;23(suppl):17–23. [PubMed] [Google Scholar]
- 72. Robbins D, Kulkarni R, Gera R, Scott Emuakpor AB, Bosma K, Penner JA. Successful treatment of high titer inhibitors in mild hemophilia A with avoidance of factor VIII and immunosuppressive therapy. Am J Hematol. 2001;68(3):184–188. [DOI] [PubMed] [Google Scholar]
- 73. White B, Cotter M, Byrne M, O’Shea E, Smith OP. High responding factor VIII inhibitors in mild haemophilia A is there a link with recent changes in clinical practice? Haemophilia. 2000;6(2):113–115. [DOI] [PubMed] [Google Scholar]
- 74. Gruppo RA, Valdez LP, Stout RD. Induction of immune tolerance in patients with hemophilia A and inhibitors. J Pediatr Hematol Oncol. 1992;14(1):82–87. [DOI] [PubMed] [Google Scholar]
- 75. Beutel K, Hauch H, Rischewski J, Kordes U, Schneppenheim J, Schneppenheim R. ITI with high-dose FIX and combined immunosuppressive therapy in a patient with severe haemophilia B and inhibitor. Hamostaseologie. 2009;29(2):155–157. [PubMed] [Google Scholar]
- 76. Thornburg CD, Ducore JM. A novel approach to immune tolerance induction in hemophilia α with factor VIII inhibitor. Haemophilia. 2019;25(1):e48–e50. [DOI] [PubMed] [Google Scholar]
- 77. Nilsson IM, Hedner U. Immunosuppressive treatment in haemophiliacs with inhibitors to factor VIII and factor IX. Scand J Haematol. 1976;16(5):369–382. [DOI] [PubMed] [Google Scholar]
- 78. Nilsson IM, Hedner U, Holmberg L. Suppression of factor VIII antibody by combined factor VIII and cyclophosphamide. Acta Med Scand. 1974;195(1-2):64–72. [PubMed] [Google Scholar]
- 79. Ruggeri Z, Mannucci P, Allain J, Frommel D. Preliminary trial of cyclophosphamide in the management of hemophiliacs with factor VIII inhibitors. Ann NY Acad Sci. 1975;240(1):412–418. [DOI] [PubMed] [Google Scholar]
- 80. Kobrinsky NL, Sjolander DE, Moser DK, Stegman DA. Ablation of hemophilic FVIII inhibitors with FVIII priming, cyclophosphamide immune suppression, and rapid tapering of FVIII immune tolerance. Am J Hematol. 2004;76(2):180–184. [DOI] [PubMed] [Google Scholar]
- 81. Lian EC, Larcada AF, Chiu AY. Combination immunosuppressive therapy after factor VIII infusion for acquired factor VIII inhibitor. Ann Intern Med. 1989;110(10):774–778. [DOI] [PubMed] [Google Scholar]
- 82. Vlot AJ, Wittebol S, Strengers PF, et al. Factor VIII inhibitor in a patient with mild haemophilia A and an Asn618 → Ser mutation responsive to immune tolerance induction and cyclophosphamide. Br J Haematol. 2002;117(1):136–140. [DOI] [PubMed] [Google Scholar]
- 83. Zettervall O, Sundqvist SB, Nilsson IM. Characterisation of the tolerant state in a patient with haemophilia B after removal of high-titre factor IX antibodies. Scand J Haematol. 1985;34(5):446–454. [DOI] [PubMed] [Google Scholar]
- 84. Nilsson I, Hedner U, Bjorlin G. Suppression of factor IX antibody in hemophilia B by factor IX and cyclophosphamide. Ann Intern Med. 1973;78(1):91–95. [DOI] [PubMed] [Google Scholar]
- 85. Edson JR, McArthur JR, Branda RF, McCullough JJ, Chou SN. Successful management of a subdural hematoma in a hemophiliac with an anti-factor VIII antibody. Blood. 1973;41(1):113–122. [PubMed] [Google Scholar]
- 86. Streif W, Ettingshausen EC, Linde R, Kropshofer G, Zimmerhackl LB, Kreuz W. Inhibitor treatment by rituximabin congenital haemophilia A. Hamostaseologie. 2009;29(02):151–154. [PubMed] [Google Scholar]
- 87. Carcao M, St Louis J, Poon MC, et al. Rituximab for congenital haemophiliacs with inhibitors: a Canadian experience. Haemophilia. 2006;12(1):7–18. [DOI] [PubMed] [Google Scholar]
- 88. Collins PW, Mathias M, Hanley J, et al. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. J Thromb Haemost. 2009;7(5):787–794. [DOI] [PubMed] [Google Scholar]
- 89. Ranta S, Koskinen S, Makipernaa A. Successful eradication of inhibitor of late recurrence and other high risk prognostic factors in a patient with severe haemophilia A. Haemophilia. 2011;17(5):e833–e834. [DOI] [PubMed] [Google Scholar]
- 90. Barnes C, Davis A, Furmedge J, Egan B, Donnan L, Monagle P. Induction of immune tolerance using rituximab in a child with severe haemophilia B with inhibitors and anaphylaxis to factor IX. Haemophilia. 2010;16(5):840–841. [DOI] [PubMed] [Google Scholar]
- 91. Aleem A, Saidu A, Abdulkarim H, et al. Rituximab as a single agent in the management of adult patients with haemophilia A and inhibitors: marked reduction in inhibitor level and clinical improvement in bleeding but failure to eradicate the inhibitor. Haemophilia. 2009;15(1):210–216. [DOI] [PubMed] [Google Scholar]
- 92. Chowdhury F, Lawrence K, Baglin T, Perry D. Rituximab failure in a patient with allo-FVIII inhibitor. Br J Haematol. 2006;135(3):412–412. [DOI] [PubMed] [Google Scholar]
- 93. Wiestner A, Cho HJ, Asch AS, et al. Rituximab in the treatment of acquired factor VIII inhibitors. Blood. 2002;100(9):3426–3428. [DOI] [PubMed] [Google Scholar]
- 94. Dunkley S, Kershaw G, Young G, et al. Rituximab treatment of mild haemophilia A with inhibitors: a proposed treatment protocol. Haemophilia. 2006;12(6):663–667. [DOI] [PubMed] [Google Scholar]
- 95. Klarmann D, Saguer IM, Funk M, et al. Immune tolerance induction with mycophenolate-mofetil in two children with haemophilia B and inhibitor. Haemophilia. 2008;14(1):44–49. [DOI] [PubMed] [Google Scholar]
- 96. Beck P, Giddings J, Bloom A. Inhibitor of factor VIII in mild haemophilia. Br J Haematol. 1969;17(3):283–288. [DOI] [PubMed] [Google Scholar]
- 97. Lin PC, Liao YM, Tsai SP, Chang TT. Immune tolerance induction therapy for patients with hemophilia A and FVIII inhibitors particularly using low-dose regimens. Pediatr Blood Cancer. 2011;57(6):1029–1033. [DOI] [PubMed] [Google Scholar]
- 98. Cross D, den Berg HV. Cyclosporin A can achieve immune tolerance in a patient with severe haemophilia B and refractory inhibitors. Haemophilia. 2007;13(1):111–114. [DOI] [PubMed] [Google Scholar]
- 99. Shaffer LG, Phillips MD. Successful treatment of acquired hemophilia with oral immunosuppressive therapy. Ann Intern Med. 1997;127(3):206–209. [DOI] [PubMed] [Google Scholar]
- 100. Mingot Castellano ME, Montes RP, Canaro M, Marco A, Gordo VC, Heiniger AI. Successful treatment of bleeding in acquired hemophilia A with activated prothrombin complex concentrate in Spain. Blood. 2017;130(suppl 1):3688. [Google Scholar]
- 101. Hedner U. Recombinant activated factor VII: 30 years of research and innovation. Blood Rev. 2015;29(suppl 1):S4–S8. [DOI] [PubMed] [Google Scholar]
- 102. Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130(23):2463–2468. [DOI] [PubMed] [Google Scholar]
- 104. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124(23):3365–3372. [DOI] [PubMed] [Google Scholar]
- 105. Waters B, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7(9):1446–1456. [DOI] [PubMed] [Google Scholar]
- 106. Batorova A, Jankovicova D, Morongova A, et al. Inhibitors in severe hemophilia A: 25-year experience in Slovakia. Semin Thromb Hemost. 2016;42(5):550–562. [DOI] [PubMed] [Google Scholar]
- 107. Santagostino E, Young G, Ettingshausen CE, Yuste VJ, Carcao M. Inhibitors: a need for eradication? Acta Haematol. 2019;141(3):151–155. [DOI] [PubMed] [Google Scholar]
- 108. Gallacher S, Deighan C, Wallace A, et al. Association of severe haemophilia A with osteoporosis: a densitometric and biochemical study. Q J Med. 1994;87(3):181–186. [PubMed] [Google Scholar]
- 109. Mansouritorghabeh H, Rezaieyazdi Z. Bleeding disorders and reduced bone density. Rheumatol Intern. 2011;31(3):283–287. [DOI] [PubMed] [Google Scholar]
- 110. Astermark J. Immune tolerance induction in patients with hemophilia A. Thromb Res. 2011;127(suppl 1):S6–S9. [DOI] [PubMed] [Google Scholar]
- 111. Ryu JE, Park YS, Yoo KY, Lee KD, Choi YM. Immune tolerance induction in patients with severe hemophilia A with inhibitors. Blood Res. 2015;50(4):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lak M, Sharifian RA, Karimi K, Mansouritorghabeh H. Acquired hemophilia A: clinical features, surgery and treatment of 34 cases, and experience of using recombinant factor VIIa. Clin Appl Thromb/Hemost. 2009;16(3):294–300. [DOI] [PubMed] [Google Scholar]
- 113. Van Staa T. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2006;79(3):129–137. [DOI] [PubMed] [Google Scholar]
- 114. Krasselt M, Baerwald C. An update on glucocorticoid-induced osteoporosis [in German]. Dtsch Med Wochenschr. 2016;141(5):352–357. [DOI] [PubMed] [Google Scholar]
- 115. Ho AY, Height SE, Smith MP. Immune tolerance therapy for haemophilia. Drugs. 2000;60(3):547–554. [DOI] [PubMed] [Google Scholar]