Abstract
Stroke is a hemostatic disease associated with thrombosis/hemorrhage caused by intracranial vascular injury with spectrum of clinical phenotypes and variable prognostic outcomes. The genesis of different phenotypes of stroke is poorly understood due to our incomplete understanding of hemostasis and thrombosis. These shortcomings have handicapped properly recognizing each specific stroke syndrome and contributed to controversy in selecting therapeutic agents. Treatment recommendation for stroke syndromes has been exclusively derived from the result of laborious and expensive clinical trials. According to newly proposed “two-path unifying theory” of in vivo hemostasis, intracranial vascular injury would yield several unique stroke syndromes triggered by 3 distinctly different thrombogenetic mechanisms depending upon level of intracranial intravascular injury and character of formed blood clots. Five major phenotypes of stroke occur via thrombogenetic paths: (1) transient ischemic attack due to focal endothelial damage limited to endothelial cells (ECs), (2) acute ischemic stroke due to localized ECs and subendothelial tissue (SET) damage extending up to the outer vascular wall, (3) thrombo-hemorrhagic stroke due to localized vascular damage involving ECs and SET and extending beyond SET to extravascular tissue, (4) acute hemorrhagic stroke due to major localized intracranial hemorrhage/hematoma into the brain tissue or space between the coverings of the brain associated with vascular anomaly or obtuse trauma, and (5) encephalopathic stroke due to disseminated endotheliopathy leading to microthrombosis within the brain. New classification of stroke phenotypes would assist in selecting rational therapeutic regimen for each stroke syndrome and designing clinical trials to improve clinical outcome.
Keywords: hemostasis, thrombosis, stroke syndromes, endotheliopathy, fibrinogenesis, vascular microthrombotic disease, microthrombogenesis, macrothrombogenesis
Background
Stroke, a very common condition, contributes to a substantial societal toll in morbidity and mortality. It is the leading cause of chronic disability, the second leading cause of dementia, and the fourth leading cause of death in the United States.1,2 In the States, the prevalence of stroke is roughly 2.5% of the adult population, which translates to approximately 7 million individuals.1 While the understanding of stroke has advanced over the years with the advent of modern imaging technology, the proliferation of therapeutic regimens utilizing various antithrombotic, anticoagulant, and fibrinolytic agents, and surgical technic and intervention have only moderately improved the outcome in general patient care when followed by recommended various guidelines.3 To further improve morbidity and mortality, it is crucial for stroke physicians to recognize not only diversified brain syndromes caused by localization of thrombus but also stroke phenotypes based on in vivo hemostasis. To date, the understanding of pathological spectrum produced by variable phenotypic stroke syndromes3,4 has been difficult task due to our shortcomings in identifying in vivo hemostasis leading to thrombogenesis. Should we be able to elucidate the role of in vivo hemostasis in different phenotypes of stroke, each stroke syndrome can be better defined and, by designing the clinical trials rationally, the outcome of stroke could be further improved with effective therapy, secondary prevention and prophylaxis.
As presented in Table 1, blood clots only occur as a result of vascular injury, which means stroke must always be proceeded by bleeding due to intravascular injury.4 The phenotypes of stroke are multiplex from transient ischemic attack (TIA) to acute hemorrhagic stroke (AHS) and also from localized neurologic syndrome due to focal neurologic dysfunction (eg, hemiplegia, speech difficulty, seizure) to generalized encephalopathy (eg, altered mental state such as confusion, disorientation, and coma). An analysis of clinical features in stroke reveals 2 critical factors govern the phenotypic expression and prognostic outcome of stroke. One is the localization of intravascular injury in a particular vasculature. The other is the level of vascular injury of the intracranial vasculature, which produces the different depth of vascular wall damage. The former creates the neurological phenotype due to the localized thrombosis,4 but the latter determines the hemostatic phenotype and severity of stroke syndrome due to molecular characteristics of vascular wall components (ie, unusually large von Willebrand factor multimers [ULVWF] and tissue factor [TF]). These different levels of vascular damage create its unique phenotypes of stroke, which are TIA, acute ischemic stroke (AIS), thrombo-hemorrhagic stroke (THS), AHS, and diffuse encephalopathic stroke shown in Table 2.
Table 1.
Three Essentials in Normal Hemostasis (Reproduced and modified with permission from Chang5).
| (1) Hemostatic principles | ||
| (1) Hemostasis can be activated only by vascular injury. | ||
| (2) Hemostasis must be activated through ULVWF path and/or TF path. | ||
| (3) Hemostasis is the same process in both hemorrhage and thrombosis. | ||
| (4) Hemostasis is the same process in both arterial thrombosis and venous thrombosis. | ||
| (5) Level of vascular damage (ECs/SET/EVT) determines different clinical phenotypes of hemorrhagic disease and thrombotic disorder. | ||
| (2) Major participating components | ||
| Components | Origin | Mechanism |
| (1) ECs/SET/EVT | Blood vessel wall/EVT | Protective barrier |
| (2) ULVWF | ECs | Endothelial exocytosis/anchoring and microthrombogenesis |
| (3) Platelets | Circulation | Adhesion to ULVWF strings and microthrombogenesis |
| (4) TF | SET and EVT | Release from tissue due to vascular injury and fibrinogenesis |
| (5) Coagulation factors | Circulation | Activation of coagulation factors and fibrinogenesis |
| (3) Vascular injury and hemostatic phenotypes | ||
| Injury-induced damage | Involved hemostatic path | Level of vascular injury and examples |
| (1) ECs | ULVWF | Level 1 damage—microthrombosis (eg, TIA [focal]; Heyde syndrome [local]; EA-VMTD/DIT [disseminated]) |
| (2) ECs/SET | ULVWF + sTF | Level 2 damage—macrothrombosis (eg, AIS, DVT, PE, AA) |
| (3) ECs/SET/EVT | ULVWF + sTF + eTF | Level 3 damage—macrothrombosis with hemorrhage (eg, THS, THMI) |
| (4) EVT alone | eTF | Level e damage—fibrin clot (eg, AHS [eg, SDH, EDH], ICH, organ/tissue hematoma) |
| Hemostatic phenotypes | Causes | Genesis |
| (1) Hemorrhage | External bodily injury | Trauma-induced external bleeding (eg, accident, assault, self-inflicted injury) |
| (2) Hematoma | Internal EVT injury | Obtuse trauma-induced bleeding (eg, tissue and cavitary hematoma in stroke, hemarthrosis) |
| (3) Thrombosis | Intravascular injury | Intravascular injury (eg, atherosclerosis, diabetes, indwelling venous catheter, surgery, procedure) |
Abbreviations: AA, aortic aneurysm; AIS, acute ischemic stroke; AHS, acute hemorrhagic stroke; DIT, disseminated intravascular microthrombosis; DVT, deep vein thrombosis; ECs, endothelial cells; EDH, epidural hematoma; EVT, extravascular tissue; ICH, intracerebral hemorrhage; PE, pulmonary embolism; SDH, subdural hematoma; SET, subendothelial tissue; TF, tissue factor; eTF, extravascular TF; sTF, subendothelial TF; THMI, thrombo-hemorrhagic myocardial infarction; THS, thrombo-hemorrhagic stroke; TIA, transient ischemic attack; ULVWF, unusually large von Willebrand factor multimers; VMTD, vascular microthrombotic disease; EA-VMTD/DIT, endotheliopathy-associated VMTD.
Table 2.
Causes and Mechanisms and Proposed Stroke Phenotypes.
| Causes | Mechanisms | Examples of contributing pathology | Examples of vascular damage | Activated hemostatic path | Examples of clinical phenotype stroke |
|---|---|---|---|---|---|
| Vascular disease or injury: In-situ stroke
Embolic stroke |
Vascular wall damage causing hemorrhage into vascular lumen | Atherosclerotic lesion(s) Physical vascular damage | Detachment of small plaque or large atheroma | ULVWF path and/or TF path | Stroke phenotypes |
| Level 1 (L1): ECs | Small plaque (L1) | Detached focal plaque | ULVWF | TIA | |
| Level 2 (L2): ECs +SET | Large atheroma (L1, L2) | Detached local atheroma | ULVWF + TF | AIS | |
| Level 3 (L3): ECs + SET + EVT | Hypertension (L1, L2, L3) | Damaged small vessel wall | ULVWF + TF | THS; embolic stroke (AIS) | |
| Vascular damage with EVT hemorrhage into brain tissue or between coverings | Vascular anomaly Obtuse head/brain trauma |
||||
| Level e (Le): Mainly EVT | Vascular anomaly (Le) | Ruptured vessel wall | TF | AHS (eg, ICH) | |
| Severe trauma (Le) | Obtuse head/brain injury | TF | AHS (ie, SAH, IVH, SDH, EDH) | ||
| Endotheliopathy due to diseases | Complement activation | ECs damage | Systemic endotheliopathy sepsis; post-surgery; pregnancy; transplant | ULVWF | TTP-like syndrome (EA-VMTD) with encephalopathic stroke |
| Oxidative stress | ECs damage | Recurrent focal endotheliopathy | |||
| Diabetes Hyperhomocysteinemia |
ULVWF (?) | Diabetic stroke | |||
| Protease enzyme disorder | ADAMTS13 deficiency | TTP | NA | ULVWF | TTP (AA-VMTD; GA-VMTD) with encephalopathic stroke |
| Other vasculopathy | Endothelial dysfunction | Genetic disease | Endotheliopathy | ULVWF | HERNS syndrome with stroke Susac syndrome with stroke |
| Vascular dysfunction | Acquired vasculopathy | Vasculopathy | ULVWF +/-TF |
Abbreviations: AF, atrial fibrillation; AHS, acute hemorrhagic stroke; AIS, acute ischemic stroke; EC, endothelial cells; EDH, epidural hematoma; EVT, extravascular tissue; HERNS syndrome, hereditary endotheliopathy, retinopathy, stroke syndrome; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; NA, not applicable; SAH, subarachnoidal hemorrhage; SDH, subdural hematoma; SET, subendothelial tissue; TF, tissue factor; THS, thrombo-hemorrhagic stroke; TIA, transient ischemic attack; TTP, thrombotic thrombocytopenic purpura; ULVWF, unusually large von-Willebrand factor multimers; VMTD, vascular microthrombotic disease; antibody-associated VMTD (AA-VMTD); endotheliopathy-associated VMTD (EA-VMTD); gene mutation-associated VMTD (GA-VMTD).
In this article, this author will depart from the conventional view of stroke, but present an alternate perspective of stroke syndrome according to the vascular physiological mechanism and in vivo hemostatic theory. The key role of the vascular wall structure contributing to hemostasis (Figure 1) will be explored, and the mechanism how hemostatic molecular components produce clinically unique stroke phenotypes will be explained by applying the concept of newly recognized 3 different molecular thrombogeneses.4 Lastly, if applicable, the potential of theory-based therapeutic options will be discussed to help in designing therapeutic clinical trials, including primary prevention, secondary prevention, and prophylaxis for different stroke phenotypes.
Figure 1.
Schematic illustration of cross section of blood vessel histology and hemostatic components (Reproduced and modified with permission from Chang5). The blood vessel wall is the site of hemostasis (coagulation) to produce hemostatic plug in vascular injury to stop hemorrhage from external vascular injury. It is also the site of hemostasis (thrombogenesis) to produce intravascular blood clots in intravascular injury to cause thrombosis. Its histologic components can be divided into the endothelium, tunica intima, tunica media, and tunica externa, and each component has different function contributing to molecular hemostasis. As shown in the illustration, ECs damage triggers exocytosis of ULVWF, SET damage promotes the release of sTF from tunica intima, tunica media, or tunica externa, and EVT damage induces the release of eTF from the outside of blood vessel wall. This depth of blood vessel injury contributes to the genesis of different thrombotic disorders such as microthrombosis, macrothrombosis, and fibrin clots/hematoma. This concept is critically important in the understanding of different phenotypes of stroke. ECs indicates endothelial cells; EVT, extravascular tissue; SET, subendothelial tissue; TF, tissue factor; eTF, extravacular TF; sTF, subendothelial tissue factor; ULVWF, unusually large von Willebrand factor multimers.
Contemporary and New Conceptual Views on Stroke
Succinctly, stroke has been defined as a disease that affects the arteries leading to and within the brain according to American Stroke Association, and is described as a “brain attack” that occurs when blood flow to an area of brain is cutoff according to National Stroke Association. However, stroke is not a simple blood clotting disorder within the cerebral arterial vasculature but is the phenomenon showing of phenotypic complexity created by thrombosis/hemorrhage resulting from the localization within the intracranial vasculature and different depth of vascular wall damage in vascular injury associated with multitude of underlying pathologies.
Hemorrhage and Thrombosis
Traditionally, stroke has been classified into 2 broad categories of stroke syndrome: hemorrhagic (bleeding) stroke and thrombotic (ischemic) stroke. These 2 phenotypes are considered to be diametrically opposite conditions because hemorrhage is characterized by bleeding into the brain tissue resulting in hematoma and brain tissue shift while ischemia is due to thrombosis characterized by “blood clots” within intracranial vasculature leading to hypoxia to a certain part of the brain due to reduced blood supply. Both may result in different clinical brain syndromes even in the same locality.6
Currently, stroke is conceptualized as acute brain syndrome caused by one of 2 different events, either hemorrhage or thrombosis, via 2 different hemostatic processes. Hemorrhage occurs as a result of intracranial bleeding due to vascular injury (eg, malignant hypertension, damage of vascular anomaly, or trauma), but thrombosis occurs as a result of blood clots (eg, detached small endothelial plaque or large atheroma) at a intracranial intravascular site. However, this author differs from this thesis that hemorrhage and thrombosis in stroke are 2 separate events occurring as the results of different hemostasis. This conceptual definition is not congruous with the logic that (1) hemorrhage activates in vivo hemostasis to form “blood clots” (hemostatic plug) and stop hemorrhage in external bodily injury and (2) thrombosis (“blood clots”) must be formed by the same activated hemostasis in intravascular injury to form intravascular “blood clots.”4,7 In fact, hemorrhage and thrombosis need the same hemostasis to stop hemorrhage or to form blood clots, often in one event of stroke in a “vascular injury.” These may be difficult concepts to accept, but the reader will understand how and why these conditions may occur simultaneously in THS once hemostatic principles shown in Table 1 5 and Table 2 are familiarized.
Thrombo-Hemorrhagic Stroke and Hemorrhagic Stroke
For examples, in a cerebral artery injury due to detachment of atherosclerotic atheroma, a tear of aortic aneurysm or malignant hypertension, could damage the endothelial cells (ECs), subendothelial tissue (SET), and/or extravascular tissue (EVT) of the vessel wall (Figure 1). A combined ECs and SET damage would trigger intravascular hemostasis in the vascular lumen and subsequent coagulation, promoting intravascular thrombosis, but additional EVT injury causes extravascular bleeding to the opposite site of blood lumen into the brain tissue. This damage releases both subendothelial TF (sTF) and extravascular TF (eTF) from the vascular wall and extravascular structure shown in Figure 1. In this situation, intravascular hemostasis produces cerebral thrombosis via release of ULVWF from ECs and sTF from SET, but extravascular bleeding due to additional EVT damage provokes bleeding into the brain tissue and initiates fibrin clots, leading to cerebral hemorrhage/hematoma.5 Thus, the combined damage of ECs, SET, and EVT from an intravascular injury would cause thrombosis in the cerebral artery and hemorrhage/hematoma within the brain tissue. This complex syndrome is called THS. In short, thrombosis and hemorrhage/hematoma are the result of one event from the same vascular injury can occur in uncontrolled malignant hypertension. This vascular wall physiologic mechanism is a very important part of in vivo hemostasis in the understanding of stroke genesis and provides major implication for the prognosis and in management of different stroke phenotypes.5,7
Unlike THS, in aneurysmal rupture of certain vascular anomaly and obtuse trauma to the head or brain, an arterial vascular damage may cause no hemostasis in the damaged intravascular arterial lumen, instead hemorrhage occurs into EVT, which could be the brain tissue or cavitary space between brain coverings. In intracranial hemorrhage into EVT (eg, brain) or cavitary space between the coverings (eg, subdural space, subarachnoid space), the release of ULVWF in insignificant amount from ECs would be nonconsequential due to absence of bleeding into intravascular lumen, primarily related to high arterial pressure gradient, but result in significant bleeding into the brain tissue or between brain coverings. This extravascular hemorrhage may activate TF and produce hematoma within the space where hemorrhage occurred in EVT. This is called AHS. It occurs in intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH)/intraventricular hemorrhage (IVH), subdural hematoma (SDH), and epidural hematoma (EDH). Currently stroke specialists have regarded the above trauma or disease-associated intracranial hemorrhage as life-threatening hemorrhagic stroke, which could occur with or without hematoma formation. It accounts for approximately 10% to 15% of all cases of stroke and is often associated with a high mortality due to limited option of treatment for acute hemorrhage.8 It is crucial to understand the axiom that every stroke always begins with a “vascular injury” in intracranial space, sometimes extending into intracranial extravascular space.
In Situ Thrombosis and Embolic Thrombosis
Depending upon the origin of the blood clot(s), stroke also can be classified into 2 groups: in situ stroke and embolic stroke. In both, the very event that initiates stroke is intravascular injury. In situ stroke occurs in intracranial vascular injury, which develops in hereditary diseases associated with underlying endotheliopathy9–11 and acquired diseases such as atherosclerosis, diabetes, hypertension, trauma,12 autoimmune diseases,13,14 infectious diseases,15,16 drugs,17,18 cancer,19–21 and others. The clinical phenotypes of stroke manifest with diversified clinical spectrum from localized neurologic deficit to diffuse encephalopathy. Although it is only recently recognized, diffuse encephalopathic stroke typically develops in acquired vascular microthrombotic disease (VMTD) in association with complement-mediated endotheliopathy in such diseases as sepsis, trauma, immune diseases, and other critical illnesses. This stroke entity is well-known to occur in thrombotic thrombocytopenic purpura (TTP) and TTP-like syndrome.4,7 Localized in situ stroke, including AHS, is responsible for approximately 70% of stroke.
On the other hand, embolic stroke commonly occurs as a result of clot(s) that travels from somewhere else in the body, usually from the heart. Embolic stroke often originates from cardiovascular pathology associated with atrial fibrillation, left atrial appendage thrombus, aortic stenosis/aneurysm, heart surgery or vascular injury, and may occur suddenly without warning signs. An embolic stroke begins when a piece of blood clot(s) called an embolus breaks loose and is carried into the vasculature and blocks an artery in the brain typically where the larger arteries branch off into smaller vessels. The blood clot reaches to a point where it cannot travel farther and causes ischemic stroke at lodged area. Embolic stroke is responsible for approximately 20% to 30% of stroke. The most of embolic stroke is associated with atrial fibrillation,22–24 which pathogenetic mechanism is a contentious issue with a serious debate at this time. This controversy will be discussed later with the thesis of recently presented novel in vivo hemostasis in the background.
Cerebral Arterial Thrombosis and Venous Sinus Thrombosis
Stroke occurs as a result of intracranial arterial thrombosis/hemorrhage leading to microthrombi, macrothrombus, fibrin clots, or hematoma, which produces cerebral ischemia, infarction, or gangrene. The molecular events of stroke are characterized by exocytosis of ULVWF from ECs, activation of platelets, and release of TF from SET/EVT, which trigger microthrombogenesis, fibrinogenesis, and macrothrombogenesis depending upon the level of vascular wall damage (Table 1 and Table 2). Typically, stroke begins with intracranial intravascular injury leading to arterial vascular occlusion or hemorrhage. The clinical manifestations are variable with localized brain syndrome depending upon the involved arterial vasculature and extent of vascular damage at the location of blood clots. However, stroke also can be occasionally expressed due to different functional nature of the vascular circulatory system (ie, arterial vs venous sinus). A stroke developing due to thrombosis involving the cerebral venous sinus (ie, CVST) tends to be less acute with little prominent neurological symptoms. It has been called stroke-like syndrome. Cerebral venous sinus thrombosis is a rare condition associated with macrothrombosis similar to deep vein thrombosis (DVT) involving the lower extremities, but develops in the sinus drainage system of the brain located between the endosteal and meningeal layers of dura mater. Clinical features may include headache, abnormal vision, any of the symptoms of stroke such as weakness of the face and limbs on one side of the body, and seizures. Although CVST is the thrombosis within intracranial venous system, it should be included in the column of stroke since it a hemostatic disease and presents with brain syndrome even though it is often atypical.
Cerebral Hemorrhage and Hematoma
Hemorrhage in ICH, SAH, and IVH means blood in liquid or semiliquid state without firm blood clot formation yet. Hematoma in SDH and EDH is in semisolid or organized state mainly composed of blood cells, plasma components and some fibrins. It is a result of modest activation of TF path due to EVT damage and is incomplete blood clots, but blood cells comingled with some fibrin meshes. Intracerebral hemorrhage occurs due to bleeding from injured blood vessel directly into the area between the outer layer of the vessel wall and brain tissue without much ULVWF and TF release. It causes hemorrhage into the brain tissue, but precludes significant hemostasis. Subdural hematoma and EDH are suspected to occur due to bleeding from the injured blood vessel located closely to the cavitary area with some release of TF from EVT site enough to activate TF-activated factor VII (TF-FVIIa) path, leading to fibrin meshes, neutrophil extracellular traps (NETs),25,26 and comingling with red blood cells to form hematoma between the brain coverings.
Macrothrombosis and Microthrombosis
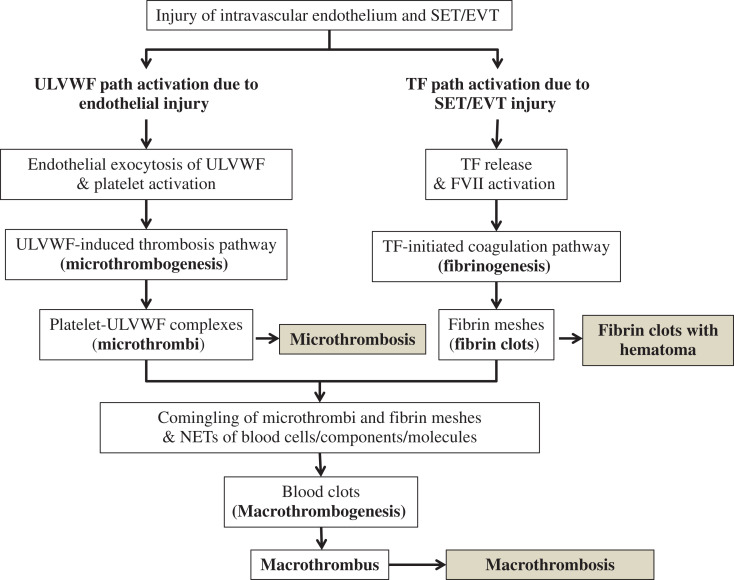
In addition to hemorrhage/hematoma leading to stroke, thrombosis-associated stroke should be understood with the concept that there are 2 different kinds of thrombosis forms present. One is microthrombosis which causes TIA, and the other is macrothrombosis which produces AIS as illustrated in Figure 2.27 To date, even in coagulation community, the different concept between macrothrombosis and microthrombosis is not clearly understood. Disseminated microthrombosis occurs in thrombotic disorders such as TTP, TTP-like syndrome, hemolytic-uremic syndrome (HUS), acute respiratory distress syndrome, disseminated intravascular coagulation (DIC), and multiorgan dysfunction syndrome (MODS).4,7,27 On the other hand, cerebral arterial thrombosis (CAT) and AIS are caused by localized macrothrombosis, but encephalopathy, which can be designated best as diffuse encephalopathic stroke, is caused by disseminated intravascular microthrombosis (DIT) as seen in sepsis and other VMTD.4,27 Current conception of thrombosis is the dogma that the essential nature of CAT/AIS and DIC/DIT is similar because they are considered to be fibrin clots made of fibrin meshes, coagulation factors, and platelets via activated TF path.28,29 This interpretation is obviously wrong because the concept of DIC has been incorrectly defined as coagulation disease although DIC is the disease of microthrombosis composed of platelet-ULVWF complexes. The correct term for DIC should be DIT.4,7,30 This author has placed quotation marks to indicate its ill-founded terminology. Thus, to convey the false concept of DIC, “DIC” with quotation marks is used in discussion of this article.5,27,30,31
Figure 2.
Normal hemostasis based on “two-path unifying theory” of hemostasis and 3 paths to thrombogenesis (Reproduced and modified with permission from Chang27). In vivo hemostasis is initiated by activation of 2 subhemostatic paths in a vascular injury: microthrombotic (ULVWF) path and fibrinogenetic (TF) path. In complete hemostasis, both paths are activated by the damaged ECs and SET/EVT of the blood vessel wall in external bodily injury and intravascular injury sites. Activated ULVWF path due to ECs damage promotes exocytosis of ULVWF that recruit platelets to produce microthrombi strings via microthrombogenesis, and activated TF path due to SET/EVT damage releases TF that activates FVII to produce TF-FVIIa complexes leading to fibrin meshes via fibrinogenesis. The final path of in vivo hemostasis is macrothrombogenesis in which microthrombi strings and fibrin meshes comingle and become unified together with incorporation of NETs. This unifying event of macrothrombogenesis produces hemostatic plug and promotes wound healing in external bodily injury, but produces macrothrombosis such as CAT in intravascular injury, leading to AIS. On the other hand, incomplete hemostasis due to lone activation of ULVWF path occurs in TIA due to small detached atherosclerotic plaque(s) and also in diffuse encephlopathic stroke commonly seen in VMTD that is often triggered by sepsis or other critical illnesses. Another kind of incomplete hemostasis is due to lone activation of TF path, which occurs in AHS (eg, subdural hematoma), resulting in partially formed fibrin clots/hematoma. AIS indicates acute ischemic stroke; AHS, acute hemorrhagic stroke; CAT, cerebral artery thrombosis; ECs, endothelial cells; EVT, extravascular tissue; FVIIa, activated factor VII; NETs, neutrophil extracellular traps; SET, subendothelial tissue; TIA, transient ischemic stroke; TF, tissue factor; ULVWF, unusually large von Willebrand factor multimers; VMTD, vascular microthrombotic disease.
The macrothrombus of CAT causing AIS occurs in the locality of the larger arterial vasculature, but the microthrombi of “DIC” and diffuse encephalopathic stroke occur systemically in the microvasculature of the brain and other organs. Then, a serious question is “how are the thrombus in the large vessel and the microthrombi in microvasculature are the same in their character and components?” To put it in another way, in interpreting of stroke, what is the difference between TIA and AIS in their character of blood clots? Transient ischemic attack and AIS must be 2 completely different stroke entities occurring in the different paths of thrombogeneses (Table 2). Logically speaking, TIA is a microthrombotic disease and AIS is a coagulation disorder caused by macrothrombotic disease.
A “macrothrombus” cannot physically occupy capillaries and arterioles. Thus, the thrombosis in the microvasculature must be made of “microthrombi.” For this simple logic alone, it can be concluded that macrothrombosis in AIS and microthrombosis in TIA are produced from 2 different hemostatic paths. Indeed, we know now the macrothrombus of AIS and DVT is formed of fibrin meshes, VWF, and platelets.32 On the other hand, the microthrombi of TTP/TTP-like syndrome and DIC are composed of platelets and ULVWF strings.33 Thus, it can be inferred that TIA is a transient and focal hemostatic event of microthrombosis, which is different from a serious and local hemostatic event of AIS occurring due to macrothrombus. These insights from DIC and diffuse encephalic stroke based on the molecular endothelial pathogenesis of VMTD have offered this author to construct “two-activation theory of the endothelium”30,31 and also identify true in vivo hemostasis based on “two-path unifying theory.”4,7 Finally, it is theorized that microthrombi composed of platelet-ULVWF complexes must interact with fibrin clots which are formed from activated TF path, and produce together the macrothrombus. This proposition supports macrothrombus is composed of the unified complex of microthrombi and fibrin clots. No doubt, recognizing the molecular pathogenetic differences amongst macrothrombus, fibrin clots and microthrombi would have major impact in the understanding of stroke syndromes and on their therapeutic individualization.
The Mechanism of Stroke
Intravascular Injury and Stroke Phenotypes
It should be emphasized that both in situ thrombosis and embolic thrombosis are always the products of intravascular injury as summarized in “Three Essentials in Normal Hemostasis” (Table 1).4,7,27 Even in thrombophilic state such as protein C deficiency and factor V Leiden, thrombosis that causes stroke cannot be formed unless intravascular hemorrhage from ECs tear and SET/EVT damage occurs first as a result of intravascular injury (eg, detachment of atherosclerotic plaque, surgery, vascular tear due to hypertension or metastatic cancer). In situ thrombosis occurs in intracranial vascular injury, and embolic thrombosis is travelled to the intracranial vasculature after thrombus formed from extracranial vascular injury. In every thrombosis, the pathophysiological mechanism forming thrombosis of stroke must begin with intravascular injury because thrombosis is the result of intravascular hemostasis. Five preconceptions in the understanding of the stroke logic based upon “two-path unifying theory” of hemostasis are formulated in Table 3.
Table 3.
Five Preconceptions on Stroke Interpretation According to “Two-Path Unifying Theory” of Hemostasis.
| Preconception 1 Stroke cannot occur without intracranial or extracranial intravascular injury, even in a person with thrombophilia (eg, FV Leiden) or hemorrhagic disease (eg, hemophilia A) as well as in normal person, but once intravascular injury is initiated, underlying thrombophilic state or hemorrhagic disease markedly potentiates the severity of stroke. Preconception 2 Stroke means either intracranial intravascular injury has triggered the formation of thrombus, or embolic thrombus from extracranial intravascular injury site has travelled to the intracranial vasculature. Preconception 3 Risk factors such as atherosclerosis, atrial fibrillation, diabetes, blood stasis, hypertension, immobility, old age, trauma, surgery, implanted vascular access device, alcohol intake, smoking, hospitalization, oral contraceptive, earthquake, accident, cancer, infection, and many others are not true “risk factors for thrombosis,” but they are the “risk factors inducing vascular injury” that could lead to thrombosis. The only risk factor for thrombosis is “vascular injury.” Therefore, the only risk event precipitating for stroke is “vascular injury.” Of course, not every vascular injury causes thrombosis. Preconception 4 Thrombo-hemorrhagic stroke (THS) is initiated by the breach of the endothelium/SET/EVT that causes hemostasis in into the vascular lumen to produce “thrombosis” and also into the brain tissue to trigger extravascular “hemorrhage/hematoma” concurrently. Preconception 5 Acute hemorrhagic stroke (AHS) such as ICH, SAH, IVH, SDH, and EDH may occur as a result of obtuse head/brain trauma. Because ECs and SET are minimally breached, hemostasis does not occur in the intravascular lumen at the injury site due to pressure gradient despite of intracranial vascular injury. Instead, a significant EVT damage results in hemorrhage into the brain tissue or between the coverings of the brain. This clinical phenotype leads to hemorrhage within EVT or hemorrhage into the space between the coverings of the brain and promotes activation of lone TF path, which produce hematoma. |
Abbreviations: AHS, acute hemorrhagic stroke; EC, endothelial cells; EDH, epidural hematoma; EVT, extravascular tissue; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; SET, subendothelial tissue; SAH, subarachnoidal hemorrhage; SDH, subdural hematoma; TF, tissue factor; THS, thrombo-hemorrhagic stroke.
Genesis of Stroke Syndromes
Figure 1 shows schematic illustrative presentation of the cross section of the vessel histology and physiologic components involved in hemostasis. The inner lining of the blood vessel is covered with the structural barrier endothelium, which releases ULVWF and FVIII from Weibel-Palade bodies in ECs damage34 and functions as the primary structure with tunica intima preventing blood leakage into SET (ie, tunica media and externa) and EVT. When a small superficial endothelial atherosclerotic plaque is detached without damage to the SET (level 1 damage), ULVWF are released from the ECs and recruit platelets, which promote microthrombogenesis and produce microthrombi strings (Figure 2). If, in addition to endothelial damage, the intravascular injury extends into SET (level 2 damage), subendothelial TF (sTF) would be released into circulation and be immediately available for intravascular hemostasis and produce macrothrombus. Further, if the vascular compromise extends beyond SET and into EVT (level 3 damage) due to intravascular injury (eg, localized aneurysm rupture; uncontrolled hypertension-related injury; surgical/procedure-related damage), hemorrhage would occur into EVT (ie, brain tissue) with release of extravascular TF (eTF). This would lead to hemorrhage and hematoma in addition to macrothrombus. The combined macrothrombus and hemorrhage cause THS.
However, generalized endotheliopathy (generalized level 1 damage) seen in sepsis, certain trauma, and other critical illnesses activates only ULVWF path, and leads to disseminated VMTD, including encephalopathy, which is the clinical phenotype of diffuse encephalopathic stroke. On the other hand, the embolic stroke migrated from external cranial vascular site (eg, heart) of level 2 damage produces embolic AIS phenotype. Separately, in a patient with obtuse external trauma to the head or brain may cause intracranial vascular injury with hemorrhage into EVT, which extends into the brain tissue, but without hemorrhage into intravascular lumen. This will lead to AHS phenotype very different from local THS.
Hemostatic Nature and Stroke
Stroke is the most common life-threatening hemostatic disorders because it includes not only in situ and embolic stroke but also very common diffuse encephalopathic stroke associated with VMTD involving the brain. The phenotype of thrombosis expressed by the different level (depth) of intracranial intravascular injury is the better predictor for the severity of the stroke than the phenotype expressed by the vessel localization of the thrombosis within the brain. Stroke syndrome is an arduous concept to comprehend for the clinician because we are only armed with partially explainable in vivo hemostasis mechanism (ie, TF-initiated extrinsic cascade/cell-based coagulation) at this time.
Unsolved issues in hemostasis
In addition to incompletely understood hemostatic mechanism, clinicians and laboratory hematologists as well as coagulation scientists have used inconsistent terminology in the lexicon of coagulation. These include “coagulation” and “thrombosis,” “blood clots” and “fibrin clots,” “hematoma” and “thrombus,” and “macrothrombus” and “microthrombi.” Further, sometimes the terms “coagulation,” “hemostasis,” and “thrombogenesis” have been used ambiguously to mean the same or similar hemostatic processes in different situations. Also, the term “coagulation” in the laboratory in vitro and that in a disease in vivo convey very different meanings.
Contemporary dogmatic theory on hemostasis has solidified the proposition that all the thromboses must be the same or similar in their character as a result of the final product of hemostasis triggered by TF release and ending with formation of “fibrin clots” via extrinsic cascade/cell-based coagulation. This current concept of the blood clots (thrombus) defines that every thrombus in arterial thrombosis and DVT are made of “fibrin clots,” but perhaps with different NETs.25 This uniformity thesis has allowed for the clinician to use the same anticoagulation therapy for every thrombotic disorder in absence of understandable thrombogenetic mechanism.4 We know better now that anticoagulation is effective in management of DVT, but it is not effective at all in certain arterial thrombosis such as “DIC” and TTP,5,27,31 which are characterized by arteriolar and capillary microthrombosis. Also, AIS caused by fibrin clot with trapped blood cells, cellular components, platelets, and VWF35 can be prevented effectively with anticoagulation therapy. These clinical and pathological differences among DVT, AIS, and “DIC” have been an enigmatic puzzle to the clinicians and could not be explicated by contemporary hemostatic theories. Clinicians have understood both DVT and DIC are similar disease from the same “blood clots” but occurring in different vasculatures (ie, large vasculature vs microvasculature). Therefore, many clinicians have called DIC is the disorder of microvascular thrombosis instead of that of vascular microthrombosis. If microthrombi of “DIC” are recognized to be different in its character from macrothrombosis of DVT and AIS, the unifying concept of microthrombi and “fibrin clots” perfectly explains the genesis of macrothrombus. Finally, we can affirm that DVT is macrothrombotic disease and “DIC” is microthrombotic disease (ie, VMTD) occurring from separate thrombogenetic paths.5,27,31 In retrospect, this ambiguity in terminology has contributed to the delay in identifying the true mechanism of in vivo hemostasis to date.5,27
The in vitro based hemostatic theory of in vitro intrinsic and extrinsic coagulation cascades has been very useful in identifying various coagulation disorders due to coagulation factor deficiency utilizing in vitro clotting tests such as prothrombin time, activated partial thromboplastin time, thrombin time, and various coagulation factor assays.36 However, when comprehensive understanding of in vivo hemostasis is needed, the role of platelets and von Willebrand factors (VWF) could not be assigned into proper places in the mechanism of fibrinogenesis represented by in vitro extrinsic cascade/cell-based model. Additionally, the pathophysiological mechanism producing microthrombosis in VMTD, such as “DIC”, TTP, TTP-like syndrome, HUS, thrombotic microangiopathy, and MODS, including encephalopathy, has not been clearly understood5,27 to date. Now, the microblood clots of “DIC” (eg, microthrombi seen in diffuse encephalopathic stroke) are established to be “microthrombi.”30,33,37 This reinterpretation of microthrombosis and the concept of microthrombogenesis have helped this author in formulating in vivo hemostasis, which can account for every aspect of hemostasis-related diseases that couldn’t be explained by extrinsic cascade/cell-based coagulation theory, including different stroke phenotypes.
In fact, the role of disseminated VMTD in stroke, which brain phenotype is characterized by encephalopathy presenting with altered mental state, confusion, seizure, and coma rather than localizing neurological symptoms and signs, has been well-known in clinical medicine.31 The support for the concept of microthrombogenesis further comes from increased activity of ULVWF/VWF that has been significantly high in certain stroke syndromes (ie, TIA and AIS).38–40 However, the issue whether stroke is at high risk due to ULVWF or it causes high ULVWF after stroke is another unresolved issue at this time. This riddle can be easily answered by “two-path unifying theory” of hemostasis.
Novel “two-path unifying theory” of hemostasis
This author has had a long-standing interest on the pathogenesis of “DIC” because this condition is very similar to TTP with the same characteristic of vascular microthrombosis and hematologic features of thrombocytopenia, microangiopathic hemolytic anemia (MAHA) associated with MODS, including the brain and renal dysfunction.5,27,31,41 The main difference between “DIC” and TTP has been “DIC” is always associated with critical illnesses and TTP is caused by the deficiency of ULVWF-cleaving protease ADAMTS13, which occurs in hereditary gene mutation-associated TTP (ie, GA-VMTD) or acquired antibody-associated TTP (ie, AA-VMTD).31 In clinical cases, another intriguing feature of VMTD has been there are 2 groups of patients with microthrombosis: one with extremely low ADAMTS13 activity less than 5% of normal and the other with mild to moderately diminished ADAMTS13 activity between 25% and 75%.31 The former group is associated with TTP, but the latter group almost always occurs in critically ill patients with endotheliopathy-associated (EA) diseases such as sepsis and polytrauma. Pathogenetically this latter group is different from immune TTP and hereditary TTP, and has been called as endotheliopathy-associated VMTD (EA-VMTD) or TTP-like syndrome.31 Oftentimes, TTP-like syndrome is associated with coagulopathy. The EA-VMTD-associated coagulopathy has occurred with thrombocytopenia, MAHA, and MODS as seen in TTP; however, unlike TTP, TTP-like syndrome is often characterized by prolonged prothrombin time and activated partial thromboplastin time and hypofibrinogenemia. The coagulopathy in critically ill patients has been termed acute “DIC” when associated with abnormal coagulation parameters, but it is called chronic “DIC” when unassociated with coagulopathy.5,27,31 However, it has become apparent that both acute and chronic “DIC” have occurred in association with complement activation, leading to endotheliopathy, molecular changes of exocytosis of ULVWF, and release of inflammatory cytokines. Certainly, this endothelial molecular pathogenesis is identical in both “DIC” and TTP-like syndrome. Thus, “DIC” is concluded to be a clinical phenotype of EA-VMTD as is other TTP-like syndromes.5,27,30,31
In recent publications, this author was able to affirm that the concept of “DIC” attributed to activated TF-FVIIa path leading to fibrin clots was ill-founded. “DIC” should be correctly redefined as DIT, which clinical disease is VMTD occurring as a result of activated ULVWF path of hemostasis. More importantly, both “DIC” and DIT with hematologic features of TTP and TTP-like syndrome are characterized by the microthrombosis expression.4,5,7,27,30,31,41 If “DIC” were not the result of TF-FVIIa path-initiated coagulopathy because microthrombi are composed of platelet-ULVWF complexes, it is logical to infer that there has to be another hemostatic path exists via activation of ULVWF and platelets, which contributes to the formation of microthrombi in “DIC.” To this author, this path perfectly fits and works well in in vivo hemostasis framework because the exocytosis of ULVWF interacting with platelets is the first event of in vivo hemostasis in external bodily injury. Now, 2 molecular events are identified; one is well-known TF role activating FVII (TF path) in vitro and in vivo hemostasis and the other is the role of ULVWF recruiting platelets (ULVWF path). Both are essential processes in in vivo hemostasis. Thus, TF path and ULVWF path can be put together and construct a novel in vivo hemostatic theory. This proposition from recognition of false concept of “DIC” has led this author to formulate “two-path unifying theory” of hemostasis as illustrated in Figure 2 and “three different mechanisms of thrombogenesis” as annotated and shaded in the Figure.4,5,7,27 Finally, a novel well-defined physiological mechanism of in vivo hemostasis is identified.
As articulated in the schematic illustration in Figure 1, the level (depth) of vascular damage is the critical determinant that produces the different character and components of blood clots as well as severity of stroke syndrome. Following vascular injury involving the damage of ECs and SET/EVT, 2 hemostatic subpaths must be activated. One is microthrombotic (ie, ULVWF) path, and the other fibrinogenetic (ie, TF) path. The third is combined path producing macrothrombosis from unifying of ULVWF and TF paths. In ULVWF path, ULVWF with FVIII are released from Weibel-Palade bodies of ECs and become anchored to the membrane of injured ECs as long elongated strings and recruit platelets to form platelet-ULVWF complexes42–45 through microthrombogenesis. The platelet-ULVWF complexes become “microthrombi” strings. But, in TF path, TF is released from damaged SET/EVT and activates FVII triggering extrinsic cascade/cell-based coagulation, promoting the sequential activation of coagulation factors and generating thrombin that converts fibrinogen to “fibrin meshes.” Following the activation of these 2 subpaths, final phase leads to the third path called macrothrombotic path in which microthrombi strings and fibrin meshes become comingled. Most likely, at this stage, neutrophils including other blood cells and molecules passively integrate into macrothrombotic path via NETosis.46 This unifying process of microthrombi, fibrin clots, and NETs together at the vascular injury site produces the final “blood clots” (hemostatic plug) to stop bleeding at external bodily injury site, but also forms “blood clots” (macrothrombus) leading to thrombosis at intravascular injury site. These mechanisms are the essence of in vivo blood coagulation and thrombogenesis integrated into the updated framework of “two-path unifying theory” of hemostasis shown in Figure 2.5 In addition, collagen and various adhesion molecules partake actively in primary event of hemostasis,47,48 but extracellular traps promoting NETosis25,46,49–51 are likely secondary cellular components and molecules that participate passively at the unifying stage.
This framework of in vivo hemostatic mechanism has been derived from reinterpretation of “DIC”4,7,27,31 that affirms the critical role of ULVWF and platelets in microthrombogenesis.5,27,31 This hemostatic mechanism in intravascular injury identifies 3 paths of thrombogenesis, which are microthrombogenesis, fibrinogenesis, and macrothrombogenesis, producing 3 different kinds of blood clots: (1) “microthrombi” for microthrombosis,52 (2) “fibrin clots” for fibrin clot disease and hematoma, and (3) “macrothrombus” for macrothrombosis. In stroke, based on 3 different levels of intravascular wall injury initiating hemorrhage and thrombosis, 5 different phenotypes of stroke are identified. The role of the different levels of vascular damage and resultant phenotypes are summarized in Table 2 and Table 4.
Table 4.
Hemostatic Characteristics of Stroke Syndrome.
| Stroke Character | Microthrombosis | Macrothrombosis | Macrothrombosis with hemorrhage | Hemorrhage/hematoma | ||
|---|---|---|---|---|---|---|
| Focal VMTD | Systemic VMTD | Thrombotic | Embolic | |||
| Stroke syndromes | TIA | Encephalopathic stroke | AIS | THS | AHS | |
| Causes | ||||||
| Vascular injury | Intravascular: focal endotheliopathy (eg, atherosclerotic plaque detachment) | Intravascular: disseminated endotheliopathy (eg, EA-VMTD) | Intravascular: local vascular injury/embolism (eg, vascular anomaly; trauma; AF (?); cardiac injury; LAA thrombosis) | Intravascular: local vascular injury beyond vessel wall (eg, uncontrolled hypertension) | Extravascular (EVT): rupture of vascular anomaly; obtuse head/brain injury (ie, AHS) | |
| Level | ECs | ECs | ECs + SET | ECs + SET + EVT | EVT | |
| Nonvascular disease/injurya | NA | Disseminated (eg, GA-VMTD, AA-VMTD) | NA | NA | NA | |
| Hemostatic path | Focal lone ULVWF path activation | Disseminated lone ULVWF path activation | Combined activation of ULVWF and TF paths | Combined activation of ULVWF and TF paths | Lone activation of TF path | |
| Mechanism of stroke genesis | Microthrombogenesis | Microthrombogenesis | Macrothrombogenesis | Macrothrombosis/fibrin clots | Fibrin clots with hematoma | |
| Character of blood clots | Platelet-ULVWF strings | Platelet-ULVWF strings | Combined platelet-ULVWF strings, fibrin meshes and trapped blood cells, DNAs, and histones | Combined platelet-ULVWF strings, fibrin meshes and trapped blood cells, DNAs, and histones; hematoma | Hematoma | |
| Examples | Focal EA-VMTD | Disseminated EA-VMTD, AA-VMTD, GA-VMTD | Cerebral arterial thrombosis; cerebral embolic thrombosis | Thrombo-hemorrhage | ICH, SAH, IVH, SDH, EDH | |
Abbreviations: AIS, acute ischemic stroke; AF, atrial fibrillation; EC, endothelial cells; EDH, epidural hematoma; EVT, extravascular tissue; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; LAA, left atrial appendage; NA, not applicable; SAH, subarachnoidal hemorrhage; SDH, subdural hematoma; SET, subendothelial tissue; TF, tissue factor; TIA, transient ischemic attack; TTP, thrombotic thrombocytopenic purpura; ULVWF, unusually large von Willebrand factor multimers; VMTD, vascular microthrombotic disease; AA-VMTD, antibody-associated VMTD; EA-VMTD, endotheliopathy-associated VMTD; GA-VMTD, gene mutation-associated VMTD.
a Nonvascular disease producing microthrombi occurs in TTP (ie, AA-VMTD and GA-VMTD), but still is aberrant hemostasis since ULVWF path is activated and participated in encephalopathic stroke.
Finally, the complete hemostatic mechanism utilizing the essential vascular wall components and coagulation factors is identified and thrombogenetic mechanisms constructed. Four major hemostatic components are ULVWF, platelet, TF, and various coagulation factors, and the involved vascular physiologic participants are ECs and SET/EVT. The application of this hemostatic and thrombogenetic mechanism in the interpretation of stroke provides several clearly defined phenotypes of stroke syndrome, which allows instructive and practical approaches not only in making the diagnosis but also for identifying rational therapy and prognostication.
Stroke Interpretation Based on Hemostasis
Redefinition of Stroke
Stroke can be redefined as a hemostatic disease characterized by neurologic syndrome due to intracranial thrombosis, hemorrhage, and/or hematoma formation as a result of intracranial intravascular or intracranial extravascular injury or travelled embolic thrombosis to the intracranial vasculature from the extracranial vasculature. It occurs due to cerebral capillary/arterial vascular thrombosis or hemorrhage, but rarely develops due to thrombosis in the cerebral venous sinus as well. Intracranial vascular injury could be trauma or disease, including internal vascular injury, external obtuse head or brain trauma, underlying vascular disease, sepsis, cancer, and coagulopathy. The rule of in vivo hemostatic mechanism in stroke is summarized in “Five preconceptions of stroke” and listed in Table 3.
Hemostatic Classification of Stroke Syndrome
First, 5 major hemostatic components/participants: (1) vessel wall (ECs/SET) and EVT, (2) ULVWF, (3) TF, (4) platelets, and (5) coagulation factors5 are fundamental elements in in vivo hemostasis and contribute to coagulation process simultaneously depending upon the nature of vascular injuries. The quality and quantity of the above components, including NETs, determine the character and size of thrombosis. Second, the most important ingredient determining the severity of thrombosis is the level (depth) of vascular injury that is summarized in Table 1 and Table 2. The level of arterial intravascular damage creates 5 distinctively different stroke syndromes as presented in Table 4 and their genesis is briefly explained as follows.
TIA: Level 1—focal damage limited to ECs without breach of tunica intima
AIS: Level 2—local damage of ECs with breach of tunica intima and extending into tunica media (SET)
THS: Level 3—local damage of ECs with breach of tunica intima and tunica media (SET) and beyond tunica externa (EVT)
AHS: Level e—local vascular damage to EVT in intracranial vascular trauma or obtuse head/brain injury with breach of EVT without hemostasis within the vascular lumen but hemorrhage into the brain tissue or into the space between membranous coverings of the brain.
Diffuse encephalopathic stroke: Level 1 generalized—disseminated but limited damage only to ECs involving intracranial vasculature as a result of disseminated endotheliopathy without breach of tunica intima.
*CVST is not discussed here because it is not arterial system.
As shown in Table 2 and Figure 2, level 1 focal damage causes the exocytosis of ULVWF, which activates lone ULVWF path of hemostasis. This level 1 damage produces only microthrombi strings in the microvasculature and leads to TIA. Level 2 damage releases ULVWF and TF, and activates both ULVWF and TF paths, which produces macrothrombus in the larger vasculature and leads to AIS. Level 3 damage releases ULVWF and subendothelial TF (sTF), and activates both ULVWF and TF paths and produces macrothrombus in the larger vasculature, and it also penetrates into EVT and causes hemorrhage into the brain tissue. This level 3 damage into the brain leads to THS. However, if level e damage occurs due to obtuse head/brain trauma, the intracranial extravascular injury does not cause bleeding into the intravascular lumen but initiate bleeding into brain tissue (EVT), sometimes causing extravascular TF (eTF) release that leads to activation of TF path and promotes the formation of hemorrhage/hematoma. This event leads to AHS seen in ICH, SAH/IVH, SDH, and EDH. Lastly, level 1 (generalized) damage (ie, disseminated endotheliopathy) triggers excessive exocytosis of ULVWF and activates lone ULVWF path in the intracranial intravascular microvasculature. This endotheliopathy produces disseminated microthrombi strings and leads to encephalopathy as a part of MODS in VMTD. This can be termed “diffuse encephalopathic stroke.”
Controversial Issues in Stroke
Encephalopathy as a stroke entity
No doubt, microthrombotic encephalopathy is a stroke phenotype occurring in critically ill patients. Encephalopathy is often provoked as a part of generalized MODS associated with VMTD (ie, TTP and TTP-like syndrome) and is characterized by diffuse intracranial intravascular microthrombosis.31 Transient ischemic attack, which microthrombosis occurs as a result of level 1 damage in focal endotheliopathy, is self-limited and reversible stroke.4,7,40 Diffuse encephalopathic stroke is also readily reversible if treated with appropriate treatment in early stage of VMTD even though it is caused by disseminated intracranial microthrombosis because microthrombi strings are composed of platelet-ULVWF complexes and ULVWF can be readily cleaved by antimicrothrombotic agents/regimen such as therapeutic plasma exchange. Diffuse encephalopathic stroke, occurring in hepatic encephalopathy,53,54 cardiac encephalopathy,55 pulmonary encephalopathy,56 uremic encephalopathy,57–59 hypertensive encephalopathy,60 and septic encephalopathy,61–64 represents the phenotype of stroke involving the brain among MODS associated with generalized VMTD. If masked and undiagnosed in early stage of VMTD and untreated with therapeutic plasma exchange, diffuse encephalopathic stroke could evolve rapidly into MODS resulting in the demise of the patient.
To date, however, because of the lack of understanding on the pathogenesis and yet not recognized as a clinical disease entity for encephalopathy associated with MODS, diffuse encephalopathic stroke of the brain syndrome has not been included in stroke syndrome. Obviously, it is a disseminated form of TIA caused by activated ULVWF path. Diffuse encephalopathic stroke does not respond to TF-based anticoagulation therapy because their pathogenetic mechanism is via microthrombogenesis occurring due to lone activation of ULVWF path, which leads to platelet-ULVWF complexes. It should be emphasized that diffuse encephalopathic stroke in sepsis is usually not due to brain tissue invasion of the pathogen through blood–brain barrier, but is the result of disseminated microthrombi within the microvasculature of the brain. Thus, diffuse encephalopathic stroke is reversible disease if treated in early stage with antimicrothrombotic therapy.
Although anticoagulation therapy is ineffective for diffuse encephalopathic stroke associated with VMTD,65 therapeutic plasma exchange as a surrogate of antimicrothrombotic treatment has been attempted in sepsis and MODS with promising potential if utilized in early stage of MODS.66–70 The pathogenesis of this stroke is an important concept derived from hemostasis, microthrombogenesis, as well as molecular function of endotheliopathy in EA-VMTD.27
Role of ADAMTS13 and ULVWF in stroke
The key role of ULVWF in stroke has been found to be significant in TIA and AIS.38–40,71 In the study of chimeric platelet derived and ECs derived VWF mice, endothelial derived VWF was found to be the major determinant that mediates VWF-dependent ischemic stroke promoting postischemic thrombo-inflammation.38 Also, a case–controlled study showing of the high level of VWF antigen and activity obtained from the post-stroke blood samples was associated with the occurrence of AIS. This relation was unaffected by the severity of the acute-phase response or by genetic variation or degradation.39 In prospective observational analytical case–control study, ADAMTS13 activity was reduced and VWF antigen expression increased within 4 weeks of TIA or ischemic stroke onset.40 The study concluded increased ULVWF caused by reduced ADAMTS13 had promoted enhanced platelet adhesion and aggregation contributing to stroke in response to stimulation with collagen and adenosine diphosphate via VWF-mediated pathways.
The implication of increased VWF and decreased ADAMTS13 in post-stroke patients is an unresolved issue. An explanation was decreased protease ADAMTS13 might be caused by genetic or acquired conditions, and the excess of ULVWF due to the decreased protease was thrombogenic, promoting stroke in both TIA and AIS.39 This has implied that elevated ULVWF was a risk factor for stroke. However, to understand the vascular biology of ULVWF, we have to go back to in vivo mechanism of hemostasis. As shown in Figure 2, and Table 1, thrombosis mediating stroke cannot occur without intravascular injury first. This preconception supports that increased ULVWF/VWF activity, which promotes the activation of microthrombotic path leading to microthrombogenesis and generating microthrombi strings,4,7,31 is the result of exocytosis of ULVWF from endothelial injury. Focal endothelial damage releases ULVWF and triggers microthrombosis of TIA (level 1 damage), and local endothelial and SET damage releases ULVWF/TF and triggers macrothrombosis of AIS (level 2 damage). Generalized endotheliopathy releases an extensive amount of ULVWF and promotes disseminated cerebral microthrombosis of diffuse encephalopathic stroke.27 Thus, increased VWF and ULVWF are not the cause of stroke but are the result of stroke occurring due to ECs damage. This recognition of the vascular biology of ULVWF in stroke may have significant implication in the management and secondary prevention.
Potential role of circulating TF-bearing microvesicles as a diagnostic marker of AIS
Another serious controversy in stroke syndrome is what is the difference between TIA and AIS. Their initial stroke features may be similar, but their subsequent clinical courses are different. How are their pathogenetic mechanisms different between 2 stroke phenotypes? The answers may come from the TF-bearing microvesicles (TF-positive MV) and perhaps other procoagulant-positive MV which are released by apoptotic and damaged cells into circulation, but TF is an integral membrane protein, normally separated from the blood by the vascular endothelium, which plays a key role in promoting blood coagulation.72 With a perforating vascular injury, TF becomes exposed to blood as a result of SET damage and binds plasma FVIIa to activate TF path and initiate extrinsic cascade/cell-based coagulation.73
The most fundamental aspect of blood coagulation is “hemostasis leading to thrombogenesis can be activated only by vascular injury” as shown in Table 1-(1) and Table 3. This means the thrombogenesis is a natural process in intravascular injury. Procoagulant ULVWF and VWF-positive MV are released/formed from ECs damage in TIA as a result of level 1 injury, but ULVWF, VWF-positive MV, TF, and TF-positive MV are released/formed from ECs and SET damage in AIS as a result of level 2 injury as shown in Figure 1 and Table 4. Therefore, it is natural to expect the expression of ULVWF, VWF-positive MV, TF, TF-positive MV, and also other MV in vascular injury. In stroke, ULVWF and TF would be utilized in the formation of microthrombosis or macrothrombosis, but some MV would be strayed in circulation as noted in clinical investigations.74–76 Although MV were suspected to be potential regulators of cardiovascular diseases, contributors of pathogenesis of thrombosis, and/or emerging marker and therapeutic targets of stroke,77–79 it is this author’s opinion that VWF-positive MV and TF-positive MV are not the regulator or cause of thrombogenesis, but simply represent the leftover “microparticles” of thrombogenesis from damaged ECs and SET following intravascular injury in stroke. This thesis is very much consistent with “two-path unifying theory” of hemostasis. However, luckily this TF-positive MV may be the best laboratory marker yet that can differentiate between TIA and AIS. Thus, theoretically strong expression of TF-positive MV supports the diagnosis of AIS due to level 2 damage of the vessel wall, which was consistently demonstrated in recently published articles.74–76
Until now, the pathogenetic difference between TIA and AIS has not been clearly understood in coagulation community even though their clinical course is very different and prognostic diversity is intriguing. These also have created anxiety for the patient when the treatment decision was discussed and decided for stroke, and at times some tension occurred between the patient and the clinician. Additionally, clinical trials often had lumped the patients with TIA and AIS together, which might have precluded fair interpretation of therapeutic results without theory-based medicine. Hopefully, this issue separating the different phenotypes between TIA and AIS in stroke could be resolved by the use of TF-positive MV. Additional investigations are needed.
Mechanisms of THS vs AHS
Among 5 major phenotypes of stroke, the pathogeneses of TIA, AIS, and diffuse encephalopathic stroke are simple and straightforward to recognize and be understand. However, 2 hemorrhagic strokes THS and AHS need special explanation from the point view of in vivo hemostasis and vascular physiology.
As shown in Table 2, TIA is the result of focal ECs damage (level 1 damage) from minor endothelial injury (eg, small atherosclerotic plaque detachment) or transient endothelial dysfunction (eg, atrial fibrillation) which leads to microthrombi strings composed of platelet-ULVWF complexes. These microthrombi strings should be resolved by circulating protease ADAMTS13 and the patient recovers after transient ischemic event. Acute ischemic stroke is the result of local endothelial injury (eg, detached atherosclerotic atheroma) causing deeper vascular damage into SET (level 2 damage) that leads to the unifying process of both microthrombi from ULVWF path and fibrin clots from TF path. The damage produces macrothrombus at the site of detached atheroma. However, in addition to level 2 damage, if the outer vascular wall tunica externa and beyond (i.e., EVT), schematically shown in Figure 1, is damaged (eg, severe hypertension), intracranial intravascular hemostasis occurs in the vascular lumen and bleeds into the brain, it leads to level 3 damage. Hemostasis in the lumen creates macrothrombosis from activated ULVWF and sTF paths, and bleeding into EVT (brain tissue) causes hemorrhage/hematoma from activated eTF path. This is called THS.
In contrast, certain intracranial vascular injury associated with vascular anomaly (eg, subarachnoid aneurysm) or obtuse trauma (eg, head/brain injury while on antiplatelet therapy) may produce intracranial extravascular injury (level e damage) leading to almost pure hemorrhagic stroke without chance to produce thrombosis because hemorrhage does not occur into the vascular lumen, but into EVT as noted in Table 1. Acute hemorrhagic stroke develops due to hemorrhage into the brain tissue (eg, ICH) or cavitary space between the coverings of the brain (eg, SAH, IVH, SDH, EDH). These stroke phenotypes collectively are called AHS. This preconception is derived from theoretical thesis based on “two-path unifying theory” of hemostasis.4,7,27 Thus, these 2 different pathogeneses between THS and AHS may dictate very different approach in their management.
Origin of embolic stroke: atrial fibrillation versus atrial cardiopathy
Approximately 20% to 40% of stroke is caused by embolic thrombi originated from the heart or other extracranial vasculature.80 According to Framingham study report in 1991, the attributed risk of stroke from all cardiovascular contributors had decreased with age except from atrial fibrillation, for which the risk increased significantly after 34 years follow-up.81 Atrial fibrillation has been assigned to be the most common cause of embolic stroke.82,83 Current views on the mechanism of thrombogenesis in atrial fibrillation rest on the century-old hypothesis that fibrillation of the atrium produces stasis of blood, which causes thrombus formation and embolism to the brain.84 Recently however, the validity of this prevailing model of atrial fibrillation and thromboembolism has been questioned22–24,80,85,86 because embolic stroke occurs without atrial fibrillation and straightforward association between atrial fibrillation and stroke does not convincingly demonstrate temporality, specificity, or a biological gradient, and it was not concordant with the totality of the available experimental evidence.22
Atrial fibrillation seems to fulfill the criteria of Virchow’s triad, which are necessary for thrombus formation: (1) blood stasis, (2) endothelial dysfunction, and (3) altered hemostasis. Blood stasis is evident in the left atrium of patients where flow velocity is markedly reduced concomitantly with impaired contractility of left atrial appendage.84 It is still not clear, however, how remodeling-related blood stasis can be implicated favoring thrombus formation in atrial fibrillation.87 This hypothesis has been recently challenged by the demonstration that atrial remodeling does not influence clotting activation and thrombus formation in canine models.88 Instead, there is clear association between atrial cardiopathy and ischemic stroke, and left atrial appendage has long been considered to be a prime site for thrombus formation in patients with atrial fibrillation.22
Hence, we are confronted with 2 very basic issues in the triangle of (1) atrial fibrillation, (2) atrial cardiopathy, and (3) thrombus formation, which are all involved in embolic stroke one way or another. The first issue is what is the primary event in thrombosis formation? Is this event atrial fibrillation or atrial cardiopathy? The second issue is what is the character of thrombus? Is it microthrombi or macrothrombus? To understand these 2 issues, we have to go back to the basics of hemostasis principle. The first principle in Table 1 is “hemostasis, leading to thrombosis, can be activated only by intravascular injury.” According to this logic, the answer to first issue comes from contentious claim of “intravascular injury” because atrial fibrillation alone does not cause vascular injury (Table 3). The answer to second question is related to the character of thrombus, which is obviously macrothrombus that is the case in embolic thrombi.
Atrial fibrillation, which does not cause intravascular injury that triggers hemostasis, could cause only endothelial dysfunction89 without SET damage. It promotes exocytosis of ULVWF without release of TF, and activates ULVWF path and produces transient “microthrombi” strings causing TIA.89 Thus, atrial fibrillation alone without vascular injury which produces damage to ECs and SET cannot cause macrothrombus that can lead to embolic AIS. It is very likely that “cryptogenic stroke” (ie, microembolic TIA) due to transient microthrombi may be prevalent in atrial fibrillation. Could it be possible that cryptogenic microembolic stroke has been included in embolic stroke (ie, macroembolic AIS) in some of clinical trials? Also, could it be possible that thrombosis within left atrial appendage due to cardiopathy promote both the onset of atrial fibrillation and embolic AIS? It is known the thrombus within left atrial appendage is macrothrombus that produces AIS.85,90,91 The hypothesis of atrial fibrillation alone promoting macrothrombus is probably incorrect according to in vivo hemostatic theory. This hemostatic theory also refutes Virchow’s concept that the triad is needed for thrombus formation since the only true cause of thrombosis is “intravascular injury.” Once intravascular injury causing ECs and SET damage occurs, ULVWF and TF paths trigger macrothrombus formation. The role of thrombophilic state or blood stasis is not the initiating cause of thrombus formation, but is the potentiating factor for thrombogenesis only after vascular injury has occurred.7
Hope is this on-going theoretical debate89,91–93 and clinical trials based on conceptual issues of the pathophysiological mechanism in cardiac thrombus formation in atrial fibrillation and cardiopathy clarify the pathogenesis of embolic stroke and microembolic stroke sooner than later and provide a firmer recommendation for the prevention of this unique stroke syndrome.
Searching for Efficient Patient Care and Therapeutic Avenues
Pharmacological options in therapeutic, primary preventive, secondary preventive, and prophylactic approaches involve complex issues due to our limited understanding of pathogenetic mechanisms and diversified clinical phenotypes of stroke syndrome. Well-organized, but ever-changing guidelines have been proposed for the management of stroke and various stroke syndromes, which this author will leave the general and specific guidance for the clinician to obtain the resources from dedicated medical societies, medical centers, and clinical scientists relevant to their disciplines.94–99 Also, the advice on surgical and interventional approaches should be obtained from highly specialized experts on these areas of treatments. Instead, for potential therapeutic selection on the newly proposed stroke phenotypes, this author will try to focus and opine on rational pharmacological approach for usage of anti-thrombotic and hemostatic agents based on hemostatic theory. This viewpoint may advocate theory-based therapeutic, preventive, and prophylactic approach prior to the consideration of clinical trials.
Because arterial and venous thromboembolisms have been the leading causes of morbidity and mortality around the world, intensive therapeutic research has been focused on the use of pharmacologic agents for the treatment, prevention, and prophylaxis in various thrombotic disorders. For almost 70 years, unfractionated heparin and vitamin K antagonists have been the available therapeutic options. Due to many limitations of these traditional anticoagulants, medical community has encouraged for the development of novel agents more than 2 decades, and new classes of oral anticoagulants that specifically target the platelet, activated FX and formation of thrombin, and more recently thrombolytic agents that promote thrombolysis have been procured and are now clinically available.100 This author will briefly summarize hemostasis inhibiting and promoting agents/regimens that may be theoretically useful in the stroke management.
Classification of Therapeutic Agents in Stroke Syndromes
The following is the list of armamentaria of hemostatic inhibitors and fibrinolytic agents utilized in clinical trials and/or used in the clinical settings of thrombotic stroke. Also, listed are scarce promoters producing hemostasis that may have potential for clinical use in AHS. Based on in vivo hemostatic mechanism of stroke syndrome, theoretical therapeutic agents used in stroke syndromes are assessed as shown in Table 5.
Table 5.
Available Hemostatic Agents That Are Potentially Useful in Stroke Syndrome.
| Category of hemostatic agents | Examples of hemostatic agents/regimens | Potential benefit for stroke | Comments |
| Antiplatelet agents | To be used to inhibit only activation of lone ULVWF path | ||
| Oral agent | |||
| Cyclooxygenase inhibitor | ASA alone or in combination with clopidogrel; ticlopidine; dipyridamole | TIA in secondary prevention | |
| ADP receptor inhibitor | |||
| Adenosine reuptake inhibitor | |||
| Antimicrothrombotic treatments | |||
| Oral agent | N-acetyl cysteine (?) | TIA in secondary prevention (?) | |
| Parenteral therapy | rADAMTS13; TPE | Encephalopathic stroke for treatment | |
| Anti-FXa agents | To be used to inhibit only in combined activation of ULVWF and TF paths producing macrothrombus | ||
| Oral agent | |||
| Direct FXa inhibitor | Rivaroxaban; apixaban; endoxaban | AIS due to embolic stroke in secondary prevention | |
| Parenteral drug | |||
| Direct FXa inhibitor | LMWH (enoxaparin); pentasaccharides (eg, fondaprinux) | AIS in progression (?) | |
| Anti-thrombin agents | |||
| Oral agent | |||
| Direct thrombin inhibitor | Dabigatran | AIS due to embolic stroke in secondary prevention | |
| Parenteral drug | |||
| Direct thrombin inhibitor | UFH; argatroban; refludan | AIS in progression (?) | |
| Anti-fibrinogenetic agents | |||
| Oral agent | |||
| Vitamin K antagonist | Coumadin | AIS due to embolic stroke in secondary prevention | |
| Parenteral drug | |||
| Direct thrombin inhibitor | UFH | AIS in progression (?) | |
| Fibrinolytic agents | tPA (alteplase); streptokinase; urokinase | AIS for therapy | |
| Hemostatic agents | Desmopressin rFVIIa |
AHS for therapy (?) | To be used as hemostatic agent Needs clinical trials |
Abbreviations: ADP, adenosine diphosphate; AIS, acute ischemic stroke; AHA, acute hemorrhagic stroke; ASA, acetyl salicylic acid; EVT, extravascular tissue; ICH, intracerebral hemorrhage; LMWH, low-molecular-weight heparin; rADAMTS13; recombinant ADAMTS13; rATIII, recombinant antithrombin III; rFVIIa, recombinant activated factor VII; TF, tissue factor; TIA, transient ischemic attack; tPA, tissue plasminogen activator; TPE, therapeutic plasma exchange; UFH, unfractionated heparin; ULVWF, unusually large von Willebrand factor multimers.
Antimicrothrombotic Agents (ULVWF Path Inhibitors)
Antiplatelet agents
An overview of major clinical trials and meta-analyses by Hackam and Spence101 has concluded that aspirin-dipyridamole is an acceptable antiplatelet therapy for patients with noncardio-embolic ischemic stroke or TIA and probably superior to aspirin alone. However, there are many limitations in the body of randomized trials evaluating antiplatelet therapy for the secondary prevention of ischemic stroke and TIA. And the evidence is insufficient to claim the benefit of aspirin-dipyridamole or clopidogrel monotherapy in long-term prevention.101
This conclusion is not surprising because TIA (level 1 vascular damage) is the result of lone activation of ULVWF path, which is a benign transient stroke probably with no lasting impact toward acute brain syndrome. Transient ischemic attack is physiologically resolved by naturally occurring protease ADAMTS13 without any specific therapy. Antiplatelet agent would not be beneficial for resolving formed microthrombi, but is expected to be efficacious in preventing recurrence of TIA. However, AIS would not be benefitted with antiplatelet agent in therapeutic intervention, secondary prevention, and prophylaxis because it is due to macrothrombosis with significant brain syndrome that occurs due to combined activation of ULVWF and TF paths (level 2 vascular damage). Besides, antiplatelet agent is ineffective against activated TF path even in secondary prevention.
In the future, clinical trials must compare the effectiveness of antiplatelet agents between TIA and AIS separately since their thrombogenetic mechanisms are different. Theoretically antiplatelet agents would inhibit ULVWF path only and be useful in secondary prevention of TIA, but antiplatelet agent alone would not be effective in secondary prevention of AIS and embolic stroke.
Recombinant ADAMTS13
Recently, decreased ADAMTS13 and increased ULVWF/VWF have been closely correlated to ischemic stroke.38–40,71 Retrospective studies have concluded that the high level of VWF was associated with increased risk of developing ischemic stroke.39,102 However, please note that the blood samples in patients with stroke were obtained after stroke for the study of ULVWF/VWF. Current interpretation is increased VWF/ULVWF is a prothrombotic marker for stroke.103 But, as discussed earlier in the text, “two-path unifying theory” of hemostasis supports transiently increased VWF and ULVWF is not the cause of ischemic stroke, but is the result of increased exocytosis from endothelial damage causing TIA and AIS in vascular injury. It is likely that decreased ADAMTS13 activity could have been related to imbalance due to excessive exocytosis of ULVWF or might have preexisted due to heterozygous mutation of ADAMTS13 gene in some patients, which potentiated TIA and AIS once intravascular injury had occurred.31 Theoretically, recombinant ADAMTS13 (rADAMTS13) would cleave ULVWF present in unresolved microthrombi strings and therapeutically benefit for “severe and prolonged” TIA in level 1 damage and for secondary prevention in a short term, but would not benefit AIS caused by level 2 damage because AIS develops due to macrothrombus from combined activation of ULVWF and TF path and cannot be countered by antimicrothrombotic therapy. Parenteral antimicrothrombotic therapy (eg, rADAMTS13) might be practical for “one” short-term therapeutic use in “severe and prolonged” TIA, and oral antimicrothrombotic therapy (eg, N-acetylcysteine) may be very safe and useful for secondary prevention of recurrent TIA. At this time, oral antiplatelet agent is indicated in secondary prevention of TIA. Oral antimicrothrombotic therapy with N-acetylcysteine needs well-designed clinical trials. Theoretically, antiplatelet agents and antimicrothrombotic agents have no place for the treatment and prevention of AIS. On the other hand, both rADAMTS13 and therapeutic plasma exchange as a surrogate for ADAMTS13 are anticipated to be effective in the treatment of diffuse encephalopathic stroke associated with generalized VMTD in very early stage.104,105
Antithrombotic Agents (TF Path Inhibitors)
Antithrombin agents and antifibrinogenetic agents
For many decades, vitamin K inhibitor (coumadin) and naturally occurring anticoagulant from bovine and porcine intestinal mucosa (unfractionated heparin) have been extensively utilized in clinical practice. Newer anticoagulants, including antithrombin agents such as low-molecular-weight heparin (LMWH), argatroban, bivalirudine, and lepirudin, have been introduced, and recently antifibrinogenetic agents, such as FXa inhibitors and pentasaccharides as well as LMWH, have been added to the armamentaria of anticoagulants for prophylaxis, secondary prevention, and therapeutic effect, particularly among patients with cardio-embolic stroke.
In regard to antithrombotic therapy for stroke, experts have expressed a broad spectrum of opinions. Surveys of practitioners have also demonstrated a lack of consensus on the use of anticoagulants for ischemic stroke. This uncertainty has existed largely due to incomplete understanding of the pathogenesis of different phenotypes of stroke and the lack of definitive clinical data based on the “hemostatic evidence of medicine.” A review by the panel of the Stroke Council of the American Heart Association found no strong evidence for effectiveness of anticoagulants in treating AIS. Several clinical trials have suggested that emergent anticoagulation regimens have no significant effect in improving clinical outcomes for patients with AIS.106
Currently, there is lack of consensus on the recommendation and benefit for using full-dose anticoagulation for treatment of patients with AIS because of limited efficacy and increased risk of bleeding complications. Latest Cochrane review107 on anticoagulation for AIS did not provide any evidence that the early use of anticoagulants is of overall benefit to people with strokes caused by blood clots. More research is needed to find out if there are ways to select the people with stroke who will benefit from anticoagulants without suffering the hemorrhagic complications.
This conclusion from therapeutic clinical trials could have been anticipated because AIS is the result of intravascular damage that is resulting from activation of ULVWF path and TF path of hemostasis in intravascular injury at the area of in situ thrombus. Once a macrothrombus is formed as a result of unifying process of microthrombi strings from ULVWF path and fibrin meshes from TF path, the macrothrombus would unlikely be resolved with any kind of anticoagulation therapy until natural fibrinolysis is activated. Further, preventive anticoagulation is impractical in isolated AIS episode. But the patient with a persistent underlying disease such as atrial cardiopathy with recurring thrombus formation in the left atrial appendage, mechanical valve associated atrial fibrillation85,89,108–110 that is at high risk for recurrent “intravascular injury” might be benefitted with TF path inhibitors in primary prevention of embolic stroke. According to in vivo hemostatic theory, nonvalvular atrial fibrillation in isolated more likely produces TIA rather than AIS. Therefore, antiplatelet agent might be beneficial for primary and secondary prevention of TIA-in isolated nonvalvular atrial fibrillation.
Thrombolytic/Fibrinolytic Agents (Fibrinolysis Activators)
Tissue plasminogen activators
The modern era of the thrombolytic/fibrinolytic therapy has begun in the early 1990s.111 Among thrombolytic agents such as streptokinase, urokinase and tissue plasminogen activator (tPA), alteplase that is a tPA approved by the US Food and Drug Administration (FDA) for the treatment of AIS has been considered to be the mainstay of thrombolytic therapy.112 Theoretically, this is a very reasonable therapeutic agent for AIS due to its character of macrothrombus if utilized in the symptom onset of stroke in less than 3 hours. The efficacy of alteplase for AIS is established.112–114 Although thrombolytic/fibrinolytic agents are theoretically ideal, it is still in early stage of clinical use as safety issue is being addressed. The usage of thrombolytic therapy remains low in clinical practice.115,116 We will wait for the results of further controlled clinical trials to provide clinicians with evidence-based medicine that is selected and initiated on the concept of theory-based medicine.
Hemostasis Promoters
Acute hemorrhagic stroke includes ICH, SAH/IVH, SDH, and EDH. It is characterized by extravascular hemorrhage or hematoma without intravascular thrombosis. Specifically, the hemostasis causing AHS does not occur within the vascular lumen in the absence of significant ECs and SET damage, but extravascular hemorrhage progresses into the brain tissue due to EVT damage or hemorrhage into the space between the brain coverings due to obtuse head/brain injury. In these situations, intracranial extravascular hemorrhage creates incomplete hemostasis because only TF path may become activated due to eTF, but ULVWF is not available and ULVWF path is not activated due to minimal penetrating endothelial injury. Thus, complete hemostasis cannot occur but only modest fibrin meshes are formed via activated TF-FVIIa complex (TF path) leading to fibrinogenesis and produce hematoma within the brain or between brain coverings. Acute hemorrhagic stroke is interpreted to be a hemostatic disease caused by lone activation of TF. Theoretically, the initial treatment of AHS could begin with hemostatic promoters.
Current medical therapy of AHS is primarily focused on adjunctive and supportive measure to minimize injury and stabilize the patient in the perioperative phase if surgical intervention were needed. If AHS is precipitated by hypertension, underlying hemorrhagic disease, anticoagulation/antithrombotic therapy whether or not vascular anomalies such as subarachnoid aneurysm is present, this underlying pathology should be treated immediately and hemorrhagic disease be corrected. Theoretically, an attractive approach to control acute bleeding due to vascular injury with/without underlying hemorrhagic disease or vascular anomalies is a hemostatic treatment with ultraspeedy administration of a hemostatic agent if available. Potential hemostatic agents based on in vivo hemostatic theory includes such as desmopressin and recombinant FVIIa that promote thrombogenesis of in vivo hemostasis.117–119
Desmopressin
Desmopressin, a synthetic analog of vasopressin, in addition to its utility in several conditions such as diabetes insipidus, uremic bleeding, and nocturnal enuresis has been approved for use in the treatment of hemophilia A, von Willebrand disease type 1, and congenital and acquired platelet dysfunction. The mechanism of action of desmopressin is its agonist activity at V2 receptors that leads to a rise in intracellular concentrations of cyclic adenosine monophosphate, which in turn induces exocytosis of ULVWF from its storage sites (ie, Weibel–Palade bodies) of ECs into circulation.120
Based on the mechanism of in vivo “two-path unifying theory” of hemostasis,5,7,27 it is expected the treatment of desmopressin could initiate and potentiate hemostasis exactly at the active bleeding site of intracranial vascular injury that could be repaired by ULVWF and activated FVII if treated in very early stage of AHS. Desmopressin administration would release highly thrombogenic ULVWF and FVIII at the vascular injury site in large quantity and activate ULVWF path, and recruit platelets to form microthrombi strings. Microthrombi strings would be unified with fibrin meshes formed from activated TF path due to hemorrhage of AHS and may induce efficient macrothrombogenesis producing natural hemostatic “patch” at the extravascular bleeding site. More than 3 decades ago, the use of desmopressin has been advocated in the treatment of hemostatic disorders.121–123 According to hemostatic theory, desmopressin might be an ideal drug if the diagnosis of AHS can be established in a very early stage.
Desmopressin has been used in both primary ICH in the absence of underlying vascular malformation or coagulopathy and secondary ICH due to underlying vascular anomaly or anticoagulation state.117,118 Hematoma expansion is a detrimental outcome of spontaneous ICH, resulting in significant neurological deficits, and enhancement of ICH-induced brain injury.124 Desmopressin stabilized the platelet function in neurosurgical patients with secondary intracranial hemorrhage,117,125 and was associated with a decreased likelihood of intracranial hemorrhage/hematoma expansion during the first 24 hours in retrospective assessment of its effectiveness and safety in patients with antiplatelet-associated ICH.126 Theoretically, desmopressin appears to be one drug that has potential for early treatment of ICH and other AHS. It would be an interesting proposal to test the hemostatic effect of desmopressin in ICH, SAH, and other AHS.
TF Path Promoter
rFVIIa
Recombinant activated FVII (rFVIIa) is a potent promoter of TF path, which triggers extrinsic cascade/cell-based coagulation leading to fibrinogenesis to produce thrombin and subsequent fibrin meshes. Although rFVIIa has been approved by the US FDA 2 decades ago for hemophilia A and B with inhibitors, bleeding episodes in hemophilia, and FVII deficiency,127,128 its off-label use has been controversial, especially to enhance hemostasis in patients with bleeding who may or may not have an underlying coagulation defect.128 While evidence-based guidelines do exist for rFVIIa treatment in hemophilia, none is available for its off-label use.
According to in vivo hemostatic theory and thrombogenetic mechanism, rFVIIa even with pharmacologic dose is expected to be ineffective in AHS because fibrin clots enhanced by rFVIIa alone cannot achieve complete hemostasis without the hemostatic plug, which needs ULVWF and platelets at bleeding intravascular injury site. The patient with AHS already has enough TF available from EVT damage (eg, brain issue area), but sufficient quantity of ULVWF would not be available at bleeding intravascular injury and EVT site. For example, in ICH, excessive bleeding at the site of a small intracranial ECs damage extending to EVT into the brain tissue does not produce sufficient ULVWF due to intravascular pressure gradient. Thus, the sufficient hemostatic patch composed of microthrombi strings does not occur. But TF present in EVT hemorrhage promotes regional fibrinogenesis and produces fibrin meshes leading to cerebral hematoma (Table 1). In this situation, intravenous treatment with pharmacologic dose of rFVIIa would further increase cerebral hematoma. Also, pharmacologic dose of rFVIIa almost surely would promote pathologic “intravascular fibrinogenesis” if continuously administered. If additional vascular injury occurs, intravascular macrothrombogenesis could produce severe macrothrombotic complication. Thomas et al129 appropriately cautioned the administration of FVIIa to patients with arterial injuries and injured mesenteric and cerebral vessels might be especially susceptible to thrombosis. This macrothrombotic complication can be explained by in vivo hemostatic theory.
Well-controlled clinical trials were done utilizing rFVIIa for ICH with an enthusiasm in earlier clinical trial,119 but at the additional confirmation trials with rFVIIa were proved to be unsuccessful in improving survival for acute ICH.130–132 In retrospect, as presaged from “two-path unifying theory” of hemostasis, the lack of benefit of rFVIIa for ICH and theoretical complications of serious of disseminated fibrin thrombi and macrothromboembolic complications could have been anticipated because hemorrhage/hematoma from EVT (ie, brain) damage in ICH cannot be corrected by induction of intravascular fibrinogenesis. It can be concluded that rFVIIa is not effective and is contraindicated in the management of AHS according to both in vivo hemostatic theory-based medicine and evidence-based medicine following clinical trials.
In reflecting on the review of past clinical trials, this author would like to caution designing clinical trials in selecting the patients for each stroke syndrome separately. A note of consideration is summarized in Table 6. First, TIA, which is focal microthrombotic disease due to level 1 vascular damage causing lone activation of ULVWF path, should be separated from AIS, perhaps using TF-positive MV marker, because AIS is local macrothrombotic disease due to level 2 vascular damage causing activation of combined ULVWF and TF paths. Transient ischemic attack and AIS occur as a result of 2 different hemostatic thrombogeneses. Second, we know that antiplatelet regimen is expected to be effective for TIA prevention according to theory-based medicine (Table 5) and also was found to be effective by evidence-based medicine.101 Third, THS due to level 3 vascular damage is also a very different stroke syndrome from AIS, which pathogenetic differences are defined in Table 2 and Table 4. Thus, any suspected THS should not be included in AIS trials and in AHS trials.
Table 6.
Proposed Theoretical Considerations in the Clinical Trial for Different Stroke Phenotypes.
|
Abbreviations: AHS, acute hemorrhagic stroke; AIS, acute ischemic stroke; NAC, N-acetyl cysteine; rFVIIa, recombinant activated factor VII; TF, tissue factor; THS, thrombo-hemorrhagic syndrome; TIA, transient ischemic attack; t-PA, tissue plasminogen activator; ULVWF, unusually large von Willebrand factor multimers.
Interestingly, anti-thrombin/antifibrinogenetic therapy of LMWH in AIS caused by large artery occlusion had reduced early neurologic deterioration.133 Perhaps this finding suggests certain macrothrombosis (eg, AIS) in in situ stroke is not a completed stroke event yet when it is diagnosed, but thrombogenesis might be a continuing process. This proposition may support thrombosis in progression could still be benefited from anticoagulation treatment in early stage of stroke. This factor should be weighed in interpreting the result of clinical trials among different groups of the same stroke phenotype.
Conclusion
The acceptable definition of stroke is a hemostatic disease occurring as a result of intracranial vascular thrombosis and/or hemorrhage due to intracranial intravascular or extravascular injury, or embolic thrombosis due to extracranial intravascular injury, leading to variety of focal, local, or disseminated brain syndromes. The thrombotic character could be microthrombi, fibrin clots, or macrothrombus, and hemorrhagic character could be intracerebral or cavitary hemorrhage, or partially clotted fibrin hematoma. Since CVST is caused by macrothrombus due to activated ULVWF and TF paths in the intracranial venous sinus system and presents with stroke-like phenotype, it also might be included in stroke syndrome because it is a hemostatic disease but with atypical brain syndrome. Certainly, vascular physiology plays a critical role in producing different phenotypes of stroke with significant therapeutic implication, which can be clearly understood through a novel “two-path unifying theory” of hemostasis.
Acknowledgments
The author expresses sincere appreciation to Miss Emma Nichole Zebrowski for her insights on the structural function of the blood vessel wall in in vivo hemostasis and excellent illustrative art works of Figure 1.
Authors’ Note: Contributed 100% by the corresponding author.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jae C. Chang  https://orcid.org/0000-0002-9401-7684
https://orcid.org/0000-0002-9401-7684
References
- 1. Benjamin EJ, Muntner P, Alonso A, et al. A report from the American Heart Association: American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update . Circulation. 2019;139(12):e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balami JS, Chen RL, Buchan AM. Stroke syndromes and clinical management. QJM. 2013;106(9):607–615. [DOI] [PubMed] [Google Scholar]
- 4. Chang JC. Thrombogenesis and thrombotic disorders based on “two-path unifying theory of hemostasis: philosophical, physiological and phenotypical interpretation. Blood Coagul Fibrinolysis. 2018;29(6):585–595. [DOI] [PubMed] [Google Scholar]
- 5. Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thrombos Hemost. 2019:25(6):1–20. doi:10.1177/1076029619887437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caplan LR. Basic pathology, anatomy, and pathophysiology of stroke In: Caplan’s T, ed. Stroke: A Clinical Approach, 4th ed Saunders Elsevier; 2009:22. [Google Scholar]
- 7. Chang JC. Hemostasis based on a novel “two-path unifying theory” and classification of hemostatic disorders. Blood Coagul Fibrinolysis. 2018;29(3):573–584. [DOI] [PubMed] [Google Scholar]
- 8. Garbossa D, Altieri R, Specchia FM, et al. Are acute subdural hematomas possible without head trauma? Asian J Neurosurg. 2014;9(6):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jen J, Cohen AH, Yue Q, et al. Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Neurology. 1997;49:1322–1330. [DOI] [PubMed] [Google Scholar]
- 10. Søndergaard CB, Nielsen JE, Hansen CK, et al. Hereditary cerebral small vessel disease and stroke. Clin Neurol Neurosurg. 2017;155(7):45–57. [DOI] [PubMed] [Google Scholar]
- 11. Amato C, Ferri R, Elia M, et al. Nervous system involvement in Degos disease. AJNR Am J Neuroradiol. 2005;26(3):646–649. [PMC free article] [PubMed] [Google Scholar]
- 12. Kowalski RG, Haarbauer-Krupa JK, Bell JM, et al. Acute ischemic stroke after moderate to severe traumatic brain injury: incidence and impact on outcome. Stroke. 2017;48(8):1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Amorim LC, Maia FM, Rodrigues CE. Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus. 2017;26(4):529–536. [DOI] [PubMed] [Google Scholar]
- 14. Kamel H, Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol. 2012;69(3):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manousakis G, Jensen MB, Chacon MR, et al. The interface between stroke and infectious disease: infectious diseases leading to stroke and infections complicating stroke. Curr Neurol Neurosci Rep. 2009;9(4):28–34. [DOI] [PubMed] [Google Scholar]
- 16. Lucchese G, Flöel A, Stahl B. Cross-reactivity as a mechanism linking infections to stroke. Front Neurol. 2019;10(3):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacco S, Merki-Feld GS, Ægidius KL, et al. ; European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European headache federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18(5):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonseca AC, Ferro JM. Drug abuse and stroke. Curr Neurol Neurosci Rep. 2013;13(8):325. [DOI] [PubMed] [Google Scholar]
- 19. Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43(5):3029–3034. [DOI] [PubMed] [Google Scholar]
- 20. Dearborn JL, Urrutia VC, Zeiler SR. Stroke and cancer—a complicated relationship. J Neurol Transl Neurosci. 2014;2(5):1039. [PMC free article] [PubMed] [Google Scholar]
- 21. Álvarez-Pérez FJ, Verde I, Uson-Matin M, et al. Frequency and mechanism of ischemic stroke associated with malignancy: a retrospective series. Eur Neurol. 2012;68(3):209–213. [DOI] [PubMed] [Google Scholar]
- 22. Kamel H, Okin PM, Elkind MS, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(2):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yaghi S, Song C, Gray WA, et al. Left atrial appendage function and stroke risk. Stroke. 2015;46(1):3554–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramlawi B, Abu Saleh WK, Edgerton J. The left atrial appendage: target for stroke reduction in atrial fibrillation. Methodist Debakey Cardiovasc J. 2015;11(6):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. 2019;45(6):86–93. [DOI] [PubMed] [Google Scholar]
- 26. Farkas ÁZ, Farkas VJ, Gubucz I, et al. Neutrophil extracellular traps in thrombi retrieved during interventional treatment of ischemic arterial diseases. Thromb Res. 2019;175(4):46–52. [DOI] [PubMed] [Google Scholar]
- 27. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thrombosis J. 2019:17(9):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levi M, van der Poll T. A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost. 2014;40(2):874–880. [DOI] [PubMed] [Google Scholar]
- 29. Gando S. Tissue factor in trauma and organ dysfunction. Semin Thromb Hemost. 2006;32(6):48–53. [DOI] [PubMed] [Google Scholar]
- 30. Chang JC. Disseminated intravascular coagulation: is it fact or fancy? Blood Coagul Fibrinolysis. 2018;29(3):330–337. [DOI] [PubMed] [Google Scholar]
- 31. Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J. 2018;16(4):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staessens S, Fitzgerald S, Andersson T, et al. Histological stroke clot analysis after thrombectomy: technical aspects and recommendations. Int J Stroke. 2019:1747493019884527. [DOI] [PubMed] [Google Scholar]
- 33. Chauhan AK, Goerge T, Schneider SW, et al. Formation of platelet strings and microthrombi in the presence of ADAMTS-13 inhibitor does not require P-selectin or beta3 integrin. J Thromb Haemost. 2007;5(6):583–589. [DOI] [PubMed] [Google Scholar]
- 34. Turner NA, Moake JL. Factor VIII is synthesized in human endothelial cells, packaged in Weibel-Palade bodies and secreted bound to ULVWF strings. PLoS One. 2015;10(2):e0140740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lippi G, Favaloro EJ. Venous and arterial thromboses: two sides of the same coin? Semin Thromb Hemost. 2018;44(2):239–248. [DOI] [PubMed] [Google Scholar]
- 36. Lippi G, Favaloro EJ. Hemostasis practice: state-of-the-art. J Lab Precis Med. 2018;3(6):67. [Google Scholar]
- 37. Bernardo A, Ball C, Nolasco L, et al. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104(7):100–106. [DOI] [PubMed] [Google Scholar]
- 38. Dhanesha N, Prakash P, Doddapattar P, et al. Endothelial cell-derived von Willebrand factor is the major determinant that mediates von Willebrand factor dependent acute ischemic stroke by promoting postischemic thrombo-inflammation. Arterioscler Thromb Vasc Biol. 2016;36(3):1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bongers TN, de Maat MP, Van Goor ML, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37(6):2672–2677. [DOI] [PubMed] [Google Scholar]
- 40. McCabe DJ, Murphy SJ, Starke R, et al. Relationship between ADAMTS13 activity, von Willebrand factor antigen levels and platelet function in the early and late phases after TIA or ischaemic stroke. J Neurol Sci. 2015;348(5):35–40. [DOI] [PubMed] [Google Scholar]
- 41. Chang JC. Molecular pathogenesis of STEC-HUS caused by endothelial heterogeneity and unprotected complement activation, leading to endotheliopathy and impaired ADAMTS13 activity: based on two-activation theory of the endothelium and vascular microthrombotic disease. Nephrol Renal Dis. 2017;2(3):1–8. [Google Scholar]
- 42. De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121(6):270–277. [DOI] [PubMed] [Google Scholar]
- 43. Mourik MJ, Valentijn JA, Voorberg J, et al. Von Willebrand factor remodeling during exocytosis from vascular endothelial cells. J Thromb Haemost. 2013;11(2):2009–2019. [DOI] [PubMed] [Google Scholar]
- 44. Huang J, Roth R, Heuser JE, et al. Integrin αvβ3 on human endothelial cells binds von Willebrand factor strings under fluid shear stress. Blood. 2009;113(2):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernardo A, Ball C, Nolasco L, et al. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(1):562–570. [DOI] [PubMed] [Google Scholar]
- 46. Jiménez-Alcázar M, Kim N, Fuchs TA. Circulating extracellular DNA: cause or consequence of thrombosis? Semin Thromb Hemost. 2017;43(3):553–561. [DOI] [PubMed] [Google Scholar]
- 47. Farndale RW, Sixma JJ, Barnes MJ, et al. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2(3):561–573. [DOI] [PubMed] [Google Scholar]
- 48. Xu X, Carrim N, Neves MA, et al. Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb J. 2016;14(suppl 1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pfeiler S, Stark K, Massberg S, et al. Propagation of thrombosis by neutrophils and extracellular nucleosome networks. Haematologica. 2017;102(3):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qi H, Yang S, Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol. 2017;8(3):928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oklu R, Sheth RA, Wong KHK, et al. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc Diagn Ther. 2017;7(suppl 3):S140–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91(7):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uemura M, Fujimura Y, Ko S, et al. Determination of ADAMTS13 and its clinical significance for ADAMTS13 supplementation therapy to improve the survival of patients with decompensated liver cirrhosis. Int J Hepatol. 2011;2011:759047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panackel C, Thomas R, Sebastian B, et al. Recent advances in management of acute liver failure. Indian J Crit Care Med. 2015;19(2): 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caplan LR. Cardiac encephalopathy and congestive heart failure: a hypothesis about the relationship. Neurology. 2006;66(5):99–101. [DOI] [PubMed] [Google Scholar]
- 56. Scala R. Hypercapnic encephalopathy syndrome: a new frontier for non-invasive ventilation? Respir Med. 2011;105(2):1109–1117. [DOI] [PubMed] [Google Scholar]
- 57. Mahoney CA, Arieff AI. Uremic encephalopathies: clinical, biochemical, and experimental features. Am J Kidney Dis. 1982;2(4):324–336. [DOI] [PubMed] [Google Scholar]
- 58. Scaini G, Ferreira GK, Streck EL. Mechanisms underlying uremic encephalopathy. Rev Bras Ter Intensiva. 2010;22(1):206–211. [PubMed] [Google Scholar]
- 59. Seifter JL, Samuels MA. Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol. 2011;31(6):139–143. [DOI] [PubMed] [Google Scholar]
- 60. Edvardsson B. Hypertensive encephalopathy and cerebral infarction. Springerplus. 2014;3(2):741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eggers V, Schilling A, Kox WJ, Spies C. Septic encephalopathy. Diagnosis und therapy [in German]. Anaesthesist. 2003;52(4):294–303. [DOI] [PubMed] [Google Scholar]
- 62. Ziaja M. Septic encephalopathy. Curr Neurol Neurosci Rep. 2013;13(10):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(3):557–566. [DOI] [PubMed] [Google Scholar]
- 64. Rivera-Lara L. The role of impaired brain perfusion in septic encephalopathy. Crit Care. 2019;23(5):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chang JC. Viral hemorrhagic fevers due to endotheliopathy-associated disseminated intravascular microthrombosis and hepatic coagulopathy: pathogenesis based on “two activation theory of the endothelium”. Clin Microbiol Infect Dis. 2017;2(2):1–6. [Google Scholar]
- 66. Sabin S, Merritt JA. Treatment of hepatic coma in cirrhosis by plasmapheresis and plasma infusion (plasma exchange). Ann Intern Med. 1968;68(6):1–7. [DOI] [PubMed] [Google Scholar]
- 67. Bektas M, Idilman R, Soykan I, et al. Adjuvant therapeutic plasma exchange in liver failure: assessments of clinical and laboratory parameters. J Clin Gastroenterol. 2008;42(4):517–521. [DOI] [PubMed] [Google Scholar]
- 68. Kes P. Therapeutic plasma exchange in severe sepsis or septic shock. Acta Med Croatica. 1998;52(3):127–132. [PubMed] [Google Scholar]
- 69. Fortenberry JD, Nguyen T, Grunwell JR, et al. Therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure: the thrombocytopenia-associated multiple organ failure network prospective experience. Crit Care Med. 2019;47:e173–e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stegmayr BG. Is there a place for apheresis in patients with severe sepsis or multi organ dysfunction syndrome? Turk J Haematol. 2000;17(6):5–11. [PubMed] [Google Scholar]
- 71. Qu L, Jiang M, Qiu W, et al. Assessment of the diagnostic value of plasma levels, activities, and their ratios of von Willebrand factor and ADAMTS13 in patients with cerebral infarction. Clin Appl Thromb Hemost. 2016;22(8):252–259. [DOI] [PubMed] [Google Scholar]
- 72. Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: which? where? when? Arterioscler Thromb Vasc Biol. 2009;29(1):1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36(2):104–107. [DOI] [PubMed] [Google Scholar]
- 74. Słomka A, Świtońska M, Sinkiewicz W, Żekanowska E. Assessing circulating factor VIIa-antithrombin complexes in acute ischemic stroke: a pilot study. Clin Appl Thromb Hemost. 2017;23(2):351–359. [DOI] [PubMed] [Google Scholar]
- 75. Lundström A, Mobarrez F, Rooth E, et al. Prognostic value of circulating microvesicle subpopulations in ischemic stroke and TIA [published online January 25, 2020]. Transl Stroke Res. 2020:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Świtońska M, Słomka A, Sinkiewicz W, Żekanowska E. Tissue-factor-bearing microparticles (MPs-TF) in patients with acute ischaemic stroke: the influence of stroke treatment on MPs-TF generation. Eur J Neurol. 2015;22(4):395–401. [DOI] [PubMed] [Google Scholar]
- 77. Zaldivia MTK, McFadyen JD, Lim B, Wang X, Peter K. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. 2017;4(3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen Y, Li G, Liu ML. Microvesicles as emerging biomarkers and therapeutic targets in cardiometabolic diseases. Genom Proteom Bioinform. 2018;16(2):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zarà M, Guidetti GF, Camera M, et al. Biology and role of extracellular vesicles (EVs) in the pathogenesis of thrombosis. Int J Mol Sci. 2019;20(11):E2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kamel H, Healey JS. Cardioembolic stroke. Circ Res. 2017;120(2):514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke.1991;22(3):983–988. [DOI] [PubMed] [Google Scholar]
- 82. Kirchhof P, Breithardt G, Bax J, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the Fifth Atrial Fibrillation Network/European Heart Rhythm Association Consensus Conference. Europace. 2016;18(3):37–50. [DOI] [PubMed] [Google Scholar]
- 83. Ceornodolea AD, Bal R, Severens JL. Epidemiology and management of atrial fibrillation and stroke: review of data from four European countries. Stroke Res Treat. 2017;2017(9):8593207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow triad revisited. Lancet. 2009;373(9658):155–166. [DOI] [PubMed] [Google Scholar]
- 85. Mac Grory B, Chang A, Atalay MK, et al. Left atrial appendage thrombus and embolic stroke. Stroke. 2018;49(2):e286–e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kamel H, Okin PM, Longstreth WT, Jr, et al. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11(4):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Violi F, Pastori D, Pignatelli P. Mechanisms and management of thrombo-embolism in atrial fibrillation. J Atr Fibrillation. 2014;7(3):1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kunihiro N, Katsuyoshi C, Yu-Ki I, et al. Atrial fibrillation-associated remodeling does not promote atrial thrombus formation in canine models. Circ Arrhythm Electrophysiol. 2012;5(6):1168–1175. [DOI] [PubMed] [Google Scholar]
- 89. Polovina MM, Lip GY, Potpara TS. Endothelial (dys)function in lone atrial fibrillation. Curr Pharm Des. 2015;21(5):622–645. [DOI] [PubMed] [Google Scholar]
- 90. Zhan Y, Joza J, Al Rawahi M, et al. Assessment and management of the left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2018;34(5):252–261. [DOI] [PubMed] [Google Scholar]
- 91. Leifer D, Rundek T. Atrial cardiopathy: a new cause for stroke? Neurology. 2019;92(1):155–156. [DOI] [PubMed] [Google Scholar]
- 92. Lip GY, Blann AD. Von Willebrand factor and its relevance to cardiovascular disorders. Br Heart J. 1995;74(4):580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mahajan R, Brooks AG, Sullivan T, et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart. 2012;98(6):1120–1126. [DOI] [PubMed] [Google Scholar]
- 94. Adeoye O, Nyström KV, Yavagal DR, et al. Recommendations for the establishment of stroke systems of care: a 2019 update. Stroke. 2019;50(2):e187–e210. [DOI] [PubMed] [Google Scholar]
- 95. Schwamm LH, Pancioli A, Acker JE, et al. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association’s Task Force on the Development of Stroke Systems. Stroke. 2005;36(4):690–703. [DOI] [PubMed] [Google Scholar]
- 96. Wright L, Hill KM, Bernhardt J, et al. Stroke management: updated recommendations for treatment along the care continuum. Intern Med J. 2012;42(5):562–569. [DOI] [PubMed] [Google Scholar]
- 97. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. [DOI] [PubMed] [Google Scholar]
- 98. Correction to: 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/America Stroke Association. Stroke. 2018;49(6):e233–e234. [DOI] [PubMed] [Google Scholar]
- 99. McCoy CE, Langdorf MI, Lotfipour S. American Heart Association/American Stroke Association deletes sections from 2018 stroke guidelines. West J Emerg Med. 2018;19(4):947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Franchini M, Liumbruno GM, Bonfanti C, et al. The evolution of anticoagulant therapy. Blood Transfus. 2016;14(6):1751–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke. 2019;50(3):773–778. [DOI] [PubMed] [Google Scholar]
- 102. Hanson E, Jood K, Karlsson S, et al. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thrombosis Haemostasis. 2011;9(1):275–281. [DOI] [PubMed] [Google Scholar]
- 103. Chen X, Cheng X, Zhang S, Wu D. ADAMTS13: an emerging target in stroke therapy. Front Neurol. 2019;10(2):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Qu L, Kiss JE, Dargo G, et al. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. J Clin Apher. 2011;26(4):208–213. [DOI] [PubMed] [Google Scholar]
- 105. Stegmayr B. Apheresis in patients with severe sepsis and multiorgan dysfunction syndrome. Transfus Apher Sci. 2008;38(2):203–208. [DOI] [PubMed] [Google Scholar]
- 106. Shahpouri MM, Mousavi S, Khorvash F, et al. Anticoagulant therapy for ischemic stroke: a review of literature. J Res Med Sci. 2012;17(8):396–401. [PMC free article] [PubMed] [Google Scholar]
- 107.Cochrane Data base of Systematic Reviews. Anticoagulants for acute ischaemic stroke https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000024.pub4/full
- 108. Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. 2018;3(4):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fauchier L, Philippart R, Clementy N, et al. How to define valvular atrial fibrillation? Arch Cardiovasc Dis. 2015;108(2):530–539. [DOI] [PubMed] [Google Scholar]
- 110. Olsson SB, Executive Steering Committee of the SPORTIF III Investigators. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362(9397):1691–1698. [DOI] [PubMed] [Google Scholar]
- 111. Ouriel K. A history of thrombolytic therapy. J Endovasc Ther. 2004;11(suppl 2):II128–II133. [DOI] [PubMed] [Google Scholar]
- 112. Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;3(7):CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (The Third International Stroke Trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Henderson SJ, Weitz JI, Kim PY. Fibrinolysis: strategies to enhance the treatment of acute ischemic stroke. J Thromb Haemost. 2018;16(7):1932–1940. [DOI] [PubMed] [Google Scholar]
- 116. McDermott M, Skolarus LE, Burke JF. A systematic review and meta-analysis of interventions to increase stroke thrombolysis. BMC Neurol. 2019;3(19):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kapapa T, Röhrer S, Struve S, et al. Desmopressin acetate in intracranial haemorrhage. Neurol Res Int. 2014;2014(7):298767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Francoeur CL, Roh D, Schmidt JM, et al. Desmopressin administration and rebleeding in subarachnoid hemorrhage: analysis of an observational prospective database. J Neurosurg. 2018;1(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med. 2005;352(2):777–785. [DOI] [PubMed] [Google Scholar]
- 120. Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J Thromb Haemost. 2003;1(6):682–689. [DOI] [PubMed] [Google Scholar]
- 121. Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997;90(4):2515–2521. [PubMed] [Google Scholar]
- 122. Drouet L, Scrobohaci ML, Boudaoud S, et al. Desmopressin (dDAVP): a powerful general hemostatic agent? Nouv Rev Fr Hematol. 1989;31(5):163–165. [PubMed] [Google Scholar]
- 123. Lethagen S. Desmopressin (DDAVP) and hemostasis. Ann Hematol. 1994;69(2):173–180. [DOI] [PubMed] [Google Scholar]
- 124. Burchell SR, Tang J, Zhang JH. Hematoma expansion following intracerebral hemorrhage: mechanisms targeting the coagulation cascade and platelet activation. Curr Drug Targets. 2017;18(2):1329–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Naidech AM, Maas MB, Levasseur-Franklin KE, et al. Desmopressin improves platelet activity in acute intracerebral hemorrhage. Stroke. 2014;45(8):2451–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Feldman EA, Meola G, Zyck S, et al. Retrospective assessment of desmopressin effectiveness and safety in patients with antiplatelet-associated intracranial hemorrhage. Crit Care Med. 2019:47(3):1759–1765. [DOI] [PubMed] [Google Scholar]
- 127. Martinowitz U, Michaelson M; Israeli Multidisciplinary rFVIIa Task Force . Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rFVIIa Task Force. J Thromb Haemost. 2005;3(7):640–648. [DOI] [PubMed] [Google Scholar]
- 128. Hoffman M, Leuung LLK, Tirnauer JS. Recombinant Factor VIIa: Clinical Uses, Dosing, and Adverse Effects. Uptodate 2019; last updated 2019. [Google Scholar]
- 129. Thomas GO, Dutton RP, Hemlock B, et al. Thromboembolic complications associated with factor VIIa administration. J Trauma. 2007;62(2):564–569. [DOI] [PubMed] [Google Scholar]
- 130. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(3):2127–2137. [DOI] [PubMed] [Google Scholar]
- 131. Yuan ZH, Jiang JK, Huang WD, et al. A meta-analysis of the efficacy and safety of recombinant activated factor VII for patients with acute intracerebral hemorrhage without hemophilia. J Clin Neurosci. 2010;17(3):685–693. [DOI] [PubMed] [Google Scholar]
- 132. Gladstone DJ, Aviv RI, Demchuk AM, et al. Effect of recombinant activated coagulation factor VII on hemorrhage expansion among patients with spot sign–positive acute intracerebral hemorrhage: the spotlight and Stop-IT randomized clinical trials. JAMA Neurol. 2019;76(2):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang Q, Chen C, Chen XY, et al. Low-molecular-weight heparin and early neurologic deterioration in acute stroke caused by large artery occlusive disease. Arch Neurol. 2012;69(8):1454–1460. [DOI] [PubMed] [Google Scholar]