Abstract
A 42-year-old woman with juvenile idiopathic arthritis was treated with anakinra, corticosteroids, and hydroxychloroquine when she developed chronic hypoxic respiratory myopathy. She was admitted to the intensive care unit for acute hypercapnic respiratory failure and required prolonged intubation, subsequent tracheostomy, and long-term ventilatory support due to multiple failed spontaneous breathing trials after discontinuation of anakinra and steroids. Muscle biopsy revealed type II fiber atrophy with the accumulation of autophagosomes and vacuoles presenting as curvilinear bodies, elevated MHC class I antigen expression, and infiltration by CD68+ macrophages and CD8+ T cells. Type II fiber atrophy was attributed to corticosteroid use and curvilinear bodies due to blockade of autophagy by hydroxychloroquine. After hydroxychloroquine was discontinued, the patient recovered to her prehospitalization baseline.
Keywords: hydroxychloroquine, corticosteroids, myopathy, autophagy, curvilinear body, type II fiber, atrophy, necrosis, juvenile inflammatory arthritis
Introduction
Hydroxychloroquine is widely utilized as a treatment for a variety of autoimmune rheumatic diseases. However, hydroxychloroquine can also lead to a number of side effects including bull’s eye maculopathy and less commonly toxic myopathy.1,2 Myopathy seen in hydroxychloroquine toxicity is thought to be due to the inhibition of lysosomal degradation of phospholipids and glycogen leading to the formation of curvilinear body seen on muscle biopsies.3 Hydroxychloroquine promotes the accumulation of autophagic vacuoles by inhibiting the lysosome acidification.4 Our case represents hydroxychloroquine-induced myopathy in a 42-year-old Caucasian female with prolonged use for treatment of JIA. The patient also suffered respiratory failure and required tracheostomy for long-term ventilatory support, which was only reversed on discontinuation of hydroxychloroquine.
Case Presentation
A 42-year-old woman with a past medical history significant for juvenile idiopathic arthritis (JIA) on anakinra 100 mg subcutaneous daily and hydroxychloroquine 200 mg twice daily, chronic adrenal insufficiency secondary to steroids, currently on hydrocortisone 20 mg every morning and 10 mg in the afternoon, seizure disorder, and a history of recent culture negative infective endocarditis with subsequent cardioembolic stroke presented from a nursing home after a mechanical fall. Imaging on arrival revealed an age indeterminate C7 vertebral fracture, which was treated with conservatively with a cervical collar and pain control. Shortly after admission, her hospital course was complicated by acute on chronic hypoxic and hypercapnic respiratory failure requiring rescue BiPAP (bilevel positive airway pressure) support and admission to the intensive care unit. The patient became encephalopathic, and arterial blood gas revealed respiratory acidosis with significant carbon dioxide retention of >105 mm Hg. The patient was intubated for impending respiratory failure.
Computed tomography thorax was performed and showed a left-sided opacification initially treated as a left-sided pneumonia. However, her respiratory status failed to improve despite being on adequate antibiotic therapy, and she failed recurrent spontaneous breathing trials while off sedation. These findings lead to the discovery of significant neuromuscular weakness and the left-sided opacification. The opacification was suspected to be due to lung collapse secondary to left-sided diaphragmatic weakness (Figure 1). On day 13 of admission, the patient underwent tracheotomy for expected long-term ventilator support and percutaneous endoscopic gastrostomy.
Figure 1.
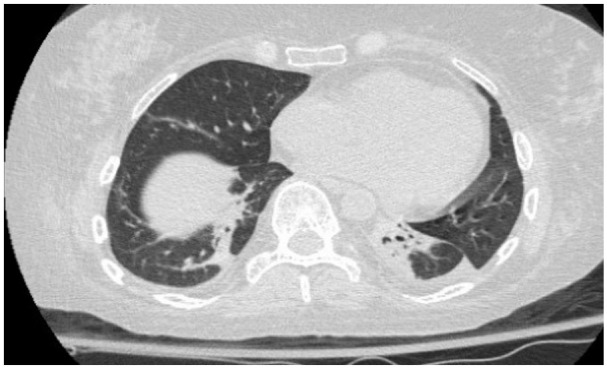
Computed tomography thorax revealing left lung opacity attributed to lung collapse due to diaphragmatic weakness.
Of note, she had recurrent admissions including an admission for a mechanical fall from bed and was found to have a subarachnoid hemorrhage. She was hospitalized yet again after falling down the stairs and sustaining a comminuted fracture of the distal left tibia. Given her recurrent hospital admissions, prolonged hospital course, and diaphragmatic weakness, there was concern the patient had been exhibiting progressive weakness prior to admission. History taking was limited due to patient mechanical ventilation and nonverbal status. She had reportedly noticed progressive muscular weakness worsening over the past year, which was attributed to her recurrent hospitalizations leading to deconditioning. Prior to this year, the patient did not require supplemental oxygen.
During admission, her serum aldolase initially was elevated at 11.8 (3.3-10.3 U/L normal), but 4 days later, it normalized to 4.6. Her creatine kinase was within normal limits at 70 U/L (normal = 20-180 U/L). Thyroid-stimulating hormone was elevated at 8.15 (normal = 0.27-4.2 IU/mL), but the free thyroxine was normal at 0.98 (normal = 0.93-1.7 ng/dL). Her alanine aminotransferase was mildly elevated at 36 U/L (her outpatient alanine aminotransferase from 3 months ago was also elevated at 58, with prior laboratory values within normal limits). Her aspartate aminotransferase was mildly elevated at 62 U/L (her outpatient aspartate aminotransferase from 3 months ago was also elevated at 46, with prior laboratory values within normal limits). Her bilirubin was within normal limits, and her creatinine has been chronically low ranging from 0.2 to 0.4mg/dL.
Rheumatology was consulted to evaluate the mechanism of her diaphragmatic weakness given her history of JIA. She was diagnosed with JIA at the age of 18 months. She resides in a nursing home and at baseline uses a wheelchair for mobility. According to her mother, she had been on various therapies for JIA. In the past, she developed complications from medications, including hematuria while on aspirin and gold salt therapy, and secondary systemic lupus erythematosus diagnosed after starting etanercept therapy at age 35 years. Based on history, it was estimated that she had been on hydroxychloroquine for about 10 years. She had a prolonged hiatus from hydroxychloroquine from her mid-20s to age 40 years, but was on a stable dose of 200 mg twice daily from age 40 to 42 years. The medication was stopped after concern for retinal maculopathy, a diagnosis that was later ruled out with ophthalmologic examination. The patient had significant synovitis in both hands and it was recommended to continue hydroxychloroquine. Anakinra was held this admission due to newly discovered Enterococcus faecalis urinary tract infection causing septic shock.
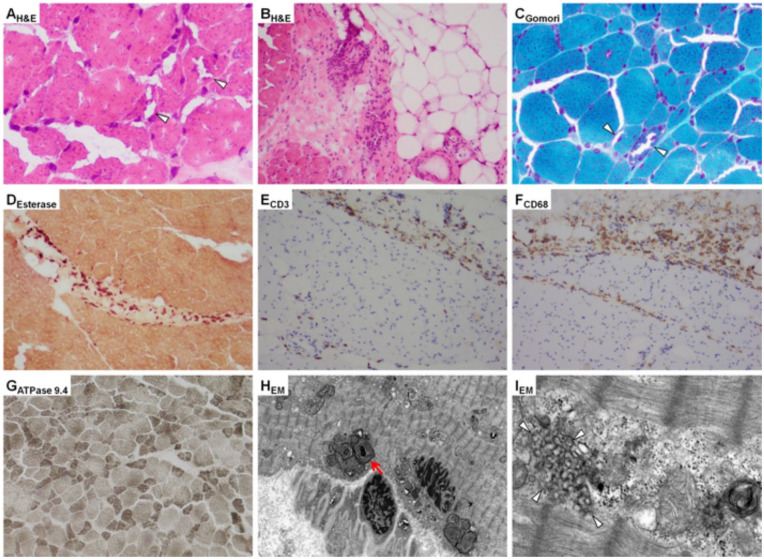
Neurology was consulted to evaluate the patient’s progressive neuromuscular decline. Physical examination revealed muscle weakness in all extremities with proximal muscles affected more than distal muscles (⅗ strength in proximal muscles, ⅘ strength in distal muscles). She was hyporeflexic with 3 to 4 beats of clonus in both ankles. Brain magnetic resonance imaging was consistent with prior stroke and bilateral encephalomalacia, but no acute pathology was identified. Spinal magnetic resonance imaging returned unremarkable and no further neurologic imaging was recommended. Electromyogram (EMG) was performed and suggested myopathy with decreased motor unit recruitment of proximal upper and lower extremities without an inflammatory pattern. Needle EMG reported myopathic changes more significant in the proximal muscles compared with distal muscles. There was some irritability detected in proximal and distal arm muscles. There were no demyelinating features and no evidence of decrement on slow repetitive stimulation. Based on diagnostic testing and clinical presentation, demyelinating diseases and central nervous system insult were ruled out, a primary muscle pathology was suspected as the likely etiology for the patient’s weakness. A myositis panel was sent and returned negative. The myositis panel included anti-Jo1 Ab, PL-7, PL12, EJ, OJ, SRP, MI-2, Fibrillarin, MDA-5, NXP-2, TIF1 gamma, Anti-PM/Scl-100, U2 snRNP, anti-U1-RNP, Ku, and Anti-SSA. Muscle biopsy was performed on the right quadriceps muscle. Muscle biopsy light microscopy reported a granular myopathy with rimmed vacuoles and type II fiber atrophy with scattered inflammation (Figure 2A-C and G). The initial findings were relatively nonspecific but have been identified in critical illness myopathy and myopathy related to chronic corticosteroid or chronic chloroquine use.
Figure 2.
Histological analysis of right quadriceps muscle biopsy. Hematoxylin and eosin–stained images demonstrate (A) significant fiber size variation with increased granularity and vacuoles (arrow heads) and (B) perifascicular mononuclear inflammatory cells. Gomori trichrome sections demonstrate significant fiber size variation with increased granularity and vacuoles (arrow heads) (C). Perifascicular inflammatory cells highlighted by esterase (D), CD3, demonstrating infiltrating T-lymphocytes, primarily in the perifascicular compartment, with infiltration into the endomysium (E), and CD68, demonstrating histiocytes, also primarily in the perifascicular compartment (F). ATPase pH 9.4 demonstrating selective type II fiber atrophy (atrophy of darker stained fibers) (G). Electron microscopy images demonstrating abnormal autophagosomes (red arrow) (H) and curvilinear bodies corresponding to the vacuoles seen on light microscopy (arrowheads) (I).
Electron microscopy (EM) as well as the addition of immunohistochemical stains; CD3, CD20, and CD68 were ordered to further evaluate the inflammatory cells found in the endomysial and perimysial regions on light microscopy (Figure 2D-F). Her serology was significant for normal JO-1 antibody at 4 [AU]/mL (normal = 0-99 [AU]/mL), borderline elevated ANA speckled pattern antibody at 80 1/dil (normal is <80 1/dil). At this time, her physical examination continued to reveal significant synovitis in the bilateral hand joints. Factoring in her uncontrolled inflammatory arthritis, her other rheumatological medications being held due to septic shock, and literature review highlighting the rarity of hydroxychloroquine toxicity, it was elected to continue the hydroxychloroquine inpatient, encourage inpatient physical therapy as tolerated, and closely await EM report. Rheumatology requested hydroxychloroquine levels to be drawn, which resulted as 763 ng/mL. The patient’s hydrocortisone was continued due to septic shock in the setting of chronic adrenal insufficiency, as she had been on chronic steroid therapy since the age 18 years.
Electron microscopy showed granular material identified by the hematoxylin and eosin stains contained a number of abnormal autophagosomes (Figure 2H) and vacuoles contained characteristic curvilinear bodies (Figure 2), consistent with hydroxychloroquine-induced myopathy. There was no significant myosin loss identified. Immunohistochemical staining for CD3, CD20, and CD68 revealed the presence of frequent T lymphocytes and histiocytes in the perimysium and endomysium with less frequent B lymphocytes in the perimysium (Figure 2E and F). These findings were thought to be consistent with an inflammatory response to muscle injury. Gomori trichrome sections, similar to the hematoxylin and eosin stains, demonstrated fiber size variation and scattered vacuoles consistent with medication-induced myopathy such as hydroxychloroquine.5
Discussion
Our patient’s profound respiratory failure became a diagnostic dilemma involving multiple subspecialists and diagnostic testing modalities. In addition to the confounding effect of the patient’s progressive JIA and recurrent hospitalizations causing deconditioning, the rarity of hydroxychloroquine-induced myopathy added to the diagnostic challenge in this case.
Hydroxychloroquine impairs acidity in lysosomes and therefore interferes with enzyme degradation of autophagic vacuoles leading to abnormal enlarged autophagosomes as seen in our patient’s muscle biopsy sample.4,6 Case series examining muscle biopsy from those with hydroxychloroquine toxicity highlight the pathologic changes to mitochondria, vacuoles, and the presence of curvilinear bodies.7 Patients being treated with antimalarials for rheumatologic diseases were followed over a 3-year period and microscopic changes consistent with chloroquine or hydroxychloroquine-induced myopathy were found in just over 12% with 6.7% experiencing clinical signs of toxicity.8
The condition is painless and predominantly affects the proximal muscles and is less severe than chloroquine myopathy.9 In 2007, Siddiqui et al described a case of respiratory failure requiring long-term mechanical ventilation.10 Existing literature identifies potential risk factors for toxicity including Caucasian race, renal failure, and the concomitant use of myotoxic medications.11 Our patient was Caucasian but did not have any evidence of renal failure during her hospital stay.11 Recovery time is not well understood and seems to vary considerably among patients and different forms of myopathy. In a retrospective review of 3 patients with antimalarial-induced myopathy, all 3 patients recovered completely within 8 weeks of discontinuing the medication.12 Khoo et al examined muscle biopsy of over 1400 patients being treated with hydroxychloroquine and of those 19 had curvilinear bodies on biopsy.13 More than 60% of these patients had muscle weakness. Additionally, there was no cumulative dose response relationship among those with curvilinear bodies on biopsy.13 Hydroxychloroquine-induced myopathy predominantly affects proximal muscles and spares sensory fibers.10 This pattern is consistent with our patient’s EMG findings of predominantly proximal muscle myopathy and lack of significant conclusive sensory deficit.
To our knowledge, this patient represents only the second case of hydroxychloroquine-induced myopathy necessitating mechanical ventilation. The 42-year-old patient in our case was considerably younger than the patient discussed in the prior case who was 88 years of age.10 This disparity is likely due to the patient’s extensive history of JIA, which was diagnosed at just 18 months old. Additionally, our case serves as an example of the diagnostic challenges of diagnosing hydroxychloroquine-induced myopathy. Patients with rheumatologic disorders are often on more than one immunosuppressive, including steroids, which are a well-known cause of myopathy.9
In our case, there were features of coexisting steroid-induced myopathy. Steroid-induced myopathy predominantly affects the proximal muscles and is dose-dependent unlike hydroxychloroquine toxicity.13 Furthermore, creatine kinase levels and EMG studies are within normal limits for the majority of cases as is the case for our patient.14,15 Occasionally, EMG studies show slow amplitude motor unit potentials.15,16 Although there is no definitive testing to diagnose steroid-induced myopathy features on muscle biopsy, such as atrophy of type II b fibers, help distinguish it from hydroxychloroquine-induced myopathy.16 Our patient did have evidence of type II fiber atrophy on biopsy in addition to curvilinear bodies, which further supports a combined process.
Other differentials considered in our patient’s case include chronic inflammatory demyelinating polyneuropathy (CIDP), critical illness myopathy, and other inflammatory myopathy. CIDP is a progressive or relapsing disease affecting both motor and sensory fibers, particularly the large sensory fibers.16 CIDP typically affects both proximal and distal muscles, as well as sensory fibers leading to diminished deep tendon reflexes.17,18 EMG in CIDP typically shows evidence of demyelination predominantly in the distal muscles.19 In our patient, EMG lacked a demyelination pattern as well as significant sensory deficits. The patient’s EMG additionally demonstrated a proximal rather than distal muscle predominance of myopathy.
Features of critical illness myopathy include elevated CK, lack of muscle excitability, motor amplitude <80% of the lower limit of normal in 2 or more nerves, and prolonged compound muscle action potential durations.20 Critical illness myopathy often leads to quadriparesis, especially in the proximal muscles and can even affect facial muscles.21 Critical illness myopathy is a term that covers a number of discrete entities. Among these is thick filament myopathy that is associated with nondepolarizing neuromuscular blocking agents, which are often used for paralysis during sedation, and glucocorticoids.22 Thick fiber myopathy leads to destruction of the muscle tissue and thus prolonged recovery time.22 Inability to wean mechanical ventilation is a common feature of critical illness myopathy and our patient failed multiple spontaneous breathing trials put this on the differential diagnosis.21 However, our patient’s lack of significant sensory involvement on EMG favors the predominant hydroxychloroquine-induced myopathy as the predominant etiology. Additionally, in critical illness myopathy, muscle biopsy pathology typically demonstrates myosin loss which was not found on EM of the muscle biopsy.21
Discontinuing hydroxychloroquine is the mainstay of treatment as demonstrated in a prospective longitudinal study, which showed improvement in muscle strength at 6-month intervals in those who initially exhibited moderate to severe clinical myopathy.8 The exact timeline and level of strength recovery the patients in the study experienced are unclear. Given pathologic findings consistent with steroid-induced myopathy, reducing the dose of steroids and potentially discontinue steroids entirely would be beneficial.23 However, this was not possible for our patient given her history of adrenal insufficiency and multiple failed attempts to wean dosages in the past. Overall, this case serves as an opportunity to highlight differential diagnoses related to myopathy in those with rheumatologic disorders. Many myopathic disorders discussed have overlapping features making isolating a diagnosis even more challenging. Additionally, it emphasizes the profound effect hydroxychloroquine-induced myopathy can have on patients, including respiratory failure necessitating mechanical ventilation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Chew AL, Sampson DM, Chelva E, Khan JC, Chen FK. Perifoveal interdigitation zone loss in hydroxychloroquine toxicity leads to subclinical bull’s eye lesion appearance on near-infrared reflectance imaging. Doc Ophthalmol. 2018;136:57-68. doi: 10.1007/s10633-017-9615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology. 2014;121:1257-1262. doi: 10.1016/j.ophtha.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 3. Stauber WT, Hedge AM, Trout JJ, Schottelius BA. Inhibition of lysosomal function in red and white skeletal muscles by chloroquine. Exp Neurol. 1981;71:295-306. doi: 10.1016/0014-4886(81)90090-x [DOI] [PubMed] [Google Scholar]
- 4. Tho JH, Blumbergs P. Hydroxychloroquine induced vacuolar myopathy mimicking acid maltase. J Clin Neurosci. 2010;17:1637. doi: 10.1016/S0022-510X(09)70541-6 [DOI] [Google Scholar]
- 5. Cai C, Anthony DC, Pytel P. A pattern-based approach to the interpretation of skeletal muscle biopsies. Mod Pathol. 2019;32:462-483. doi: 10.1038/s41379-018-0164-x [DOI] [PubMed] [Google Scholar]
- 6. Shukla S, Gultekin SH, Saporta M. Pearls & Oy-sters: hydroxychloroquine-induced toxic myopathy mimics Pompe disease: critical role of genetic test. Neurology. 2019;92:e742-e745. doi: 10.1212/WNL.0000000000006914 [DOI] [PubMed] [Google Scholar]
- 7. Khosa S, Khanlou N, Khosa GS, Mishra SK. Hydroxychloroquine-induced autophagic vacuolar myopathy with mitochondrial abnormalities. Neuropathology. 2018;38:646-652. doi: 10.1111/neup.12520 [DOI] [PubMed] [Google Scholar]
- 8. Casado E, Gratacos J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390. doi: 10.1136/ard.2004.023200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doughty CT, Amato AA. Toxic myopathies. Continuum (Minneap Minn). 2019;25:1712-1731. doi: 10.1212/CON.0000000000000806 [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui AK, Huberfeld SI, Weidenheim KM, Einberg KR, Efferen LS. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest. 2007;131:588-590. doi: 10.1378/chest.06-1146 [DOI] [PubMed] [Google Scholar]
- 11. Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27:2927-2931. [PubMed] [Google Scholar]
- 12. Avina-Zubieta JA, Johnson ES, Suarez-Almazor ME, Russell AS. Incidence of myopathy in patients treated with antimalarials. A report of three cases and a review of the literature. Br J Rheumatol. 1995;34:166-170. doi: 10.1093/rheumatology/34.2.166 [DOI] [PubMed] [Google Scholar]
- 13. Khoo T, Otto S, Smith C, et al. Curvilinear bodies are associated with adverse effects on muscle function but not with hydroxychloroquine dosing. Clin Rheumatol. 2017;36:689-693. doi: 10.1007/s10067-016-3408-5 [DOI] [PubMed] [Google Scholar]
- 14. Askari A, Vignos PJ, Jr, Moskowitz RW. Steroid myopathy in connective tissue disease. Am J Med. 1976;61:485-492. doi: 10.1016/0002-9343(76)90327-2 [DOI] [PubMed] [Google Scholar]
- 15. Bowyer SL, LaMothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol. 1985;76:234-242. doi: 10.1016/0091-6749(85)90708-0 [DOI] [PubMed] [Google Scholar]
- 16. Khaleeli AA, Edwards RH, Gohil K, et al. Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol (Oxf). 1983;18:155-166. doi: 10.1111/j.1365-2265.1983.tb03198.x [DOI] [PubMed] [Google Scholar]
- 17. Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve. 2001;24:311-324. doi: [DOI] [PubMed] [Google Scholar]
- 18. Dyck PJ, Lais AC, Ohta M, et al. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc. 1975;50:621-637. [PubMed] [Google Scholar]
- 19. Chio A, Cocito D, Bottacchi E, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. 2007;78:1349-1353. doi: 10.1136/jnnp.2007.114868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latronico N, Friedrich O. Electrophysiological investigations of peripheral nerves and muscles: a method for looking at cell dysfunction in the critically ill patients. Crit Care. 2019;23:33. doi: 10.1186/s13054-019-2331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shepherd S, Batra A, Lerner DP. Review of critical illness myopathy and neuropathy. Neurohospitalist. 2017;7:41-48. doi: 10.1177/1941874416663279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasper DM, Fauci A, Hauser SL, Longo DLM, Jameson JL, Joseph L. Neurologic critical care. In: Harrison’s Principles of Internal Medicine. 19th ed. McGraw Hill Education; 2015. [Google Scholar]
- 23. Haran M, Schattner A, Kozak N, Mate A, Berrebi A, Shvidel L. Acute steroid myopathy: a highly overlooked entity. QJM. 2018;111:307-311. [DOI] [PubMed] [Google Scholar]