Abstract
Background
Transient osteoporosis of the hip (TOH) is a rare and temporary clinical condition characterised by bone marrow edema (BME), severe pain, and functional limitation. It commonly occurs in middle-aged men or in women in the last trimester of pregnancy. TOH usually resolves with conservative therapy but may predispose to hip fracture or progression to avascular necrosis (AVN). Etiology is still unclear, although several pathophysiological mechanisms underpinning this condition has been proposed. We describe the management of an unusual case of TOH occurred in a patient with subclinical hypothyroidism.
Case presentation
A clinical case of a 46-year-old man with severe pain in the left anterior thigh is presented. After a comprehensive clinical and radiological approach, a TOH was diagnosed. Moreover, biochemical assessment suggested the presence of subclinical hypothyroidism. After 3 months of treatment with clodronate, physical therapy and hormone replacement therapy (HRT) a significant improvement of clinical and radiological outcomes was observed.
Conclusion
Several pathological conditions have been related to development of TOH. In our case, we suggested for the first time a role of subclinical hypothyroidism as novel contributory factor for the onset of this condition, providing pathophysiological mechanisms and a scientific rationale for pharmacological treatment.
Keywords: Case report, Transient osteoporosis of the hip, Hypothyroidism, Diphosphonates, Rehabilitation
Background
Transient osteoporosis (TO) is a rare disease characterized by bone edema as main finding, so that this condition is included among the bone marrow edema syndromes (BMES) [1]. In clinical practice, BMES are commonly underestimated and referred to interchangeably as bone marrow lesions (BMLs) that are characterized by progressive musculoskeletal pain with insidious onset, usually affecting a single joint, and functional impairment with limitations of activities of daily living (ADLs) [1]. The term “BMLs” defines conditions characterized by high bone marrow signal intensity on fluid-sensitive sequences on magnetic resonance imaging (MRI) [2]. This finding can be found in several traumatic, degenerative, inflammatory, vascular, metabolic, neoplastic and iatrogenic disorders [3].
Among BMLs, TO is characterized by low bone mineral density (BMD) affecting one skeletal site (such as hip or knee) and sometimes other bones [3–6]. Transient osteoporosis of the hip (TOH) is the most prevalent TO, mainly affecting men in middle age, even if pregnancy is considered as the most common risk factor [7], as well as the first cause of TOH described in the literature [8]. However, it is essential to identify primary (idiopathic) or secondary forms of TOH [7], where radiological finding of BMLs could be suggestive of systemic conditions.
In this case report we describe an unusual form of TOH related to subclinical hypothyroidism describing clinical and diagnostic work-up as well as therapeutic management and proposing a pathophysiological hypothesis.
Case presentation
A 46-year-old Caucasian man referred to our outpatient rehabilitation service in January 2019 for spontaneous pain at the left thigh. He was 1.80 m tall and weighed 72 kg (BMI 22.2 kg/m2). He reports a lifestyle characterized by occasional alcohol intake (less than one alcohol unit per day) and sedentary work (office worker). Medical history is significant for a trimalleolar fracture of the right ankle treated with open reduction and internal fixation in October 2011. Moreover, in July 2018 he practiced stool culture, faecal occult blood test, celiac disease screening, urinalysis, and abdominal ultrasound, because of the occurrence of several episodes of acute diarrhoea, that were responsive to a short course of antidiarrheal and antispasmodic agents and probiotics.
Clinical complaints started in January 2019 with gradually increasing groin pain without any previous trauma requiring the use of a crutch. The primary care physician prescribed oral diclofenac 150 mg and omeprazole 20 mg per day for 1 week, but the symptoms did not regress. Therefore, his doctor advised him to consult a physiatrist at our service.
On physical examination, patient reported severe groin pain (Numeric Rating Scale, NRS, 8/10) radiating to the antero-medial thigh and at the knee, worse during the night and weight-bearing activity. Moreover, passive and active range of motion (ROM) of the left hip (internal and external rotation 15°, flexion 95°) were limited and patients was forced to use walking sticks. We planned a diagnostic workup including laboratory exams, including complete blood count (CBC), serum erythrocyte sedimentation rate (ESR), C-Reactive Protein (CRP), alanine transaminase (ALT), aspartate transaminase (AST), creatinine, uric acid, alkaline phosphatase (ALP), calcium, phosphate, parathyroid hormone (PTH), 25(OH) vitamin D, thyroid-stimulating hormone (TSH), total testosterone, protein electrophoresis, urinary free kappa and lambda light chains, and magnetic resonance imaging (MRI) scan of the hips. Laboratory tests were normal, except for increased serum TSH (3.28 μIU/ml, normal range 0.2–2.5 μΙU/ml), while remarkable and diffuse bone edema in epiphyseal and metaphyseal region of the left proximal femur was reported, supporting the diagnosis of TOH (Fig. 1).
Fig. 1.
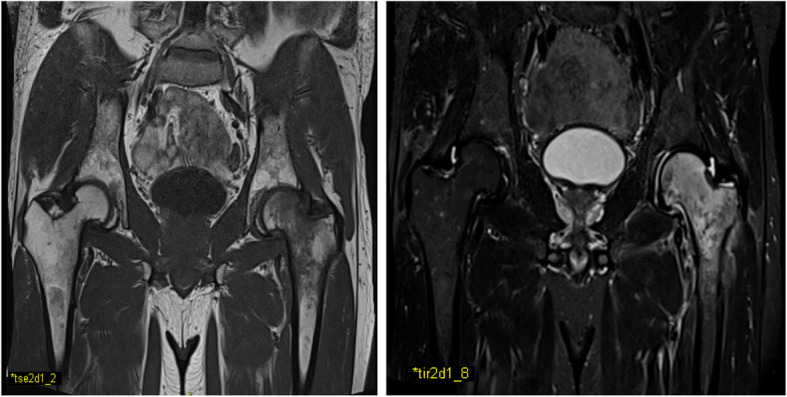
Left lower limb MRI showing remarkable and diffuse bone marrow edema
Therefore, we prescribed clodronate (200 mg i.m. for 10 days and then 200 mg i.m. every other day for 20 days) [9], oral calcium citrate (1 stick of 500 mg per day for 1 month), and cholecalciferol (1 oral solution of 25,000 IU weekly for 1 month). Pharmacological approach was associated with instrumental physical therapy, including Pulsed Electromagnetic Fields (PEMFs) stimulation [8 h per day (night use) for 6 weeks; the device generated single-voltage pulses of 1.3 milliseconds in duration, with a frequency of 75 Hz, and was positioned on the lateral thigh] [10–12], and Neuromuscular Electrical Stimulation (NMES) [13] of the left quadriceps (1 session per day for 3 weeks; electrodes were placed around the thigh for 30 min each session, generating a frequency of 50 Hz, pulse duration of 250 ms, and 10 s on and 30 s off). Moreover, protected weight bearing for 3 weeks was advised. Finally, we recommended a consultation with an endocrinologist to address putative thyroid disorders.
The endocrinologist performed thyroid ultrasound revealing hypoechoic nodule of 5 mm and new laboratory assessment confirming increased TSH levels (3.67 μΙU/ml) and normal level of free thyroxine, (FT4, 15.18 pg/ml, normal range 6–18 pg/ml) and free triiodothyronine, (T3, 3.67 pg/ml, normal range 2.57–4.43 pg/ml). The specialist made the diagnosis of subclinical hypothyroidism and prescribed a nutraceutical containing both myo-inositol (600 mg) and selenium (83 mcg) (1 tablet per day for 2 months).
Clinical and instrumental follow-up performed after 2 months from the beginning of therapies, showed significant pain relief (NRS 2/10), improved ROM of the hip, and a significant reduction of bone edema at MRI examination of the left hip (Fig. 2). Moreover, the patient was able to walk without aids. However, due to persistent high level of TSH (3,56 μΙU/ml, normal range 0,2–2,5 μΙU/ml), endocrinologist prescribed levothyroxine 25 μg per day resulting in serum TSH reduction (2,64 μΙU/ml) after 2 months. Finally, the patient did not report any adverse events.
Fig. 2.
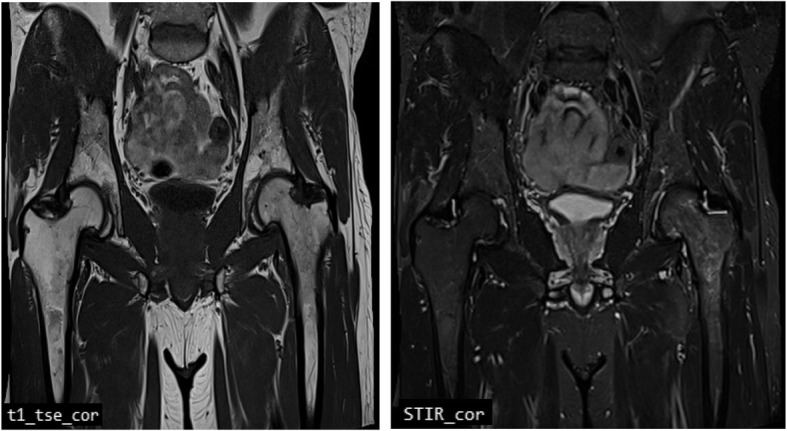
Left hip MRI showing a significant reduction of bone edema at proximal femur
Discussion and conclusion
Bone marrow lesions (BMLs) are characterized by high signal on both T2/proton density with fat suppression and short tau inversion recovery (STIR) MRI sequences with or without low signal intensity on T1-weighted images [14]. These conditions are generally defined as bone marrow edema (BME), a nonspecific MRI finding in both symptomatic and asymptomatic patients. Transient osteoporosis of the hip is included in non-traumatic BMES [14], whose pathogenic mechanisms are not well defined. Our patient is an office worker conducting a sedentary lifestyle, thus suggesting a non-traumatic trigger of TOH. BMES is usually burdened by a challenging differential diagnosis [14–16], considering that trauma, infection, inflammation, degenerative process, ischemic injury, neoplasia, surgery, drugs, neurologic or metabolic disorders [7] might be associated to its occurrence. Moreover, all these conditions might contribute to impaired bone metabolism. Particularly, severe hypothyroidism negatively influences bone modeling and skeletal growth in children, whereas in adults it leads to delayed remodeling of cortical and trabecular bone because of abnormal osteoblastic and osteoclastic activity [17–20].
Some cases of TOH due to severe hypothyroidism have been reported in the literature. In 1938 Albright first described radiological lesions of hip simulating Legg-Perthes disease, reversible after hormone replacement therapy (HRT) in a 13-year-old hypothyroid child with growth retardation [21]. In 1959 Weissbein et al. described a case of a 22-year-old man affected by primary myxedema that referred to orthopedic service for severe pain of anterior region of the thigh to the knee aggravated by walking. Plain x-ray demonstrated an osteolytic lesion of the right femoral head improved after HRT [22]. More recently, McLean and Podel reported a case of a 25-year-old man with severe hypothyroidism and right hip and knee pain on weight-bearing and effusion of the right hip at MRI evaluation, improving within 2 to 3 months of HRT [17]. About 15 years later, Mepani and Findling described a case of a 32-years-old man with severe primary hypothyroidism, with 3-to-4 months hip discomfort and BME of the femoral head at MRI improving at 3 months and 1 year after HRT [23].
However, the cases mentioned share the occurrence of spontaneous bone pain due to lesions at femoral head in patients with severe hypothyroidism.
On the other hand, our case is unique because the occurrence of TOH in a patient with subclinical hypothyroidism has not previously been described in the literature so far. We speculate that subclinical hypothyroidism may be a metabolic trigger for TOH as reported for severe hypothyroidism [17, 18], resulting in fatty replacement of bone marrow [24], cellular infiltration (lymphocytes, plasma cells, histiocyte) [25], impaired blood flow (impaired venous return), local hyperemia [26–29], and pro-inflammatory milieu (cytokines) [3]. These pathways may cause a vicious circle with an increased bone turnover and acceleration of biological processes called Regional Acceleratory Phenomen (RAP) [30, 31]. The rationale for using antiresorptive drugs like bisphosphonates (BPs) could be their action on increased regional high bone turnover state, pro-inflammatory milieu (cytokines) and vasoactive agents, resulting in clinical and radiological improvements [3].
The role of BPs in relieving bone pain has been extensively hypothesized. Osteoclastic activity and/or bone inflammation produce an acidic microenvironment that activates specific chemoreceptors (TRVP1 and ASICs) that may be involved in the pathogenesis of pain [32, 33]. Therefore, by inhibiting osteoclast activity BPs might be effective in reducing bone pain. Moreover, in rat models, alendronate raises pain threshold, reducing the number of c-Fos + neurons (proto-oncogene expressed by neurons following both nociceptive and non-nociceptive stimuli) in lamina 1 and 2 of the dorsal horns of the spinal cord [34]. Also, the release of substance P and other neuropeptides may be implicated in mechanisms of bone pain. In a rat model of sciatic nerve injury, it has been hypothesized that substance P may be implicated even in BMD changes [35]. Bisphosphonates may reduce levels of substance P via the TNF-α pathway playing a key role in inflammatory pain transmission by primary sensory nerves [36–38]. Among BPs, clodronate seems to have peculiar anti-inflammatory activity thanks to its intracellular metabolites (such as AppCCl2p produced by RAW 264 macrophages) acting on cytokines and NO release reducing DNA binding activity of NF-kB [39].
Alongside anti-resorptive and anti-inflammatory action, analgesic mechanisms of clodronate have been proposed. This non-nitrogen-containing BPs (non-N-BPs) blocks phosphate transporter family SLC17 and inhibits vesicular transporters of ATP and/or glutamate [40]. Hydrolyzed products of ATP and ATP released in extracellular environment stimulate purinoceptors (P2X or P2Y and P1 adenosine receptor) on peripheral sensory nerves involved in pain transmission and modulation. Clodronate is a presynaptic blocker of vesicular ATP release from neurons that might decrease neuropathic and inflammatory pain by slowing down purinergic chemical transmission [41].
On the other hand, anti-resorptive effects of clodronate depends by intracellular accumulation of toxic ATP analogs [42, 43] and inhibition of mitochondrial ATP translocases [44] in osteoclasts resulting in their apoptosis.
Anti-resorptive, anti-inflammatory and analgesic properties of clodronate make it a viable strategy to manage BMES and several studies have evaluated its efficacy in these conditions. In two RCTs, daily intravenous clodronate (300 mg daily for 10 or 12 consecutive days) has been investigated to treat algodystrophy with clinical recovery in 1–2 months [38]. The same dosing regimen was used in three patients with TOH followed by physical therapy (3 weeks of flexibility exercises) with clinical recovery and BMD improvement at 3–4 months [45]. Similar results were reported in a 30-year-old woman with TOH using clodronate in association with calcium and vitamin D supplementation after 2 months [46].
Even if this therapeutic protocol seems to be commonly suggested for BMES [7], recently, some authors assert that a global dose of 3000 mg of clodronate would appear insufficient [9]. In our case we used for the first time intramuscular clodronate for the management of TOH. Our protocol consists of intramuscular clodronate at the dose of 200 mg daily for 10 consecutive days and followed by 200 mg every other day for 20 days reaching a total dose of 4000 mg, as proposed by Frediani et al. [9, 47].
However, clodronate and other BPs must be carefully administered in patients with low serum ALP, commonly noticed in patients with severe hypothyroidism [18], because of higher risk of atypical femoral fractures as reported for patients with hypophosphatasia [48]. Our patient had normal serum serum ALP (68.3 U/l, normal range 40–129 U/l) that allows to administer BPs safely.
We combined instrumental physical therapy with pharmacological treatment for the first time in the management of TOH. It has been hypothesized that PEMFs may preserve subchondral bone from marrow edema and stimulate osteogenic activity reducing the risk of trabecular fracture in femoral head osteonecrosis [12]. Moreover, PEMFs may promote bone formation, antioxidant and adenosine receptors synthesis reducing pro-inflammatory cytokines in CRPS I [49]. Concerning NMES, encouraging results were also reported in reducing pain after 6 weeks in patients with osteonecrosis of the femoral head [13].
Based on the available evidence, we assumed that combining PEMFs, NMES and clodronate may have been a suitable treatment strategy for our case of TOH.
Thyroid HRT would seem to be an effective approach in patient with TOH and severe hypothyroidism [17, 21–23]. However, in our case we used promptly a higher dose of clodronate (4000 mg in 1 month). Only belatedly endocrinologist added levothyroxine, when bone edema already seemed in resolution.
We postulate that a multimodal intervention based on the use of clodronate and physical therapy could improve the clinical outcome in patients with TOH caused by subclinical hypothyroidism. In conclusion, TOH is suggestive of abnormal bone metabolism. Diagnostic process has a pivotal role for revealing secondary forms of TOH. Starting an appropriate and timely intervention is mandatory for a complete recovery from TOH to avoid the progression towards avascular necrosis of the hip.
Acknowledgments
None.
Abbreviations
- TO
Transient osteoporosis
- TOH
Transient osteoporosis of the hip
- BME
Bone marrow edema
- AVN
Avascular necrosis
- HRT
Hormone replacement therapy
- BMES
Bone marrow edema syndromes
- BML
Bone marrow lesion
- ADL
Activities of daily living
- BMI
Body mass index
- MRI
Magnetic resonance imaging
- BMD
Bone mineral density
- NRS
Numeric Rating Scale
- ROM
Range of motion
- CBC
Complete blood count
- ESR
Erythrocyte sedimentation rate
- CRP
C-Reactive Protein
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- PTH
Parathyroid hormone
- TSH
Thyroid-stimulating hormone
- IU
International unit
- i.m.
Intramuscular injection
- PEMF
Pulsed Electromagnetic Field
- NMES
Neuromuscular Electrical Stimulation
- STIR
Short tau inversion recovery
- RAP
Regional Acceleratory Phenomen
- BPs
Bisphosphonates
- TRVP1
Transient receptor potential cation channel subfamily V member 1
- ASICs
Acid-sensing ion channels
- AppCCl2p
Adenosine 5′-(beta,gamma-dichloromethylene) triphosphate
- TNF-α
Tumor necrosis factor-α
- NO
Nitric oxide
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- ATP
Adenosine triphosphate
- SLC
Solute carrier family 17
- RCT
Randomized controlled trial
Authors’ contributions
MP, AM, and GI designed the report; MP, SL, GT, and MB collected the patient’s clinical data; AM, MP, and MB analyzed the clinical data and drafted the paper. All authors read and approved the final manuscript.
Funding
The authors would like to acknowledge the Vanvitelli per la Ricerca (VALERE) program for the allocation of funding that aims to publish University of Campania “Luigi Vanvitelli” research products.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards as laid down in the Declaration of Helsinki and its later amendments. Written informed consent was obtained from the patient to participate in the study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel S. Primary bone marrow oedema syndromes. Rheumatology. 2014;53:785–792. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
- 2.Kon E, Ronga M, Filardo G, et al. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1797–1814. doi: 10.1007/s00167-016-4113-2. [DOI] [PubMed] [Google Scholar]
- 3.Eriksen EF, Ringe JD. Bone marrow lesions: a universal bone response to injury? Rheumatol Int. 2012;32(3):575–584. doi: 10.1007/s00296-011-2141-2. [DOI] [PubMed] [Google Scholar]
- 4.Emad Y, Ragab Y, El-Shaarawy N, Rasker JJ. Transient osteoporosis of the hip, complete resolution after treatment with alendronate as observed by MRI description of eight cases and review of the literature. Clin Rheumatol. 2012;31:1641–1647. doi: 10.1007/s10067-012-2060-y. [DOI] [PubMed] [Google Scholar]
- 5.Guardiano SA, Katz J, Schwartz AM, Brindle K, Curiel R. Fracture complicating the bone marrow edema syndrome. J Clin Rheumatol. 2004;10:269–274. doi: 10.1097/01.rhu.0000141509.18395.3c. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17:1008–1012. doi: 10.1007/s00198-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 7.Asadipooya K, Graves L, Greene LW. Transient osteoporosis of the hip: review of the literature. Osteoporos Int. 2017;28(6):1805–1816. doi: 10.1007/s00198-017-3952-0. [DOI] [PubMed] [Google Scholar]
- 8.Curtiss PH, Jr, Kincaid WE. Transitory demineralization of the hip in pregnancy. A report of three cases. J Bone Joint Surg Am. 1959;41-A:1327–1333. doi: 10.2106/00004623-195941070-00014. [DOI] [PubMed] [Google Scholar]
- 9.Frediani B, Giusti A, Bianchi G, et al. Clodronate in the management of different musculoskeletal conditions. Minerva Med. 2018;109(4):300–325. doi: 10.23736/S0026-4806.18.05688-4. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli N, Bianchi A, Sartorelli E, Dondi A, Bonifacini C, Malerba F. Treatment of bone marrow edema of the talus with pulsed electromagnetic fields: outcomes in six patients. J Am Podiatr Med Assoc. 2015;105(1):27–32. doi: 10.7547/8750-7315-105.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Leo M, Milena F, Ruggero C, Stefania S, Giancarlo T. Biophysical stimulation in osteonecrosis of the femoral head. Indian J Orthop. 2009;43(1):17–21. doi: 10.4103/0019-5413.45319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massari L, Fini M, Cadossi R, Setti S, Traina GC. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 13.Ji QH, Qiao XF, Wang SF, et al. Effectiveness of neuromuscular electrical stimulation and ibuprofen for pain caused by necrosis of the femoral head: a retrospective study. Medicine (Baltimore) 2019;98(11):e14812. doi: 10.1097/MD.0000000000014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klontzas ME, Zibis AH, Vassalou EE, Karantanas AH. MRI of the hip: current concepts on bone marrow oedema. Hip Int. 2017;27(4):329–335. doi: 10.5301/hipint.5000527. [DOI] [PubMed] [Google Scholar]
- 15.Klontzas ME, Vassalou EE, Zibis AH, Bintoudi AS, Karantanas AH. MR imaging of transient osteoporosis of the hip: an update on 155 hip joints. Eur J Radiol. 2015;84(3):431–436. doi: 10.1016/j.ejrad.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Klontzas ME, Zibis AH, Karantanas AH. Osteoid osteoma of the femoral neck: use of the half-moon sign in MRI diagnosis. AJR Am J Roentgenol. 2015;205(2):353–357. doi: 10.2214/AJR.14.13689. [DOI] [PubMed] [Google Scholar]
- 17.McLean RM, Podell DN. Bone and joint manifestations of hypothyroidism. Semin Arthritis Rheum. 1995;24(4):282–290. doi: 10.1016/S0049-0172(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 18.Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin N Am. 1990;19(1):35–63. doi: 10.1016/S0889-8529(18)30338-4. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7(4):379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen EF, Mosekilde L, Melsen F. Kinetics of trabecular bone resorption and formation in hypothyroidism: evidence for a positive balance per remodeling cycle. Bone. 1986;7(2):101–108. doi: 10.1016/8756-3282(86)90681-2. [DOI] [PubMed] [Google Scholar]
- 21.Albright F. Changes simulating Legg-Perthes disease due to juvenile myxoedema. J Bone Joint Surg. 1938;20:764–769. [Google Scholar]
- 22.Weissbein AS, Darby JP, Jr, Lawson JD. An unusual bone lesion in an adult with myxedema. Report of a case and review of the literature. Arch Intern Med. 1959;104:643–648. doi: 10.1001/archinte.1959.00270100129023. [DOI] [PubMed] [Google Scholar]
- 23.Mepani JB, Findling JW. Reversible bone marrow edema of the hip due to severe hypothyroidism. J Clin Endocrinol Metab. 2009;94(4):1068. doi: 10.1210/jc.2008-1523. [DOI] [PubMed] [Google Scholar]
- 24.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 25.Axelrod AR, Berman L. The bone marrow in hyperthyroidism and hypothyroidism. Blood. 1951;6(5):436–53. [PubMed]
- 26.Rosen RA. Transitory demineralization of the femoral head. Radiology. 1970;94:509–512. doi: 10.1148/94.3.509. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann S, Engel A, Neuhold A, Leder K, Kramer J, Plenk H., Jr Bone-marrow oedema syndrome and transient osteoporosis of the hip: an MRI-controlled study of treatment by core decompression. J Bone Joint Surg (Br) 1993;75-B:210–216. doi: 10.1302/0301-620X.75B2.8444939. [DOI] [PubMed] [Google Scholar]
- 28.Arnstein RA. Regional Osteoporosis. Orthop Clin North Am. 1972;3:585–600. [PubMed] [Google Scholar]
- 29.Bray ST, Partain CL, Teates CD, Guilford WB, Williamson BR, McLaughlin RC. The value of the bone scan in idiopathic regional migratory osteoporosis. J Nucl Med. 1979;20:1268–1271. [PubMed] [Google Scholar]
- 30.Trevisan C, Ortolani S, Monteleone M, Marinoni EC. Regional migratory osteoporosis: a pathogenetic hypothesis based on three cases and a review of the literature. Clin Rheumatol. 2002;21(5):418–425. doi: 10.1007/s100670200112. [DOI] [PubMed] [Google Scholar]
- 31.Frost HM. Perspectives: bone’s mechanical usage windows. Bone Miner. 1992;19:257–271. doi: 10.1016/0169-6009(92)90875-E. [DOI] [PubMed] [Google Scholar]
- 32.Bonabello A, Galmozzi MR, Bruzzese T, Zara GP. Analgesic effect of bisphosphonates in mice. Pain. 2001;91(3):269–275. doi: 10.1016/S0304-3959(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 33.Varenna M, Adami S, Sinigaglia L. Bisphosphonates in complex regional pain syndrome type I: how do they work? Clin Exp Rheumatol. 2014;32(4):451–454. [PubMed] [Google Scholar]
- 34.Abe Y, Iba K, Sasaki K, et al. Inhibitory effect of bisphosphonate on osteoclast function contributes to improved skeletal pain in ovariectomized mice. J Bone Miner Metab. 2015;33(2):125–134. doi: 10.1007/s00774-014-0574-x. [DOI] [PubMed] [Google Scholar]
- 35.Gaus K, Moriwaki H, Suyama M, Kawamoto M, Yuge O. Capsaicin treatment inhibits osteopenia and heat hyperalgesia induced by chronic constriction injury to the sciatic nerve in rats. Hiroshima J Med Sci. 2003;52:43–51. [PubMed] [Google Scholar]
- 36.Ohtori S, Akazawa T, Murata Y, et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17(2):209–213. doi: 10.1016/j.jocn.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Van Offel JF, Schuerwegh AJ, Bridts CH, Bracke PG, Stevens WJ, De Clerck LS. Influence of cyclic intravenous pamidronate on proinflammatory monocytic cytokine profiles and bone density in rheumatoid arthritis treated with low-dose prednisolone and methotrexate. Clin Exp Rheumatol. 2001;19:13–20. [PubMed] [Google Scholar]
- 38.Varenna M, Zucchi F, Ghiringhelli D, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27:1477–1483. [PubMed] [Google Scholar]
- 39.Makkonen N, Salminen A, Rogers MJ, et al. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. Eur J Pharm Sci. 1999;8(2):109–118. doi: 10.1016/S0928-0987(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 40.Shima K, Nemoto W, Tsuchiya M, et al. The bisphosphonates clodronate and etidronate exert analgesic effects by acting on glutamate- and/or ATP-related pain transmission pathways. Biol Pharm Bull. 2016;39(5):770–777. doi: 10.1248/bpb.b15-00882. [DOI] [PubMed] [Google Scholar]
- 41.Moriyama Y, Nomura M. Clodronate: a vesicular ATP release blocker. Trends Pharmacol Sci. 2018;39(1):13–23. doi: 10.1016/j.tips.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 43.Ghinoi V, Brandi ML. Clodronate: mechanisms of action on bone remodelling and clinical use in osteometabolic disorders. Expert Opin Pharmacother. 2002;3:1643–1656. doi: 10.1517/14656566.3.11.1643. [DOI] [PubMed] [Google Scholar]
- 44.Lehenkari PP, Kellinsalmi M, Näpänkangas JP, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 45.Varenna M, Sinigaglia L, Binelli L, Beltrametti P, Gallazzi M. Transient osteoporosis of the hip: a densitometric study. Clin Rheumatol. 1996;15(2):169–173. doi: 10.1007/BF02230335. [DOI] [PubMed] [Google Scholar]
- 46.Schapira D, Braun Moscovici Y, Gutierrez G, Nahir AM. Severe transient osteoporosis of the hip during pregnancy. Successful treatment with intravenous biphosphonates. Clin Exp Rheumatol. 2003;21(1):107–110. [PubMed] [Google Scholar]
- 47.Frediani B, Bertoldi I. Clodronate: new directions of use. Clin Cases Miner Bone Metab. 2015;12:97–108. doi: 10.11138/ccmbm/2015.12.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Righetti M, Wach J, Desmarchelier R, Coury F. Teriparatide treatment in an adult patient with hypophosphatasia exposed to bisphosphonate and revealed by bilateral atypical fractures. Joint Bone Spine. 2018;85(3):365–367. doi: 10.1016/j.jbspin.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Pagani S, Veronesi F, Nicoli Aldini N, Fini M. Complex regional pain syndrome type I, a debilitating and poorly understood syndrome. Possible role for pulsed electromagnetic fields: a narrative review. Pain Physician. 2017;20:E807–E822. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


