Abstract
Background
Obtaining informed consent from research study participants continues to meet difficulties. New ways to connect with potential participants are necessary to address barriers, expand enrollment and offer more services to underserved populations.
Objectives
Electronic consent is designed to complete consenting sessions remotely and may help combat the obstacles inherent in the traditional informed consent process. We investigate the implementation of an electronic consent platform, Teleconsent, to broaden and diversify recruitment for clinical research.
Methods
Semi-structured interviews were conducted with community members to assess their perceptions regarding the acceptability and usability of Teleconsent, a form of electronic consent. Interviews were structured to determine the main benefits, challenges and concerns as detailed by each participant. Participants were divided into rural and urban groupings.
Results
We interviewed 40 participants to gather first-time perceptions of Teleconsent. We found overall positive results. Predominately in urban communities, participants possessed the technological skills and amenities to support smooth implementation of this technology. However, many participants reflect on the challenges regarding logistics, privacy and reliability of utilizing Teleconsent in underserved, rural areas. 5 of 19 participants, more than a quarter for the rural group, experienced Teleconsent software problems. During these sessions, an alternative process with paper templates was employed to complete interviews.
Conclusion
Perceptions regarding Teleconsent demonstrate current challenges along with potential acceptance within different communities. This is despite the fact that on its own it will not be able to overcome the barriers currently found in the informed consent process. Still, investment in electronic consent, including the development of enhanced and interactive content, can potentially revolutionize this process. Our findings offer a preliminary step towards determining the feasibility and acceptance of Teleconsent, a form of electronic consent, in different communities. More research surrounding the logistics of adoption is necessary in order to determine success.
Keywords: Informed Consent, Informatics, Telemedicine, Videoconference
1. Introduction
Amidst a growing national discourse surrounding health privacy, access to data and consumer comprehension, concerns regarding informed consent in the context of health care and clinical research continue to grow [1, 2]. Informed consent is the voluntary acknowledgement of a study’s procedures, risks and benefits; and the individual’s autonomous decision to fully participate in the research study [3, 4, 5]. While the informed consent process (ICP) is grounded as an ethical value - participant autonomy - the process itself is shrouded in legal protections often creating more confusion [4, 6, 7, 8]. Obtaining consent not only requires research personnel to enroll participants in a study, but to also convey and explain their rights as human subjects [3, 9]. The ICP is often executed as a clearly defined, binary choice for participants, and several concerns have arisen regarding this strategy for study enrollment [7, 10, 11, 12].
Recent reports suggest that informed consent documents are often written with an abundance of legalese, making them difficult to read and understand [6]. The consent document is generally meant to provide institutions with protection from litigation as opposed to creating a document to support comprehension of study procedures [5, 13, 14]. These documents are often written far above literacy guidelines and can present readability challenges, undermining participants’ full comprehension during the consent process [7]. However, the primary goal of the ICP is knowledge and comprehension gathered from a clear discussion between research personnel and the potential participant. This communication is vital for participant understanding, yet studies have shown that consenting sessions are often generic, lacking explanation regarding risks and alternatives, devoid of details and often leave many participants feeling ill-informed with unanswered questions [9, 11]. Although the ICP may seem straightforward, the lackluster review of research communication coupled with health literacy and readability barriers create a much more complex problem when it comes to the application of new technologies in the area of informed consent.
While informed consent can be obtained by a variety of means (verbal, telephone, fax, etc.), the most commonly used method is a traditional face-to-face paper consent. This can add a significant travel and expense burden on participants, especially when the study design requires participants to be physically present at a research facility for the initial visit. This added burden can deter potential participants, limiting a research study’s pool of recruits [12, 15]. A recent expert panel researching improvements to the ICP cited investing in electronic consent platforms to help widen participant pools and bring more underserved populations into clinical research, where they are often underrepresented [10, 16, 17, 18]. While such platforms can theoretically help with the task of signing a consent document, there is no evidence that demonstrates a direct impact on participant recruitment and retention after the legal agreement is signed.
Teleconsent is a type of electronic consent. It differs from other informed consent options by providing sessions via an online, internet enabled communication platform. The traditional paper consent process asks research participants to sign a paper consent document at the conclusion of an in-person face-to-face conversation. Imperative to successful informed consent is 1) The discussion between research personnel and participant, and 2) The legal signature of the consent document acknowledging all the risks and benefits of the study. Alternatives to the paper in-person process include a telephone or video call to fulfill the discussion requirement and separately, signatures on the consent form with a witness present if completed remotely. The signed document is then returned to study personnel by means of mail, fax, electronic patient portal or other secure means such as WhatsApp. As another alternative to these established processes, Teleconsent is a web-based application optimized for both clinical trial recruitment and informed consent. It is software specifically designed for the completion of electronic consent remotely. The platform is innovatively designed to allow research personnel to conduct live video sessions with potential study participants [19]. These sessions allow participants to connect virtually with research personnel to discuss study details and clarify questions. In addition to the communication aspect of the platform, Teleconsent also allows for the review and signature of informed consent documents in real time by creating an electronic signature. This functionality further differentiates Teleconsent from other remote consent options, such as telephone or video, since users can view and legally sign the consent form in real time during the session. With video capability, personnel are able to monitor participants for comprehension or confusion, allowing each session to be tailored to that specific participant’s informational needs. Once the consent document is signed by both parties, a PDF version of the form is available and can be stored or shared electronically.
In this study, we examine individual perceptions of Teleconsent, within rural and urban communities, following up on our previous work [20]. Our previous research tested the functionality of Teleconsent within clinical research and its ability to successfully provide a virtual space for informed consent discussions. Here, we investigate, from a user and patient perspective, the attitudes surrounding the use of the platform, questions about technical capabilities, and offer insights regarding the Teleconsent process as a new and convenient option for informed consent sessions. Regarding the potential acceptability and usability in different communities, we illustrate the benefits of Teleconsent, as well as challenges and concerns, as identified by a diverse group of participants. Furthermore, these findings will ground our future comparative research between the Teleconsent and traditional paper informed consent processes.
2. Methods
2.1. Study Overview
Initial recruitment was completed via Join the Conquest (JTC)-a web-based recruitment portal connecting potential participants to research studies-and word-of-mouth [21]. Initial eligibility criteria for online recruitment included English as a first language to control for potential language barriers, NC residency, and access to a computer with a microphone and camera, but this was amended for rural participants who were only required to live in a rural area and have English as a first language, as they were supplied with the necessary hardware to facilitate the study if required.
This recruitment method yielded a total of 336 possible candidates. From this pool, 25 participants were chosen to ensure a diverse study sample in terms of race, ethnicity, and age. Since the initial recruitment yielded a high percentage of candidates who self-identified as White with tertiary education (having at least some college), direct participant recruitment at a rural clinic in Eastern NC was also completed to help ensure a diverse participant group and study the feasibility of Teleconsent within an underserved population. Demographic information from the initial recruitment cycle through JTC was collected via Qualtrics® (Provo, UT) survey instrument, while those at the rural Eastern clinic provided this information in-person during their meeting with research personnel. During the initial recruitment, participants consented to the Teleconsent interview session. All participants were compensated for their participation. Institutional Review Board (IRB) approval was obtained prior to initiating the study.
Participants were scheduled for one-hour remote mock consent sessions with a trained study Research Assistant (RA). Participants recruited via JTC were asked to complete the sessions from wherever they felt most comfortable and were emailed a link to the Teleconsent platform upon scheduling. Participants from the rural Eastern NC clinic completed the sessions in a private room at the clinic via laptop with a remote RA. An on-site RA was present to assist with setting up the platform.
The RA first provided a brief overview and tutorial of the Teleconsent platform and then proceeded to walk participants through an electronic mock consent form, which was a four-page mock biobank specimen consent form provided by our collaborators at the Medical University of South Carolina. This session was meant to familiarize participants with the application of Teleconsent by highlighting functionalities of the software. Tutorials were not intended to complete a true informed consent session. Following the tutorial, semi-structured interviews were conducted and participant responses were recorded via audio. The semi-structured interview questions were developed by the site researchers in conjunction with three Community Advisory Board members. Findings from the interviews serve as the source of our results regarding participant experience and perceptions of Teleconsent and its application within healthcare.
In the event that the Teleconsent platform was inaccessible, a paper template was used to guide participants through the consent form and process. 5 rural clinic participants experienced this study via paper tutorial and their responses were the same as those provided by peers in their group. They did not express difficulty answering interview questions due to the use of a paper tutorial.
2.2. Measurements
Our recruitment efforts yielded a diverse group of participants with various backgrounds and education levels. This study measured the various perceptions around Teleconsent and its possible application in different communities. Participants were asked to provide general thoughts on the experience of using Teleconsent, and identify problematic challenges or concerns, as well as perceived benefits of the technology. Information was recorded about each participant’s technology comfort level and their access to various types of technology in their everyday life. Note, in this study, a distinction was not made between smart phones and telephones when collecting this information, therefore further investigation into the use of smart phones for Teleconsent would require new findings. As this study sought to highlight first time perceptions regarding the feasibility, usability and acceptability of Teleconsent, a high-level data analysis approach was utilized.
2.3. Data Analysis
Interview responses, collected via written notes and audio recordings, were collated together into a single spreadsheet and paired with collected demographic data. Paired data was then divided into two groups, urban and rural, as described by The North Carolina Rural Center. According to this metric, counties are classified as rural (population density of 250 people or less per square mile), suburban (population density between 250 and 750 per square mile), or urban (population densities greater than 750 people per square mile) based on the 2014 U.S. Census population estimates [22].
All responses were then analyzed for common themes. Each full response was broken into its elemental, single theme topics, allowing all points stated by the participant to be equally evaluated in the analysis. By this methodology, a single participant’s response could yield more than one thematic topic. Since the interview questions were designed to be open-ended, several responses touch on different ideas in a single answer. An inductive analysis approach was used to identify common themes, group similar statements together and uncover larger, overall patterns found within the pool of responses. These themes were analyzed both within and between both groups. Themes were also used to identify commonalities and develop categories for analysis, as agreed upon by the RA and the Principle Investigator.
3. Results
A total of 40 participants were enrolled with an even split between two different communities; rural (n=19) and urban (n=21), Table 1. While most were recruited online, 15 (37.5%) were directly recruited from a clinic in Eastern NC. Of the group recruited online (n=25), 4 (16.0%) were rural and 21 (84.0%) were urban. Overall, we were able to obtain an even gender split (45% male), with the largest represented age group being 50–64 years old.
Table 1:
Descriptive characteristics of participant sample (N=40).
| Urban | Rural | |||
|---|---|---|---|---|
|
|
N (Mean) |
% (SD) |
N (Mean) |
% (SD) |
| Gender | ||||
| Female | 10 | 47.60% | 12 | 63.20% |
| Male | 11 | 52.40% | 7 | 36.80% |
| Age (years) | 36.5 | 14.8 | 58 | 12.8 |
| Race | ||||
| White | 11 | 52% | 3 | 16% |
| Black or African | 3 | 14% | 15 | 79% |
| American 2 or more races | 4 | 19% | 0 | 0% |
| Other | 3 | 15% | 1 | 5% |
There were 155 separate responses, 125 from the urban participants and 30 from the rural participants. Interview responses fell into two different categories: those perceptions pertaining to the use of the Teleconsent platform and those perceptions about the process, i.e. the consent discussion, the consent form, etc. In order to capture the various levels of individual technology access, participants were questioned about phones and personal computers, Figure 1. Please note that 3 participants (1 urban and 2 rural) declined to answer our questions pertaining to technology access. A majority of participants detailed having access to these types of technologies, however 3 rural participants stated no access at all to any technology. Additionally, several participants also possessed other devices with internet capability such as tablets. This was particularly reported in a high volume within the urban grouping. Interviews also revealed that technology access appeared to vary distinctly between the communities, likely due to their different locations and infrastructures. Our data show a mismatch between what Teleconsent would require to be successful as a standalone measure (i.e. infrastructure and smart devices), and what technology is currently available in rural communities.
Figure 1:
Community technological accessibility.
3.1. Emerging Themes
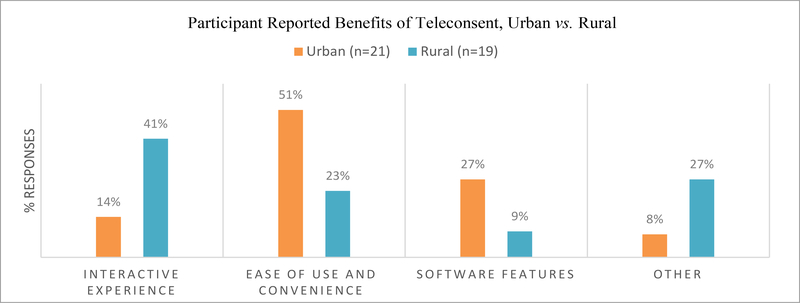
The emerging themes from our interviews can be easily categorized into three distinct groups-Teleconsent benefits, challenges and concerns. For clarification, Figures 2–4 illustrate each group as the volume (percentage) of responses, not number of participants. In terms of Teleconsent benefits, participants identified several but a majority remarked on Teleconsent’s ease and convenience as its best feature, allowing for the freedom to determine a time and location which works best with their own personal schedules, Figure 2. Many reflected on the simple design of the tool with only a small number of participants requiring extra tutorials in order to complete tasks. Over a third of rural participants especially liked the interactive aspect of the interface, and ease of use was a highlighted theme throughout most responses. “Quick and easy” was often remarked on during interviews, as well as the simplicity of the interface. Software features, such as the chat capability, allowed participants to feel as if they were still receiving quality attention. Many remarked that the process was straightforward and easy to follow due to the highlighting functionalities and electronic signature. However, a number of participants provided answers outside the question scope and the interviewer did not ask for clarification. For example, one response when asked about Teleconsent benefits was “it was nice to look up the weather.” These responses as categorized as ‘other’ in our analysis.
Figure 2:
Benefits reported by participants.
Figure 4:
Concerns expressed by participants.
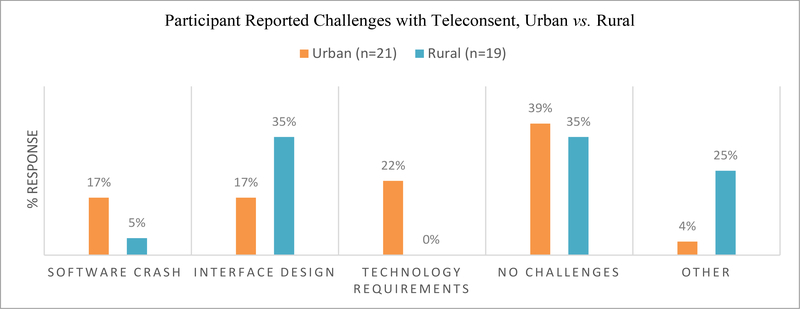
Teleconsent challenges are described as issues participants experienced with the software during the interview and tutorial session. These consistently fall into technical problems related to the platform and most of the challenges that arose during interviews stemmed from software malfunctions such as inaccessibility (US-based cloud connectivity problem) or issues with a specific feature such as the electronic signature, Figure 3. While a majority of participants stated that they didn’t have any problems with Teleconsent as a concept, there were instances of confusion when working with the online platform. Some were challenged by the design of the software and/or workflow of the process, lacking an instinctual understanding of how to use electronic platforms. A small number of participants experienced technical glitches with a specific feature, such as with the photo signature or browser requirements, while others expressly wanted compatible software for devices such as touchscreen tablets, to make the e-consent process easier. The interface for Teleconsent, currently, doesn’t contain zoom capability of the scanned consent document; therefore readability could become a critical issue. This set of interview questions yielded a number of no answer or inaudible responses, particularly from the rural grouping. These are also included in the ‘other’ categorization.
Figure 3:
Challenges with Teleconsent.
Responses categorized as Teleconsent concerns detail participant feedback regarding the larger impact of this technology in different situations. Most of the concerns voiced by interviewees revolved around the realistic feasibility of implementing this process in rural locations, along with worries regarding privacy and security, Figure 4. Responses revealed that many interviewees could foresee complications to implementation of Teleconsent, such as in areas without consistent technology access or infrastructure. This was mainly reflected within urban responses. Teleconsent inherently requires internet access in some form, making this point a large obstacle for successful deployment in rural communities. Subsequently, privacy and security were continually mentioned throughout a significant portion of responses. These concerns reflect the current national conversation surrounding telecommunication privacy and data access [23], and would need to be addressed to each participant’s satisfaction before activating a Teleconsent session. When referring to accessibility in public spaces, concerns with privacy were especially heightened. Similar to implementation, participants voiced a likewise apprehension regarding the required literacy level for successful use of electronic consent, namely comprehension regarding the software set up for an online session and understanding the consent document. Interestingly, the level of concern was lower in the rural grouping, a community that also reported the lowest levels of technological access. About a quarter of responses also mention that older participants may have a more difficult time with Teleconsent since they are accustomed to the traditional paper process and may resist this new method (defined as generational acceptance). Again, as indicted, no answer responses, along with out of scope answers are included in the ‘other’ categorization of our analysis.
A sample of participant responses is detailed in Table 2. These responses highlight various thoughts and opinions categorized by the three overall themes of benefits, concerns and challenges. While an equal number of male and female participants offered a variety information, the urban group was the most verbose in providing a large amount of feedback. Urban participants would often speak about how Teleconsent could benefit their own life and work schedule, and also theorize how this technology would work in other situations. Rural participants, in contrast, offered short direct answers. Unfortunately, most rural responses consisted of one-word answers. Interviewers did not re-ask questions for clarification or prompt for more description of their thoughts. We acknowledge this is a shortcoming in the execution of our study and is evidenced in our response data. While the interviews were designed as open-ended, allowing participants to provide as little or as much information they feel is appropriate, encouraging participants to offer more information was not consistently demonstrated by interviewers. Therefore, total responses vary from short one word answers to several trains of thought.
Table 2:
Selection of participant quotes by theme.
| “Face to face communication and ability to keep the document but also get clarification. Easy to use”. (Male, Urban) | |
| “Super convenient, don’t have to leave their own home. Can look something up on Google if don’t understand. Can highlight a part you want to show/talk about. Format is good.” (Female, Urban) | |
| BenefitS | “Just to be able to talk face to face. Kind of a luxury to be sitting here at my own house. I can drink my cup of coffee. Just to be able to talk face to face. If cold, don’t have to bundle up and go outside. Like being able to talk face to face.” (Female, Urban) |
| “I really like the idea and accessibility and I think if it runs correctly it could really change the lives of a lot of people that are living in remote locations. I think it could be a big step forward in the health care industry.” (Male, Urban) | |
| “Privacy concerns because with research comes a lot of protected HIPPA information. I would be concerned about security of the connection and the software because if you're storing people’s data, especially if taking pictures, would have to have some type of adequate cyber security system, department. Concerns also with how well software actually runs and how easy for people who aren’t very familiar with laptops, webcams, stuff like that to be able to use it with ease.” (Male, Urban) | |
| Concerns | “Download options will be unfamiliar for some people. Should be a pop up screen. If catering to rural area, need to think about those hardships and how to overcome them. This population also doesnť reach out on own. Still limitations.” (Female, Urban) |
| “Hesitant about patient population group it would serve, would be least comfortable with technology, so needs to be incredibly easy to use. Rural areas will not have good internet access (in rural areas, it is expensive). So, if have resources for satellite internet connection would also need have resources to follow up with medical/research personnel on their own.” (Male, Urban) | |
| “Getting checked in. It seems like it would be really easy to check in but the software crashed three times, and it required a specific browser. So, then I had to go find another piece of technology. For me Interface was fine because I do work on computers but for someone who doesn’t necessarily use computers often, it would be confusing.” | |
| Challenges | (Female, Urban) |
| “Not being able to do it on an iPad. This day and age anything done on the technology realm has to work across all platforms. Dramatically limiting yourself if you don’t do that.” (Male, Urban) |
4. Discussion
Findings of this study were favorable, lending credence to the idea that Teleconsent, as a virtual platform and a concept process, could be a viable and feasible option for connecting new clinical research to a larger radius of potential study recruits. Nevertheless, our findings also uncovered a variation in preparedness concerning the deployment of such a tool in different communities and settings, making theses perceptions and results decidedly mixed. While in urban locations, where technological infrastructure is reliable, Teleconsent could potentially transition seamlessly into study recruitment processes. In rural and underserved populations, the outlook for successful acceptance and usability of this tool is less optimistic. Concerns surrounding access to equipment, privacy and infrastructure make the potential application of e-consent in rural and underserved settings a more complex discussion, requiring additional attention to the realities of small, rural communities in this region. This leads us to state that while Teleconsent could help bring these communities closer to new research studies; it will have to be deployed in tandem with new policies and programs which focus on related concerns such as access, privacy, education, etc.
Teleconsent creates a more convenient way to complete informed consent tasks but it does not necessarily make these tasks easier for participants in terms of addressing the larger informed consent problems of document readability, study comprehension and autonomous decision making. Those problems still rely on the ability of the research personnel administering the consent session and the effectiveness of the discussion between the two parties. Arguably, allowing this important conversation to take place at the participant’s convenience, potentially in a comfortable environment, as may be the case in a remote session, can afford a sense of safety and possibly allow the participant to be more responsive to the ICP. At best, this theory is speculation and would require further research to determine its merit; nevertheless, a quiet, non-threatening space is recommended when completing the ICP [10]. This convenience would also help support methods such as the teach-back or teach-to-goal, where study personnel have participants relay their understanding of study procedures, risks and benefits in their own words to determine a quality level of comprehension [10]. A convenient time and place will likely help with a quicker teaching timeframe as well as enhance the goal of a balanced discussion. Both techniques have been shown to increase comprehension regarding informed consent [10, 11].
Teleconsent does not address the ongoing issues of consent documentation. Due to the complexity of prose in informed consent documents, participants generally have difficulty understanding phrases and/or paragraphs, making a clear verbal explanation of the document by study personnel all the more imperative [6, 7]. The video chat capability of Teleconsent can potentially help with this discussion, although the platform does not currently offer other functionalities, such as multimedia, to help participants comprehend the legal document. Similar research has found small increases in comprehension when using enhanced consent documents, providing icons that include layman definitions to commonly used consent phrases or multimedia interventions such as converting some documents into video [9, 24]. Teleconsent could also help address the readability of scanned consent documents by including more functionality, such as zoom, to help participants easily read, scroll, interact and notate the document prior to signature.
Relatedly, our findings align with similar research exploring electronic consent via electronic health records. These studies found preliminary support for the electronic consent process when supplemented with interactive features, allowing for a customizable experience [25]. As with our study, participants were similarly concerned regarding data privacy and security. Previous research has also established the importance of trust between research personnel and participants, relating level of trust to willingness to participant in the study [25, 26]. While our study does not focus directly on the concept of trust, Teleconsent does both provide a new media for the concept of trust in informed consent to be explored. Trust is related to security and privacy, fears that are assuaged when a genuine rapport is established as a trusted relationship [26]. Related studies have also uncovered similar findings regarding the perceptions of elderly persons and their ability to complete an electronic consent process [25].
In comparison with Teleconsent specific research, our findings show favorable consistency in terms of perceived usability and ease of use. While prior research focused on the perspective of research coordinators and how Teleconsent could support their workflows [19], our study highlights the distinct impressions from the participant point of view. Often, both these perspectives, the research team and the participant, are needed for a successful ICP and unquestionably, Teleconsent must benefit both groups. However, we hope that with our research, the participant perspective will inform changes on the research side, initiating a workable dialog between the two groups to improve the process overall.
Teleconsent has the capacity to provide clinical research with the means to attract more remote individuals, at least in terms of the informed consent session, and we hope that our findings will encourage eligible research candidates to explore new clinical studies. These sentiments are echoed in a recent series of panel recommendations, brainstorming ideas to innovate the ICP with a larger focus on increasing the enrollment and diversity of participants [10]. These findings suggest that while the barriers to true informed consent still exist, investment and research in electronic consent may demonstrate it as a superior process to traditional paper consent, especially with the addition of interactive and educational material tailored to different types of individual learning [7, 9, 27].
Teleconsent has the potential for interactive and easy consent sessions, particularly allowing for increased convenience between research personnel and potential participants. While it remains an alternative solution to overcome some barriers found in the ICP and clinical research, Teleconsent would benefit greatly from further refinements before it can be used effectively in underserved communities. While it is not a solution for many informed consent issues on its own, Teleconsent has the potential to support innovative ICP alternatives, as an integral piece in tandem with other methods to overcome lack of infrastructure, accessibility, literacy concerns, readability issues and comprehension barriers.
5. Future Work
In research with study designs that do not require the physical presence of participants for an initial visit or throughout the study, Teleconsent may become the preferred process for informed consent. Future research should examine different metrics such as satisfaction, comprehension, shared decision making and study retention [10]. Our work will continue to investigate comparisons between Teleconsent and other informed consent options. Our next step will focus on comparative research between Teleconsent usability and comprehension, and the standard paper consent process. Future directions should also explore the development of new Teleconsent functionalities to individually target problems in the ICP, such as educational content for helping in cases of health illiteracy. Future considerations should include gathering age-specific impressions of the tool to determine the validity and/or any opportunities to introduce Teleconsent to the growing geriatric population, also deemed underserved by the US health system [28].
6. Limitations
While our study offers preliminary perceptions of the Teleconsent process, there are limitations to be found. We acknowledge that Teleconsent is a progress emerging domain. Developers continue to address the technical issues which occurred during the course of our study. Technical difficulties with the software hindered a small number of interviews (5 rural participants). When the software was offline or unavailable, a paper-based template was used to guide individuals through the process. These participants did not have the full range of functionalities displayed to them during their session but they did receive the same information as others experiencing the Teleconsent online tutorial. However, we acknowledge that this could influence some recorded perceptions. Ideally, this would have been avoided in order to keep participant experience uniform. Our small sample size and purposive selection process would need to be enlarged in order to make broader generalizations regarding the acceptance and feasibility of Teleconsent in different populations and locations. Future research should not only compare e-consenting sessions to those of traditional paper consent and other options, but also explore how this technology would function in a myriad of different communities. Furthermore, during the recruitment phase, participants were introduced to an idea of Teleconsent technology. Therefore, they had an established view of this technology, similar remote consent options and/or telehealth platforms, prior to their interview. This bias could have influenced responses to favor the Teleconsent process. Given this and the small sample size, we cannot generalize that Teleconsent would be favorable to the larger general public.
Age is also a variable not thoroughly explored in our analysis. While our participant selection was aimed to provide a diverse group of individuals originating from different communities, the rural and urban groupings were not age matched. On average, the rural group is older and this may influence their interpretation and reaction to Teleconsent. While several participants mention potential hardships and/or obstacles Teleconsent may pose to an older generation, they are generally not speaking of themselves. These participants are, on average, younger and from the urban grouping. Therefore, this information, while included in our analysis, should be acknowledged for its bias. Culture, age and acceptance of new technology is an ever-changing and dynamic state, therefore these results, while preliminary, can only offer so much insight. Nevertheless, we believe that our findings show the potential Teleconsent has to bring new opportunities to research personnel and administrators. It offers an alternate solution and creates an opening for larger Teleconsent research, with findings that can, eventually, be generalized to the larger population.
Furthermore, we admit to limitations in the study execution. When participants provided answers that were short and nondescript, or off topic, the interviewers failed to prompt for more information or clarify whether a question needed to be repeated. In instances where questions were skipped or responses not recorded, personnel did not return to missed questions during the interview session and this makes our response set incomplete. We acknowledge these shortcomings.
7. Conclusion
We believe that the results of this study establish a foundation for gathering valuable and useful feedback specific to the Teleconsent process, and should inform various approaches for the introduction of this technology within different settings while addressing potential obstacles to succeed. By leveraging different applications, Teleconsent could potentially streamline a new and more effective informed consent process.
8. Acknowledgments
This project described was supported by National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number R21TR002088–01, as well as the Clinical and Translational Science Awards at the University of North Carolina at Chapel Hill UL1TR002489 and 2KR981704 and the Medical University of South Carolina UL1 TR001450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix
Teleconsent Prompt Questions
Where are you right now? Who are you with? Do you feel comfortable talking out loud with me right now?
Do you have any prior experience as a study participant? (If yes, how did consenting using Teleconsent compare to your experience before?)
What kind of access to technology, like phones, computers or the internet, do you have at home? What about other places like work or school?
What kind of technology are your comfortable or uncomfortable with? What type of technology do you use most often and how often do you use it? What makes using technology easier or harder for you?
What were your initial thoughts when you were first approached/read about Teleconsent? Had you ever heard of it before? What questions did you have about it?
Do you think more people would participant in research if they could use Teleconsent or would it be the same as consenting in person? Why or why not?
What are your concerns about the Teleconsent? What was challenging about the process for you?
What did you like about Teleconsent?
What are some suggestions you have on things we can do to improve the system or the way we used Teleconsent?
Is there anything else we should consider or that you would like to share that I haven’t asked about?
References
- 1.Grady C Enduring and Emerging Challenges of Informed Consent. N Engl J Med. 2015; 372(9): 855–862. [DOI] [PubMed] [Google Scholar]
- 2.Hayden EC. Informed consent: A broken contract. Nature. 2012; 486(7403): 312–314. [DOI] [PubMed] [Google Scholar]
- 3.The Natpional Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. 1979. [Google Scholar]
- 4.Cocanour CS. Informed consent-It’s more than a signature on a piece of paper. Am J Surg. 2017; 214(6): 993–997. [DOI] [PubMed] [Google Scholar]
- 5.Spatz E, Krumholz H, Moulton B. The New Era of Informed Consent: Getting to a Reasonable Patient Standard through Shared Decision Making. JAMA. 2016; 263(2): 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrowatka A, Brehaut JC, Saginur R, Fergusson D, Kimmelman J, Elwyn G, et al. Elements of informed consent and decision quality were poorly correlated in informed consent documents. J Clin Epidemiol. 2015; 68(12): 1472–1480. [DOI] [PubMed] [Google Scholar]
- 7.Simonds VW, Garroutte EM, Buchwald D. Health Literarcy and Informed Consent Materials: Designed for Documentation, Not Comprehension of Health Research. J Health Commun. 2016; 25(3): 289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spertus JA, Bach R, Bethea C, Chhatriwalla A, Curtis JP, Gialde E, et al. Improving the process of informed consent for percutaneous coronary inter. Am Heart J. 2015; 169(2): 234–241.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: A systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics. 2013; 14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentz J, Kennett M, Perlmutter J, Forrest A. Paving the way to a more effective informed consent process: Recommendations from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2016; 49: 65–69. [DOI] [PubMed] [Google Scholar]
- 11.Lorell BH, Mikita JS, Anderson A, Hallinan ZP, Forrest A. Informed consent in clinical research: Consensus recommendations for reform identified by an expert interview panel. Clin Trials. 2015; 12(6): 692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller C, Balls-Berry JE, Nery JD, Erwin PJ, Littleton D, Kim M, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemp Clin Trials. 2014; 39(2): 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garonzik-Wang JM, Brat G, Salazar JH, Dhanasopon A, Lin A, Akinkuotu A, et al. Missing consent forms in the preoperative area: A single-center assessment of the scope of the problem and its downstream effects. JAMA Surg. 2013; 148(9): 886–889. [DOI] [PubMed] [Google Scholar]
- 14.Grauberger J, Kerezoudis P, Choudhry AJ, Alvi MA, Nassr A, Currier B, et al. Allegations of failure to obtain informed consent in spinal surgery medical malpractice claims. JAMA Surg. 2017; 152(6): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English RA, Lebovitz Y, Griffin RB. Transforming Clinical Research in the United States, Challenges and Opportunities: Workshop Summary. National Academies Press. 2010. p. 17. [PubMed] [Google Scholar]
- 16.NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research.
- 17.Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016; 23(4): 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsky PF, Ford M, Gamito E, Higgins D, Jenkins V, Lamerato L, et al. Enrollment of racial and ethnic minorities in the prostate, lung, colorectal and ovarian cancer screening trial. J Natl Med Assoc. 2008; 100(3): 291–298. [DOI] [PubMed] [Google Scholar]
- 19.Welch BM, Marshall E, Qanungo S, Aziz A, Laken M, Lenert L, et al. Teleconsent: A novel approach to obtain informed consent for research. Contemp Clin Trials Commun. 2016; 3: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newlin T, McCall T, Ottmar P, Welch B, Khairat S. Assessing the satisfaction of citizens using teleconsent in clinical research. Stud Health Technol Inform. 2018; 247: 685–689. [PubMed] [Google Scholar]
- 21. https://jointheconquest.org/
- 22.North Carolina Department of Commerce. Rural Center Expands Its Classification of North Carolina Counties.
- 23.Asghar MR, Lee TH, Baig MM, Ullah E, Russello G, Dobbie G. A review of privacy and consent management in healthcare: A focus on emerging data sources. Proceedings of the 2017 IEEE 13th International Conference on e-Science (e-Science); 2017 Oct 24–27; Auckland, New Zealand: IEEE; 2017: 518–522. [Google Scholar]
- 24.Sonne SC, Andrews JO, Gentilin SM, Oppenheimer S, Obeid J, Brady K, et al. Development and pilot testing of a video-assisted informed consent process. Contemp Clin Trials. 2013; 36(1): 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagmajer D, Mainous III AG, Krieger JL, Golembiewski EH, Rahmanian KP, Harle CA, et al. Patient preferences toward an interactive e-consent application for research using electronic health records. J Am Med Informatics Assoc. 2017; 25(3): 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kongsholm NCH, Kappel K. Is Consent Based on Trust Morally Inferior to Consent Based on Information? Bioethics. 2017; 31(6): 432–442. [DOI] [PubMed] [Google Scholar]
- 27.Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood). 2014; 33(2): 194–199. [DOI] [PubMed] [Google Scholar]
- 28.Sidani M, Reed BC, Steinbauer J. Geriatric Care Issues. Prim Care. 2016; 44: 77098. [DOI] [PubMed] [Google Scholar]