Abstract
Background/Objective
Type-I Interferons (IFNs) contribute to pathogenesis in Systemic Lupus Erythematosus (SLE), including nephritis. The IFNs consist of a family of 16 proteins, yet are often characterized in patients without knowledge of the specific IFN subtypes involved. Different IFNs may function in kidneys, and other organs, relative to what is often measured in patient blood. Moreover, antibodies to interferons may potentially modulate systemic or organ-specific IFN activity. The aim of this study was to characterize global IFN activity levels and identify autoantibodies (AAbs) to the twelve IFNα subtypes in patient serum and urine.
Methods
IFN activity levels in serum and urine were measured using an IFN bioassay. Anti-IFN and anti-cytokine AAbs were measured by ELISA. Serum and urine samples were also characterized for their ability to neutralize the biological activity of exogenously added IFNs.
Results
Serum IFN activity was increased in 62% of SLE patient samples, relative to healthy donor controls (HDC), while binding IFNα AAbs, to at least one IFNα subtype, were found in 68% of the samples evaluated. High SLEDAI scores were significantly (p = 0.001) associated with patient samples containing IFNα AAbs to three or more IFNα subtypes in their serum. IFNα AAbs that potently block IFN activity were rare (~5% of samples), but collectively bound to all 12 IFNα subtypes. Urine IFN activity and IFNα AAb profiles did not correlate with their serum counterparts, suggesting immune responses in SLE kidneys can be distinct from those measured in serum. Analysis of AAbs to fifteen additional cytokines in serum, identified higher frequencies of GMCSF and IL17A AAbs, suggesting these signaling pathways may potentially contribute, with IFNs, to SLE pathogenesis.
Conclusions
The measurement of AAbs to multiple IFN subtypes in serum and urine may provide an alternative method for following IFN-mediated SLE disease activity. The results suggest AAbs might be used for patient monitoring and/or identifying additional cytokine signaling pathways that are functioning in different SLE patients.
Keywords: Systemic Lupus Erythematosus, interferon subtypes, cytokines, autoantibodies, serum, urinalysis
Introduction
Type-I interferons (IFNs) play a critical role in protecting the host against microbial infection 1. The type-I IFN family consists of 16 different proteins (subtypes), which include 12 IFNαs, IFNβ, IFNє, IFNκ, and IFNω that share 30%−95% amino acid sequence identity 2. The IFNs adopt a common alpha-helical structure 2, 3 and bind to the same cell surface complex consisting of IFNAR1 and IFNAR2 receptor chains 3, 4. IFN binding to the IFNARs induces the phosphorylation of JAK1 and TYK2 kinases, and the subsequent induction of multiple signaling programs that allow the host to combat diverse pathogens 5.
Individuals with systemic lupus erythematosus (SLE) often exhibit an aberrant IFN-mediated immune response. Gene profiling has revealed increased levels of IFN stimulated genes (ISGs, “IFN signature”) in SLE patient blood cells and tissues 6–11. Consistent with these observations, IFN bioactivity is often elevated in SLE patient serum 12–14. Increased serum IFN activity has been associated with increased skin involvement 13 and renal disease 15–17. Data from animal models suggests IFNs drive nephritis and end organ damage 18, 19 constituting the most severe manifestations of SLE 20.
Due to the importance of IFNs in SLE pathogenesis, measurement of serum IFN activity may be useful for stratifying SLE patient disease status for guiding therapy decisions 15, 21–23. However, IFN bioactivity measurements can also be influenced by patient-derived anti-IFN autoantibodies (IFNAAbs, refs. 24–29). In addition to altering IFN activity measurements, SLE patient IFN AAbs have been associated with blockade of IFN signaling and lower disease activity 24. Thus, endogenous IFN AAbs may have a significant impact on studies monitoring IFN activity and may influence the course of SLE patient disease. Although humans produce 16 different IFN subtypes 2, SLE patient IFN AAb status is currently inferred by measuring AAbs to one, or a small subset, of the IFN subtypes 24, 26, 29. These studies have reported variable frequencies of IFN AAbs (5–25%) in patient serum 24, 26, 29. The extent to which IFN AAbs impact SLE disease activity, and whether measuring AAbs to a subset of IFN subtypes is sufficient to accurately define a patient’s IFN AAb status, is unknown.
Variable clinical responses to IFN pathway blockade suggest that heterogeneity in IFN, and other cytokine signaling pathways may influence SLE disease pathogenesis and potentially inform therapy decisions 30–33. The goal of this report was to define serum IFN activity, and IFN AAb levels against all IFNα subtypes, in a cross-sectional analysis of randomly selected SLE patients with variable disease activity. Given the reported role of IFNs in renal disease 15, the relationship of IFN activity and IFNα AAbs in serum and urine was examined for a subset of matched patient samples. To determine if IFNαs are uniquely targeted for AAb generation in SLE patients, AAbs to additional interferons and cytokines that may be dysregulated in SLE, were also evaluated.
Methods
Samples for study
SLE patient (n=38, randomly selected), and healthy donor control (HDC) blood and urine samples were collected at the University of Alabama at Birmingham (UAB) Kirklin Clinic, Alabama Vaccine Research Clinic (AVRC), and the UAB-CCTS biorepository under UAB IRB protocols IRB-120115004, IRB-160125005, and IRB-N150417008, respectively. Written informed consent was obtained for all human specimen collections. De-identified patient data were obtained as part of routine clinical care and were not provided for analysis until data processing was completed. Five additional serum samples were purchased from BioIVT (Westbury, NY). Serum and urine samples were aliquoted, snap frozen in liquid nitrogen, and stored at −80°C until use. Test serum samples (CTL1, CTL2) used to characterize the assay were obtained from rheumatoid arthritis patients.
IFN activity measurements
Serum IFN activity was measured as previously described 34. Briefly, diluted serum, with or without a pan IFN antagonist IFNAR12-FC 35 (1nM) was incubated for 15 minutes at 25°C and then added to HL116 reporter cells for 5 hrs at 37°C. Luciferase counts were measured using a Biotek Synergy 4 Plate reader. IFN levels were determined by subtracting luciferase counts from IFNAR12-FC containing samples from those without IFNAR12-FC.
IFN and cytokine proteins and autoantibody measurements
Serum and urine IFN and cytokine AAb levels were determined by difference Enzyme-Linked-Immunosorbent Assay (dELISA). dELISAs were performed by incubating IFNs, cytokines, and rabbit IFNγ (rIFNγ) overnight at 4°C (1μg/mL) on medisorp plates (Nunc). Plates were blocked (PBS, 0.05% Tween 20, 0.05% Proclin 300, 1% BSA) for 1 hr at room temperature (RT). Serum (diluted 1:100) and urine (diluted 1:10) samples were incubated for 1 hr at RT, washed 3 times (wash buffer, PBS, 0.05% Tween 20), and incubated for 1 hr with sheep anti-human IgG-HRP (The Binding Site). Following IgG incubation, the plates were washed 3 times with wash buffer, followed by addition of TMB stop solution (Biorad) for 15 minutes. Plates were read at 450nm and 670nm (background) on a Biotech Synergy 4 plate reader. For each sample, final difference optical densities (dODs) were obtained by subtracting ODs measured in wells containing rIFNγ, from OD values measured in wells containing the IFNs or other cytokines. rIFNγ was used as the control protein since it has a similar molecular weight to other human cytokines, shares only 60% sequence identity and has distinct N-linked glycosylation sites compared human IFNγ, and performed better than other proteins in test assays. All experiments were performed in duplicate. Autoantibodies to histone H2B (NEB) were measured using the same strategy. Anti-dsDNA levels were measured using a Quanta Lite dsDNA ELISA (Inova Diagnostics). The 12 IFNαs, and most cytokines, were produced in the lab as previously described 34, 36. IL1β, IL6, IL17A, GMCSF, and TNFα were obtained from Preprotech. IFNβ was obtained from PBL Assay Science.
IFN neutralization scores
The ability of SLE serum and urine to neutralize exogenous IFN activity was defined by a neutralization score (NS). Six concentrations of IFNα2 for serum, or IFNα6 for urine (0, 0.5 pM, 1 pM, 2 pM, 4 pM, and 6 pM), were added to media or SLE samples (1:10 dilution). After 15 minutes, the mixtures were added to HL116 cells in duplicate and incubated at 37°C for 5 hours. Luciferase counts were normalized to media only (no patient serum or urine) and averaged across the six IFNα2, or IFNα6, concentrations to yield an average normalized count (ANC) for each sample. The NS was calculated as 1-ANC. Thus, SLE samples that neutralize 100% of exogenous IFNα2/IFNα6 will have an NS equal 1, while samples that stimulate the cells more than IFNα2/IFNα6 in media will have negative NS scores.
Statistical methods
Statistical calculations were performed using Graphpad Prism version 8. Based on analysis of the distributions, Kruskal-Wallis tests, with Dunn’s post testing for multiple comparisons (p=0.05) were used to test for significant differences between groups. Correlations between IFN activity levels and SLEDAI were calculated using Spearman tests with 2-tailed p values.
Data clustering
Unsupervised hierarchical clustering of IFN and cytokine AAb data was performed using dOD values divided by SDs, derived from the analysis of HDCs. Clustering and heatmap graphics were obtained using Morpheus 37, with the Euclidian distance similarity metric and average linkage method. NS and SLEDAI data were not used for clustering.
Results
Serum IFN activity and SLEDAI scores are correlated for individuals with high IFN levels
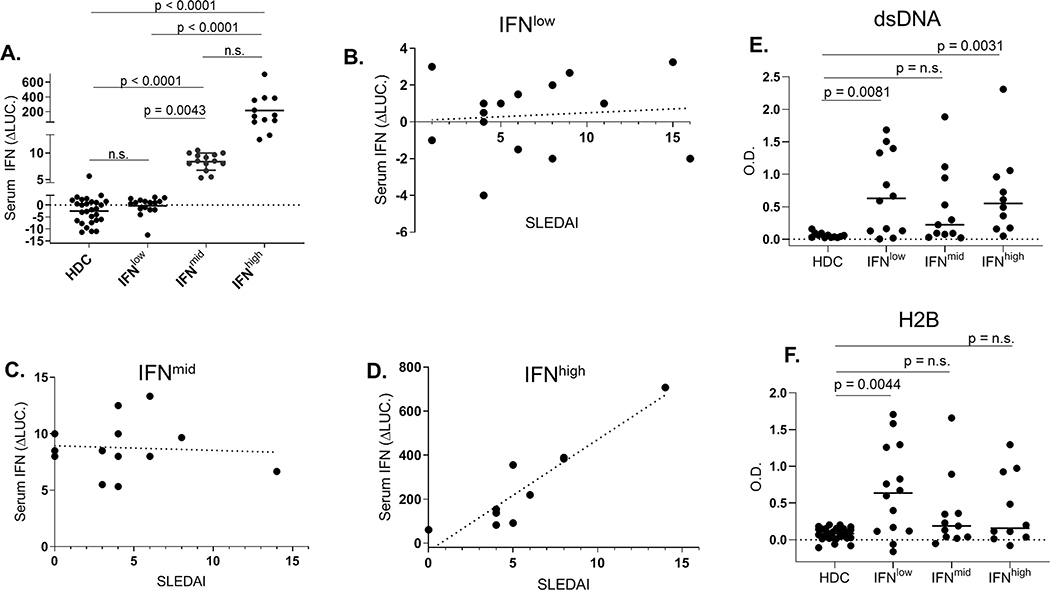
Type-I IFN serum bioactivity was measured for 42 SLE patients and 25 healthy donor controls (HDCs). Clinical information for this cohort of SLE patients is found in Table S1. HDC serum IFN levels were measured to establish a baseline of expected IFN bioactivity in healthy individuals. Based on this analysis, 26 of 42 (62%) SLE patient serum samples exhibited IFN bioactivity levels greater than HDC serum (mean HDC IFN level + 3 * S.D.), while other patients exhibited IFN levels equivalent to HDCs (IFNlow group, n=16). Hierarchical clustering further separated these 26 patients into IFNhigh (n=10; 24%) and IFNmid groups (n=16, 38%), while the IFNlow patient group remained the same (Fig. 1).
Figure 1.
IFN serum activity levels and correlation with disease activity. A, IFN activity in SLE samples measured by luciferase induction shown in IFNlow, IFNmid, and IFNhigh groups. Correlation of IFN activity and SLEDAI scores for (B) IFNlow (r = 0.15, p = n.s.), (C) IFNmid (r=−0.07, p = n.s.), and (D) IFNhigh (r = 0.92, p = 0.0002) groups. Levels of anti-dsDNA (E) and anti-H2B (F) AAbs in each IFN group are also shown.
The median SLEDAI scores for each IFN group were not statistically significant between the respective IFN groups (Table S1). However, SLEDAI was highly correlated with serum IFN levels for IFNhigh individuals (Fig. 1D, r = 0.92, p=0.0002). However, IFN levels and SLEDAI scores were not significantly correlated in the IFNmid (Fig. 1C, r =0.−07, p = n.s.) and IFNlow (Fig. 1B, r =0.15, p = n.s.) groups. Thus, IFN serum bioactivity strongly reflects disease activity for only a subset of SLE patients (24% in this cohort), which exhibit the highest IFN levels.
Relationship of IFN bioactivity and nuclear auto antigens
Serum samples were tested for anti-dsDNA and anti-histone H2B AAbs (Figs. 1D, 1E). Relative to HDCs, anti-dsDNA levels were significantly higher for IFNlow and IFNhigh patient samples, but not for IFNmid samples. In contrast, H2B AAb titers were significantly higher than HDC titers for the IFNlow group only. Thus, increasing AAb epitope specificities are observed within the three IFN groups, with IFNmid exhibiting no significant AAbs, IFNhigh individuals having significant dsDNA AAbs, and H2B and dsDNA AAbs were observed in the IFNlow group.
IFN neutralizing activity of SLE serum
The ability of SLE serum samples to neutralize the bioactivity of exogenously added recombinant IFNα2 protein was characterized (Fig. 2). The data were used to calculate a neutralization score (NS) for each sample, where an NS of one corresponds to 100% neutralization, 0 equals no neutralization, and negative values define serum that induces greater IFN activity than media alone. For the SLE samples that could be tested (n=36), IFNhigh NS scores were significantly different from IFNmid and IFNlow groups (Fig. 2), with the NS correlating inversely with endogenous IFN activity levels (Fig. 1A). IFNhigh samples induced greater IFN activity than media alone for 7 of 10 samples tested, with NS scores ranging from 0.36 to −1.66 (mean score = −0.39); IFNmid samples exhibited minimal stimulatory, or inhibitory activity (n=12, score range −0.09 to 0.13, mean = 0.11), while IFNlow samples had the greatest inhibitory activity (n=14, score range −0.24 to 0.98, mean 0.30).
Figure 2.
Assignment of a neutralizing score (NS) for SLE Serum samples. A, Examples of activating (e.g. SLE-115) or inhibitory (e.g. SLE-146) curves. B, NSs for SLE serum samples. Calculation of NS is described in the methods section.
Relationship of IFN activity with anti-IFNα subtype auto antibodies and SLEDAI
Anti type-I IFN auto antibodies (IFN AAbs) may influence the correlations between serum IFN activity and SLEDAI scores (Fig. 1). To address this hypothesis, a difference ELISA (dELISA) was established to measure anti-IFNα IgG AAbs (IFNα AAbs) against each of the twelve IFNα subtypes (Fig. 3). The dELISA subtracts IgG binding signals to a non-human control protein (rabbit IFNγ), from the IgG signal obtained for each IFNα subtype. Using test serum, specific IFNα AAb signals were observed at dOD values as low as 0.05 (Fig. 3B). Furthermore, IFNα AAbs identified in the test serum, could neutralize IFNα2a biological activity (Fig. 3C).
Figure 3.
The dELISA used to measure IFNαAAbs. A, Analysis of test serum samples for IFNαAAbs using the dELISA. SLE serum (1:100 dilution) was incubated in duplicate with each IFNα subtype and rIFNγ. Data are displayed as mean dOD values and SD from the duplicate wells. B, The sensitivity and specificity of the signal observed in the dELISA was characterized and validated by diluting the CTL1 test serum and by blocking the signal with recombinant IFNα2a (5 μM), or all IFNα subtypes (5 μM of each), except IFNα2. The results show the serum exhibits the greatest affinity for IFNα2a, but can also bind to high concentrations (11x of IFNα2 alone) of the other IFNα subtypes, which prevents interaction with plate bound IFNα2a. C, CTL1 serum, but not CTL2 serum partially blocks IFNα2a biological activity.
Using the dELISA, patient serum samples were defined as positive for an IFNα subtype AAb (IFNα AAb+) if the dELISA signal was greater than the mean dOD plus 3*SD, defined from the analysis of 25 HDC serum samples. Using this criteria, 28 of 41 (68%) SLE patient samples contained IFNα AAbs to at least one IFNα subtype, with IFNα AAbs to three or more different IFNα subtypes identified in 13 of the 41 (32%) samples tested. IFNα AAbs against IFNα2a were most prevalent, being detected in 20 of 28 (71%) IFNαAAb+ serum samples. The second most observed IFNαAAb specificity was against IFNα21 (11 of 28, 39%), while IFNα AAbs to five IFNαs (IFNα5, IFNα8, IFNα10, IFNα16, and IFNα17) were found in nine samples (9 of 28, 32%). IFNα AAbs to IFNα1 (2 of 28, 7%) and IFNα4 (3 of 28,11%) were rarely observed (Table S2).
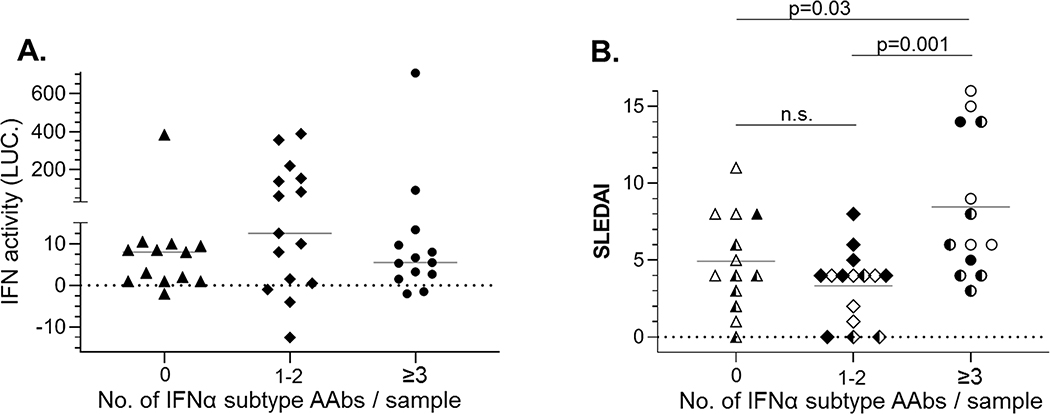
To further estimate the influence of IFNα AAb status on IFN activity, serum IFN activity was plotted according to the number of IFNα AAbs identified in the samples (e.g. 0, 1–2, or ≥3, Fig. 4A). Although not significant, the median levels of IFN activity were lowest in the samples containing IFNα AAbs to three or more IFNα subtypes, slightly higher in samples with no IFNα AAbs, and the highest in samples containing IFNα AAbs to one or two IFN subtypes. In fact, 8 of 10 (80%) IFNhigh samples had IFNα AAbs to just one or two subtypes (Fig. 4A). To evaluate the impact of IFNα and IFNα AAbs on disease activity, SLEDAI scores were plotted according to their IFNα AAb profiles (e.g. 0, 1–2, or ≥3 IFNα subtypes bound by IFNα AAbs). Despite having the lowest IFN activity levels, SLE patient serum samples containing IFNα AAbs to three or more IFNαs exhibited significantly higher SLEDAI scores than samples with AAbs that bound 2 or fewer IFNα subtypes (Fig. 4B). Three SLE serum samples exhibited low IFN activity and no IFNα AAbs yet exhibited high disease activity (SLEDAI scores 8–11). This suggests IFN may play a limited role in disease pathogenesis in some patients.
Figure 4.
Relationship of IFNαAAbs to IFN serum activity and disease activity. A, SLE serum samples were binned based on the number of IFNα subtype AAbs / sample and serum IFN activity was plotted on the y axis. B, SLE serum samples are binned as in Fig. 5A, and SLEDAI values are plotted for each sample. The IFN status of each sample is defined by the coloring of the data points, where open points correspond to IFNlow; half filled, IFNmid; and fully filled, IFNhigh.
Consistent with the idea that neutralizing IFNα AAbs can contribute to the IFNlow serum phenotype 24, IFNlow samples contained the greatest number of IFNα AAbs (50% of the total), relative to the IFNmid and IFNhigh groups (Table S2). However, the increased number of IFNα AAbs in the IFNlow group were almost entirely due to two samples that contained high titer IFN AAbs (1.3 – 2.8 dOD values) to all 12 IFNα subtypes (Fig. 5) and potently inhibited (~100%) IFNα bioactivity (Fig. 2). Thus, high titer IFNα neutralizing AAbs can be responsible for the IFNlow phenotype, but high IFNα AAb titers were only observed in 2 of the 41 (~5%) samples evaluated. In contrast to these high titer samples, the other IFNα AAb+ samples (n=26) exhibited lower titers (0.07–0.45 dOD units), bound varying numbers (1 to 11) of IFNα subtypes, and exhibited variable IFN-neutralizing activity (Fig. 5). Despite this variability, AAbs to three or more IFNα subtypes was significantly associated with increased disease activity.
Figure 5.
Diverse SLE immune responses identified by serum IFNαAAbs, anti-cytokine AAbs, and NS values. Unsupervised hierarchical clustering of dODSLE/SDHDC values for SLE serum samples was performed and then NS values (circles) were added to the clustered matrix. SLE sample labels include their IFN status L-, M-, H- for IFNlow, IFNmid and IFNhigh, respectively. SLEDAI scores of 0 are colored white, scores of 1–5 colored light to dark blue, and scores ≥6 are colored red.
Relationship of IFNα AAbs and AAbs to other cytokines
To determine if IFNs are uniquely targeted for AAb generation, the serum samples were screened for AAbs against four additional interferons (IFNω, IFNβ, IFNγ, and IFNλ1) and eleven cytokines that may be involved in SLE pathology (Table S3). With the exception of IFNα2a (49%), anti-cytokine AAbs were found at similar frequencies (2–27%) in patient serum samples compared to IFNα AAbs (5–27%). Of the 41 SLE serum samples screened, AAbs against GMCSF were identified most often (11 of 41 samples, 27%), followed by IL17A (8 of 41 samples, 20%), while AAbs against TNFα, and IL24 were found in 7 of 41 (17%) samples (Table S3). Anti-GMCSF AAbs were observed most often in IFNlow samples, while anti-IL17A and anti-TNFα were observed most often in IFNmid and IFNhigh samples, respectively. Similar to IFNα1 AAbs and IFNα4 AAbs, IFNβ AAbs were rarely observed (5% of samples). AAbs to other IFN species (IFNω, IFNγ, and IFNλ1) were observed more often (12% of samples) than IFNα1, IFNα4, and IFNβ AAbs, but at frequencies lower than AAbs to nine of the twelve IFNαs (IFNα2, IFNα5, IFNα7, IFNα8, IFNα10, IFNα14, IFNα16, IFNα17, and IFNα21).
Global analysis of IFNα AAbs and anti-cytokine AAbs
To characterize the entire distribution of IFNα AAbs and anti-cytokine AAbs found in HDC and SLE patients, dOD values were divided by their SDs, determined from the HDC samples, and subjected to unsupervised hierarchical clustering (Fig. 5). IFNα AAb+ SLE samples (e.g. samples with AAbs to at least one IFNα subtype, defined by mean+3SD) clustered into two groups, separate from most IFNα AAb- and HDC samples. Group A contained five IFNα AAb+ samples, of which three were classified as IFNhigh and two were IFNmid samples. Although these samples contained IFNα AAbs, the NS scores ranged from 0.04 to −1.23, implying the samples do not inhibit IFN bioactivity. The larger IFNα AAb+ group B, contained 26 SLE samples that exhibited higher numbers of IFNα AAbs, higher NS scores (e.g. samples are IFN inhibitory), as well as higher numbers of anti-cytokine AAbs. Although IFNα AAbs appear to be driving the clustering of most samples, three SLE samples in group B are miss-classified as IFNα AAb+, presumably due to anti-GMCSF AAbs, and other anti-cytokine AAbs, that are observed in these samples (Fig. 5). Two of the three miss-classified samples do not exhibit serum IFN activity and are negative for IFNα AAbs (Fig. 4B). However, closer inspection shows one of the samples (L-SLE-111) is positive for AAbs to IFNω and IFNβ (Fig. 5), suggesting IFN-mediated pathogenesis might be induced by distinct IFN subtypes in some patients. Also notable, Group B contains two HDC samples that mimic aspects of SLE patient AAb profiles. Overall, Figure 5 highlights the spectrum of IFNα AAbs and anti-cytokine AAbs found in SLE patient samples.
IFN activity and IFNα AAbs in matched urine samples
For a subset (22 of 41) of SLE patients, urine was collected at the same time as their blood donation, to compare IFN activity and IFNα AAb levels in two distinct body fluids. As observed in SLE serum, IFN activity in SLE urine was significantly higher than in HDC urine (Fig. 6A). However, serum and urine bioactivity did not significantly correlate with one another (Fig. 6B). The urine samples were screened for IFNα AAbs to determine if this could, at least partially, explain the lack of correspondence between IFN activity in urine and blood. The analysis revealed the number of IFNα AAb+ urine and serum samples were identical (16 of 22, 73%) for the matched group (Table S4). However, only three of the 22 samples (14%) exhibited the same IFNα AAb profile in both blood and urine. One contained high titer IFNα AAbs to all 12 IFNαs in both their serum and urine, and the other two patients did not have IFNα AAbs to any IFNα subtype in either their serum or urine (Fig. 6C). The remaining SLE patient samples contained different numbers and/or specificities of the IFNα AAbs in their urine, relative to their serum. For example, six urine samples (22%) contained IFNα AAbs to three or more different IFNαs, while their serum samples contained IFNα AAbs to one or fewer IFNαs (Figs. 6C, 6E). In contrast to serum, where IFNα2 AAbs were the most prevalent AAbs (59.1% serum vs. 31.8% in urine, Table S4), IFNα6 AAbs were most prevalent in the urine samples (50% vs. 9.1% in serum samples). In addition, IFNα1 AAbs were observed at much higher frequency in SLE urine samples (31.8%) than in serum (4.6%) Table S4). Due to the prevalence of IFNα6 AAbs in urine, NS scores were determined using IFNα6, instead of IFNα2 that was used for the serum analysis. Urine samples that contained the greatest numbers of IFNα AAbs generally exhibited the largest NS scores. Together, these results suggest SLE patient immune responses may be different within the kidney and associated tissues, represented by the analysis of urine, compared to those measured in serum.
Figure 6.
Matched SLE patient serum and urine exhibit distinct levels of IFN activity and IFNαAAbs. A, IFN activity in SLE urine compared with HDC. B, IFN activity levels measured in serum and urine do not correlate with one another (r = 0.15, p = n.s.). C, For matched SLE serum and urine samples, the number of IFNα subtype AAbs in each sample was plotted on the y axis. D, Percentage of IFNαAAbs to each IFNα subtype in matched serum and urine samples (n=22). E, Hierarchical clustering of dODSLE/SDHDC values for SLE patient and HDC urine samples, and matched serum samples, performed as described in Figure 5.
Discussion
The goal of this study was to provide a more granular analysis of serum IFN levels in SLE patients, characterize the impact of IFN AAbs on serum IFN bioactivity and disease activity, and evaluate anti-cytokine AAbs to identify other signaling pathways operating in our patient population. Towards these goals, the analysis reaffirmed that high serum IFN activity levels are observed in a majority (62% in this study) of SLE patients, relative to HDCs 13, 14. African American (AA) patients were over-represented in the IFNhigh (70% AA) group compared to Caucasian (CA) SLE patients (30%). High IFN levels were previously associated with AAbs to nucleic acid, including anti RNA and dsDNA 38. Consistent with these findings, serum IFN (r = 0.91, p = 0.002) and dsDNA AAb (r = 0.72, p = 0.02) levels strongly correlated with disease activity (SLEDAI) in the IFNhigh group, but robust correlations were not found in the IFNmid and IFNlow groups. Despite the lack of correlations with IFN levels, dsDNA AAbs were also significantly increased in the IFNlow group. In contrast, significant levels of anti-H2B AAbs were only observed in the IFNlow group. Thus, the highest overall AAb levels are found in IFNlow patients, while dsDNA levels, but not H2B AAbs, strongly correlate with serum IFN activity and SLEDAI for the IFNhigh patients.
IFNlow patients may not produce excessive IFN, or their detectable IFN levels might be reduced by neutralizing IFN AAbs. To address this question, we first focused on IFN AAbs to all IFNα subtypes. The analysis revealed IFNα AAbs are found in SLE sera at higher frequencies (68%) than previously reported 24. More importantly, 32% of the samples with IFNα AAbs to three or more subtypes exhibited lower serum IFN activity levels and significantly higher disease activity than those with two or less. These data confirm that endogenous IFNα AAbs can be responsible for reduced serum IFN activity in SLE patient samples. However, in contrast to the work of Morimoto et al., who suggested endogenous IFNα AAb-mediated blockade of IFN activity is responsible for lower disease activity 24, we found potent neutralizing IFN AAbs to be associated with higher SLEDAI scores. We did identify serum from some patients with lower SLEDAI scores that partially neutralized exogenous IFNα2a activity, based on NS scores. However, the IFNα AAb titers in these samples, were not consistent with the extent of IFN neutralization observed. In fact, we observed two samples that strongly neutralized IFNα2 activity, but did not contain any IFNα AAbs by dELISA. A possible explanation is that IgM and/or IgA IFN AAbs are responsible for the observed IFN neutralization, which were not measured in our study.
In light of discontinued and ongoing clinical trials of IFN blockade therapies 39, our IFN AAb data suggests endogenous IFN AAbs cannot be used to support or explain anti-IFNα therapies, or their apparent lower responses, relative to anti-IFNAR1 (Anifrolumab) therapy40. One reason is that most endogenous IFN AAbs, relative to anti-IFNα drug therapy, exhibit weak IFN neutralizing potential and appear to be generated over longer time periods, compared to bolus injections of drugs. It is possible that some endogenous non-neutralizing IFN AAbs might compete with anti-IFN drugs, limiting their efficacy, but this is highly speculative and many other mechanistic arguments for why receptor targeting may be more appropriate than targeting the 16-member type-I IFN family have been previously outlined 41. Our data also emphasize that IFNα AAbs may render traditional serum IFNα bioactivity assays unreliable in identifying patients in whom (multiple) type-1 IFNs may be playing a significant role in lupus disease activity.
Although IFNα AAbs rarely potently inhibited IFN biological activity, our data suggests measuring IFNα AAbs may provide an additional marker of patient disease activity. In addition, they may help us better understand the IFNlow patient phenotype. For example, IFNlow patients with multiple IFN AAbs in their serum have clearly been exposed to IFNs. Thus, in a variety of patient studies and clinical trial designs, analysis of IFN AAbs may provide a unique “history” of past IFN exposures. Thus, our data suggests many IFNlow patients may have experienced repeated IFN exposures that are pathogenic and associated with IFNα AAb development. In addition, we also find IFNlow patients with no IFN AAbs and yet they exhibit high SLEDAI scores. We anticipate that through further gene expression and serological studies we will be able to further improve our understanding of IFN-independent and IFN-dependent disease to accurately identify SLE patients that will optimally respond to anti-IFNAR1 therapy.
The high frequency of IFNα2a AAbs we observe in SLE patients has previously been observed (41–44% IFNα2a AAb+) in patients treated with IFNα2a as an anti-cancer therapeutic 42, 43. In these studies, the high incidence of IFNα2a AAbs was linked to aggregated protein in the drug preparations. In autoimmune settings, such as SLE, it is possible that aggregated endogenous IFNα2a could be released from apoptotic cells. Interestingly, the highest incidence of IFNα2a AAbs (44%) were found in renal cell carcinoma patients, compared to 4% in leukemia patients 42. Since IFN activity is associated with kidney damage in SLE 44, we evaluated IFN activity and IFNα AAbs in urine, which is a body fluid that could potentially report on local kidney immune responses. Although IFN activity was elevated in SLE patient urine, relative to HDCs, urine and serum IFN activity did not correlate with one another. It was equally surprising that when IFNα AAb levels were high in serum they were low in urine and vice versa. In addition to differences in the overall frequency of IFNα AAbs in serum and urine samples, the frequency of AAbs to specific IFNα subtypes were different. In particular, AAbs to IFNα1, which were rarely observed in patient serum (5% of samples), were observed in 32% of SLE patient urine samples. Likewise, AAbs to IFNα6 are observed in 9% of matched serum samples, but are observed in 50% of SLE urine samples. Overall, the differences in IFN and IFNα AAbs in urine versus serum, suggest that local immune responses in the kidney may be distinct from serum. However, this serum/urine distinction may be lost in patients with extreme glomerular damage, such as observed for one patient with lupus nephritis (L-SLE-116) that exhibited high-titer neutralizing IFNα AAbs to all IFNα subtypes in both their serum and urine.
The data argue that testing SLE serum for AAbs to multiple cytokines can potentially identify signaling pathways involved in SLE disease pathogenesis. Interestingly, the cytokines most often targeted by AAbs in our patient samples are often produced by pathogenic Th17 cells, including IL17A, GMCSF, and TNFα 45. IL17 has been found to be increased in SLE patient serum and could reflect alterations in Th1, Th2, Th17 cell numbers and function 46, 47. Additional studies show IL17 promotes AAb production through B-cell differentiation, class switch recombination, and synergizes with IFNα to produce BLyS 48, 49 GMCSF is known to activate monocytes and DC, resulting in enhanced antigen presentation, phagocytosis, and inflammatory cytokine production 50. In mice, T-cell and renal cell produced GMCSF has been associated nephritis, 51. These observations suggest sensitive measurement of AAbs against IFNs and cytokines may be useful in understanding SLE heterogeneity and ultimately aide in therapy decisions.
Supplementary Material
Acknowledgements
We thank Gilles Uzé for HL116 cells, Jeffrey C. Edberg and Neva Garner for assisting in sample procurement, and S. Louis Bridges Jr. and Robert P. Kimberly for encouragement and helpful discussions.
Funding
This work was supported by a Novel Research Award from the Lupus Research Alliance, #336550
Footnotes
Declaration of conflicting interests
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778–809, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, et al. Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure. 1996;4(12):1453–63. [DOI] [PubMed] [Google Scholar]
- 4.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. [DOI] [PubMed] [Google Scholar]
- 6.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker AM, Dao KH, Han BK, Kornu R, Lakhanpal S, Mobley AB, et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS One. 2013;8(6):e67003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng X, Reder NP, Yanamandala M, Hill A, Franek BS, Niewold TB, et al. Type I interferon signature is high in lupus and neuromyelitis optica but low in multiple sclerosis. Journal of the neurological sciences. 2012;313(1–2):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiche L, Jourde-Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, et al. Modular Transcriptional Repertoire Analyses of Adults With Systemic Lupus Erythematosus Reveal Distinct Type I and Type II Interferon Signatures . Arthritis & rheumatology (Hoboken, NJ). 2014;66(6):1583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36(8):481–90. [DOI] [PubMed] [Google Scholar]
- 11.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. The Journal of Experimental Medicine. 2017;214(5):1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dall’era MC, Cardarelli PM, Preston BT, Witte A, Davis JC, Jr. Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis. 2005;64(12):1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25(4):401–6. [DOI] [PubMed] [Google Scholar]
- 15.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–503. [DOI] [PubMed] [Google Scholar]
- 16.Castellano G, Cafiero C, Divella C, Sallustio F, Gigante M, Pontrelli P, et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res Ther. 2015;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney international. 2010;77(10):848–54. [DOI] [PubMed] [Google Scholar]
- 18.Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, et al. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009;183(10):6831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairhurst AM, Mathian A, Connolly JE, Wang A, Gray HF, George TA, et al. Systemic IFN-alpha drives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008;38(7):1948–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lash AA, Lusk B. Systemic lupus erythematosus in the intensive care unit. Crit Care Nurse. 2004;24(2):56–60, 2–5. [PubMed] [Google Scholar]
- 21.Oke V, Gunnarsson I, Dorschner J, Eketjäll S, Zickert A, Niewold TB, et al. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Research & Therapy. 2019;21(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CC, Kao AH, Manzi S, Ahearn JM. Biomarkers in systemic lupus erythematosus: challenges and prospects for the future. Therapeutic advances in musculoskeletal disease. 2013;5(4):210–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–16. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto AM, Flesher DT, Yang J, Wolslegel K, Wang X, Brady A, et al. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(8):2407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panem S, Check IJ, Henriksen D, Vilcek J. Antibodies to alpha-interferon in a patient with systemic lupus erythematosus. J Immunol. 1982;129(1):1–3. [PubMed] [Google Scholar]
- 26.Slavikova M, Schmeisser H, Kontsekova E, Mateicka F, Borecky L, Kontsek P. Incidence of autoantibodies against type I and type II interferons in a cohort of systemic lupus erythematosus patients in Slovakia. J Interferon Cytokine Res. 2003;23(3):143–7. [DOI] [PubMed] [Google Scholar]
- 27.Sibbitt WL Jr., Gibbs DL, Kenny C, Bankhurst AD, Searles RP, Ley KD. Relationship between circulating interferon and anti-interferon antibodies and impaired natural killer cell activity in systemic lupus erythematosus. Arthritis Rheum. 1985;28(6):624–9. [DOI] [PubMed] [Google Scholar]
- 28.von Wussow P, Jakschies D, Hartung K, Deicher H. Presence of interferon and anti-interferon in patients with systemic lupus erythematosus. Rheumatology international. 1988;8(5):225–30. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Tatouli IP, Rosen LB, Hasni S, Alevizos I, Manna ZG, et al. Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis & rheumatology (Hoboken, NJ). 2016;68(7):1677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192(12):5459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crow MK, Olferiev M, Kirou KA. Type I Interferons in Autoimmune Disease. Annual review of pathology. 2019;14:369–93. [DOI] [PubMed] [Google Scholar]
- 32.Ronnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23(2):113–21. [DOI] [PubMed] [Google Scholar]
- 33.Thorlacius GE, Wahren-Herlenius M, Ronnblom L. An update on the role of type I interferons in systemic lupus erythematosus and Sjogren’s syndrome. Curr Opin Rheumatol. 2018;30(5):471–81. [DOI] [PubMed] [Google Scholar]
- 34.Kuruganti S, Accavitti-Loper MA, Walter MR. Production and characterization of thirteen human type-I interferon-alpha subtypes. Protein Expr Purif. 2014;103:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande A, Putcha BD, Kuruganti S, Walter MR. Kinetic analysis of cytokine-mediated receptor assembly using engineered FC heterodimers. Protein Sci. 2013;22(8):1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logsdon NJ, Deshpande A, Harris BD, Rajashankar KR, Walter MR. Structural basis for receptor sharing and activation by interleukin-20 receptor-2 (IL-20R2) binding cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morpheus, https://software.broadinstitute.org [Available from: Morpheus, https://software.broadinstitute.org. [Google Scholar]
- 38.Weckerle CE, Franek BS, Kelly JA, Kumabe M, Mikolaitis RA, Green SL, et al. Network analysis of associations between serum interferon-alpha activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 2011;63(4):1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalunian KC. Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells? Lupus. 2016;25(10):1097–101. [DOI] [PubMed] [Google Scholar]
- 40.Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an Anti-Interferon-alpha Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis & rheumatology (Hoboken, NJ). 2017;69(2):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng L, Oganesyan V, Wu H, Dall’Acqua WF, Damschroder MM. Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-alpha receptor 1 antibody. mAbs. 2015;7(2):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itri LM, Campion M, Dennin RA, Palleroni AV, Gutterman JU, Groopman JE, et al. Incidence and clinical significance of neutralizing antibodies in patients receiving recombinant interferon alfa-2a by intramuscular injection. Cancer. 1987;59(3 Suppl):668–74. [DOI] [PubMed] [Google Scholar]
- 43.Ronnblom LE, Janson ET, Perers A, Oberg KE, Alm GV. Characterization of anti-interferon-alpha antibodies appearing during recombinant interferon-alpha 2a treatment. Clin Exp Immunol. 1992;89(3):330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica. 2011;6(4):330–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Qian Y, Kang Z, Liu C, Li X. IL-17 signaling in host defense and inflammatory diseases. Cellular & Molecular Immunology. 2010;7(5):328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muhammad Yusoff F, Wong KK, Mohd Redzwan N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity. 2020;53(1):8–20. [DOI] [PubMed] [Google Scholar]
- 47.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(2):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107(32):14292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez P, Rodriguez-Carrio J, Caminal-Montero L, Mozo L, Suarez A. A pathogenic IFNalpha, BLyS and IL-17 axis in Systemic Lupus Erythematosus patients. Scientific reports. 2016;6:20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100(3):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. Journal of the American Society of Nephrology : JASN. 2002;13(2):350–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.