Abstract
Background:
More than 30 million individuals participate in marathon running every year worldwide. As the popularity of marathon running continues to increase, it is essential for the purposes of injury prevention to understand the effects of marathon running on the knee cartilage.
Purpose:
To investigate the immediate effects of marathon running on knee articular cartilage and to determine the relationship between body mass index and cartilage biochemical composition.
Study Design:
Descriptive laboratory study.
Methods:
T2-relaxation magnetic resonance imaging (MRI) of knees in 18 nonprofessional marathoners (mean age, 35.6 ± 6.4 years) was performed before and after a full-length marathon. Three-dimensional models of the knee articular cartilage were reconstructed and divided into different regions of interest. The 3-dimensional models were then applied to corresponding T2-relaxation MRI maps to calculate T2 values in each region of interest. The mean values of the T2-relaxation times in each region of interest before and after the marathon were compared by use of the paired Student t test. The Pearson correlation coefficient between T2 change and runner body mass index (BMI) was calculated.
Results:
Postmarathon T2-relaxation times were significantly higher than premarathon values for patellofemoral cartilage (32.6 ± 12.1 vs 34.1 ± 10.9 ms; P < .01) and medial tibial cartilage (35.6 ± 11.7 vs 34.6 ± 12.0 ms; P = .01). The greatest increase was observed in the anterior part of the medial tibial cartilage. No statistically significant changes were seen in the T2-relaxation times of the lateral tibial and femoral cartilage. Postmarathon T2-relaxation elevation in the anteromedial knee tibiofemoral joint cartilage strongly correlated with body weight (R = 0.6746; P = .03) and BMI (R = 0.6989; P = .001). Changes in T2-relaxation times did not correlate with marathon time, height, age, or sex in any regions of interest.
Conclusion:
Marathon running leads to immediate postmarathon elevated T2-relaxation values within knee articular cartilage, suggesting biochemical content alteration. Additionally, runners with higher BMI may have greater changes in cartilage biochemical composition after a marathon. Further studies should investigate whether these changes are sustained over time to determine the relationship between immediate biochemical changes in cartilage composition and cartilage degeneration.
Clinical Relevance:
Runners with a higher BMI may carry a higher risk of anteromedial tibiofemoral cartilage degeneration compared with runners with lower BMI.
Keywords: articular cartilage, marathon, T2 mapping, knee
More than 30 million individuals participate in marathon running every year worldwide.15 Running is beneficial, as it improves fitness, helps with weight loss, and decreases the risk of cardiovascular disease and diabetes mellitus.24 Marathon running involves repetitive loading of the knee joint. These compressive loads are absorbed and dissipated by the viscoelastic articular cartilage. However, excessive repetitive forces may increase the risk of cartilage degeneration. Highly repetitive mechanical loading has been shown to result in increased chondrocyte death,12 which will quickly lead to molecular-level cartilage dysregulation, degradation, and eventually osteoarthritis (OA) of the knee.13 A large-scale longitudinal follow-up study indicated that recreational runners who ran less than 10 km per week had a low risk (3.5%) of developing knee OA. However, competitive runners have a much higher risk (13.5%) of OA.1 In particular, runners with higher body mass index (BMI) have increased mechanical loads and stress on their knees.10 As the popularity of marathon running continues to increase, it is essential for the purposes of injury prevention to acquire an accurate understanding of the effects of marathon running on knee cartilage.
Magnetic resonance imaging (MRI) is commonly used in the evaluation of meniscal and cartilage degeneration after marathon running. Krampla et al15,16 evaluated the knee MRI scans of 8 nonprofessional runners before, immediately after, and 6-8 weeks after a Vienna city marathon. At 8 weeks after the race, signs of progressive OA were noted in 1 participant with a preexisting grade III meniscal lesion. Increased meniscal signal alteration and minor signal changes in the bone marrow were observed in other participants immediately after the race, and these changes were transitory. Mosher et al21 reported a measurable decrease in cartilage thickness in their study. The effects of marathon running on the morphologic features of the knee cartilage are not consistent in the literature; this may be because different imaging modalities have been used. Therefore, an experimental design using a more sensitive technique for quantifying the cartilage quality of the knee may provide more information.
Quantitative MRI techniques such as T2 mapping,20 T1ρ,3 and delayed gadolinium-enhanced MRI of the cartilage4 allow detection of biochemical changes. T2-mapping MRI measures T2-relaxation times, which are affected by collagen structure and hydration owing to dipolar interactions.22 Several studies have demonstrated that elevated T2 values are related to the development of OA.7,18 Although several researchers have used T2 mapping in orthopaedic studies, only a few have used it to evaluate knee joint cartilage in athletes. Changes in knee articular cartilage in runners are also affected by individual BMI and marathon time, as a higher BMI and a shorter marathon time indicate a higher degree of repetitive impact loading during running. However, variations in loading intensity during marathon running has not been considered in previous studies.
The objective of this study was to investigate (1) the immediate effects of marathon running on knee cartilage and (2) the relationship between joint loading intensity and the biochemical composition of the knee articular cartilage. T2 values of the knee joint cartilage, before and after participants ran a marathon, were measured through use of a high-resolution structure scan and T2 mapping. Body weight, BMI, and marathon running time were included as parameters contributing to loading intensity. We hypothesized that running a marathon would result in an elevation of T2 values of the knee articular cartilage and that higher T2 values would correlate with shorter marathon time, higher body weight, and higher BMI.
Methods
Participants
The study was approved by the institutional review board of the participating institutions. Written informed consent was obtained from all participants involved in this study. We recruited 18 healthy, nonprofessional runners who were participating in the Shanghai International Marathon on November 11, 2018 and November 17, 2019 (mean ± SD age, 35.6 ± 6.4 years; height, 1.71 ± 0.06 m; weight, 65.4 ± 7.4 kg; BMI, 22.2 ± 2.2 kg/m2; 16 males; 2 females). Body weight and height were obtained from all the participants. The inclusion criteria were as follows: (1) those who had not participated in other marathons in the past 4 months, (2) those without knee pain in the past 6 months, (3) those with no clinical findings of knee joint pathology, and (4) those with no history of lower limb surgery. All of the participants ran at least 20 km per week 2 months before the marathon. MRI scans of the dominant lower limb were performed in all participants (17 left knees, 1 right knee). All participants finished the Shanghai International Marathon, and their completion times were recorded.
Imaging Protocols and T2 Map Calculation
The dominant knee of each participant underwent two 3.0-T MRI scans with an 8-channel knee coil (Magnetom Verio; Siemens). A premarathon MRI scan was conducted within 24 hours before the race. To reduce the effects of other activities on the knee cartilage, we obtained a postmarathon MRI scan within 10 hours after the race. Before each MRI, all participants were asked to rest in a relaxed sitting position for 1 hour. All participants underwent an MRI scan in the supine position with their knee in extension within the knee coil. Sandbags and sponges were used to stabilize the knee to prevent any motion artifacts. Two sequences were performed: a T1-weighted fat-suppression sequence (repetition time [TR], 17 ms; echo time [TE], 6.4 ms; slice thickness, 0.8 mm; field of view, 150 mm; flip angle, 12°) and a multiple-TE fast-spin echo sequence (TR, 2620 ms; TE, 11.7, 23.4, 35.1, 46.8, 58.5, 70.2, 81.9, 93.6, 105.3, 117 ms; slice thickness, 3 mm; field of view, 160 mm; flip angle, 180°). The T1-weighted fat-suppression sequence was used to reconstruct a 3-dimensional (3D) cartilage model for each participant’s knee. The multiple-TE fast-spin echo sequence was used to evaluate T2 maps. The T2 value for each voxel was computed with the function , where S 0 is the signal intensity at zero TE.
Cartilage Segmentation and Image Processing
The 3D knee cartilage model reconstruction was performed by use of Amira 6.7.0 (Thermo Fisher Scientific). A semiautomatic segmentation watershed function was used for cartilage segmentation. The segmentation results were exported as triangulated surface models in stereolithography (STL) format. After cartilage 3D modeling, several regions of interest (ROIs) were manually divided as follows. The articular cartilage of the patellofemoral joint (PFJ) was divided into 3 ROIs: lateral patella, medial patella, and trochlea. The tibiofemoral joint (TFJ) cartilage was divided into 4 ROIs: lateral femoral condyle (LFC), medial femoral condyle (MFC), lateral tibial plateau, and medial tibial plateau (MTP). Each TFJ cartilage region was divided into 4 sub-ROIs: cartilage contacting with the anterior meniscus, cartilage contacting with the middle meniscus, cartilage contacting with the posterior meniscus, and cartilage not contacting with the meniscus (Figure 1). Image processing was performed with an in-house self-developed script in MATLAB (The MathWorks). The 3D cartilage models were transformed into volumetric binary masks for the calculation of T2 values in each ROI and sub-ROI.
Figure 1.
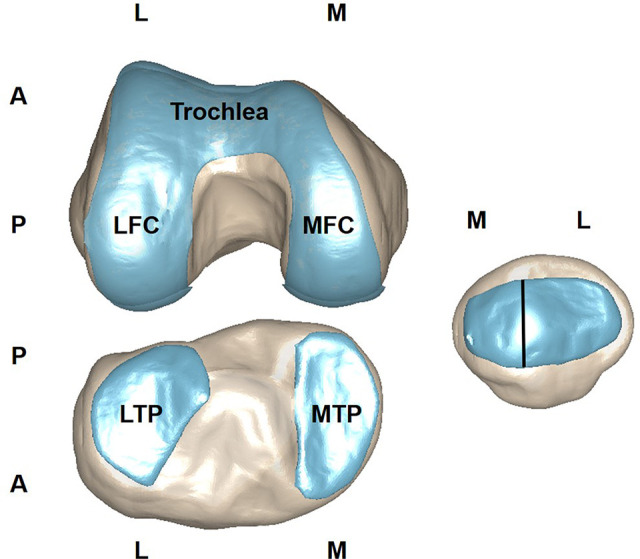
Knee joint cartilage segments assessed in this study. LFC, lateral femoral condyle; LTP, lateral tibial plateau; MFC, medial femoral condyle; MTP, medial tibial condyle; A, anterior; P, posterior; M, medial; L, lateral.
Statistical Analysis
All statistical analyses were performed with SPSS software (version 21.0; IBM Corp). The mean and standard deviation were calculated for T2 values in each ROI and sub-ROI. A paired t test was used to compare T2 values in each ROI before and after the participants ran the marathon. Pearson correlation was used to determine the relationship between T2 values and weight, BMI, and running time of each marathoner. Linear regression analyses were performed to determine the relationships between T2-value alteration and body weight, BMI, and running time. For all statistical analyses, the significance level was set at .05.
Results
T2-Value Changes in the PFJ Cartilage
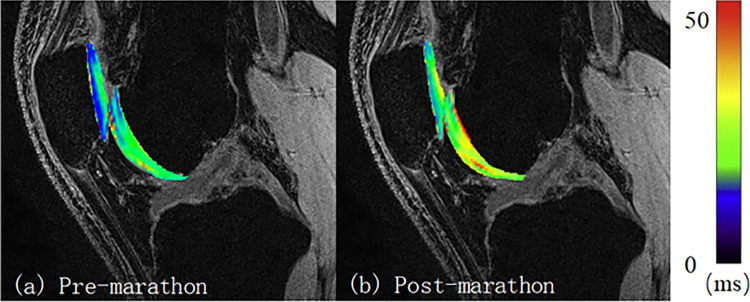
The PFJ cartilage showed significantly higher T2 values (32.6 ± 12.1 vs. 34.1 ± 10.9 ms; P < .0001) after the marathon. At the patella cartilage, both the medial and the lateral patellar cartilage showed significantly higher T2 values after the marathon versus before, with an increase of 3.5 milliseconds (+8.2%; P < .0001) and 3.3 milliseconds (+5.6%; P < .0001), respectively. At the trochlea, the cartilage T2 values increased by 1.9 milliseconds (+5.8%; P = .0011) after the marathon (Table 1, Figure 2).
TABLE 1.
Premarathon and Postmarathon T2 Values for Each Cartilage Region of Interest and the Rate of Changea
| T2 Value, Mean ± SD, ms | ||||
|---|---|---|---|---|
| Region of Interest | Premarathon | Postmarathon | Change, % | P Value |
| Patellofemoral joint | ||||
| Medial patella | 31.8 ± 10.8 | 34.4 ± 10.2 | 8.2 | < .0001 |
| Lateral patella | 32.0 ± 11.8 | 33.8 ± 11.1 | 5.6 | .0001 |
| Trochlea | 33.2 ± 10.9 | 34.1 ± 11.3 | 5.8 | .0011 |
| Tibiofemoral joint | ||||
| Medial tibial plateau | 34.6 ± 12.0 | 35.6 ± 11.7 | 2.9 | .0130 |
| Lateral tibial plateau | 34.9 ± 11.6 | 34.4 ± 11.7 | –1.4 | .1369 |
| Medial femoral condyle | 35.5 ± 11.7 | 35.2 ± 12.3 | –0.8 | .3309 |
| Lateral femoral condyle | 35.2 ± 11.7 | 35.9 ± 12.4 | 2.0 | .0531 |
aA P value less than .05 (given in boldface) indicates a statistically significant difference between the premarathon and postmarathon T2 values.
Figure 2.
T2 map comparison of the patellofemoral joint for 1 participant (male; 31 years old; weight, 70 kg; body mass index, 23.4 kg/m2).
T2-Value Changes in the TFJ Cartilage
The T2 value of the MTP was significantly (>1 ms) higher after the marathon versus before (+2.9%; P = .0130). No statistically significant changes were noted in the T2 values of the MFC or LFC cartilage (Table 1).
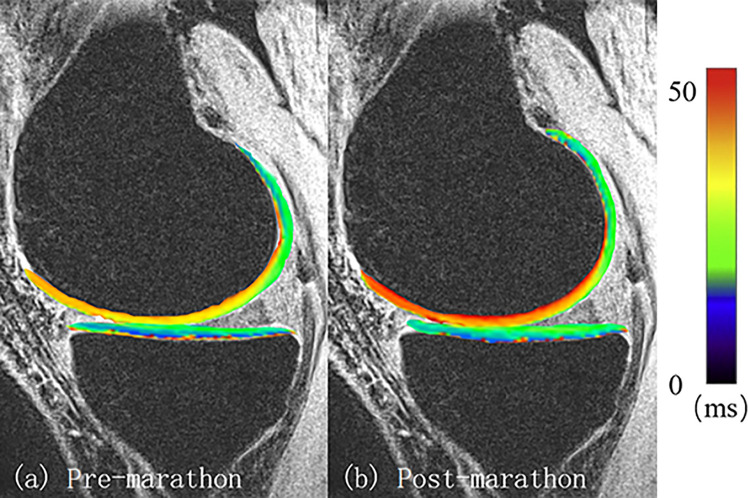
The sub-ROI analysis showed detailed T2-value changes after the marathon. With regard to the medial TFJ cartilage, only the T2 values of the anterior parts of the MTP and MFC increased significantly, by 3.0 milliseconds (+9.2%; P = .0001) and 3.0 milliseconds (+9.2%; P = .0001), respectively. The T2 values of the middle MTP or posterior MTP were not significantly different after participants ran the marathon. Similar results were found in the LFC cartilage, where the T2 values of the anterior part of the LFC cartilage were significantly higher (+6.4%; P = .0038) after the marathon, with no difference in the middle or posterior compartments (Table 2, Figure 3). No significant differences in the T2 values were found in any other cartilage compartments.
TABLE 2.
Premarathon and Postmarathon T2 Values for Each Subregion of Interest of the Tibiofemoral Joint and the Rate of Changea
| T2 Value, Mean ± SD, ms | ||||
|---|---|---|---|---|
| Subregion of Interest of Tibiofemoral Joint | Premarathon | Postmarathon | Change, % | P Value |
| Medial tibial plateau | ||||
| Anterior | 32.7 ± 11.2 | 35.7 ± 12.2 | 9.2 | .0001 |
| Middle | 35.8 ± 12.5 | 35.4 ± 11.7 | –1.1 | .8034 |
| Posterior | 35.3 ± 11.6 | 35.9 ± 11.7 | 1.7 | .8006 |
| Medial femoral condyle, anterior | 32.7 ± 11.2 | 35.7 ± 12.3 | 9.2 | .0001 |
| Lateral femoral condyle, anterior | 32.7 ± 10.7 | 34.8 ± 12.1 | 6.4 | .0038 |
aA P value less than .05 (given in boldface) indicates a statistically significant difference between the premarathon and postmarathon T2 values. In the anterior medial part of the tibiofemoral cartilage, elevated T2 values were found after participants had run the marathon. The anterior medial part of the tibiofemoral cartilage contributed the greatest elevation of T2 values.
Figure 3.
T2 map comparison of the tibiofemoral joint for 1 participant (female; 30 years old; weight, 52 kg; body mass index, 21.6 kg/m2).
Relationship Between Marathon Loading Intensity and T2-Value Changes
The change in T2 values in the anterior part of the MTP cartilage after the marathon strongly correlated with body weight (R = 0.6746; P = .0324) and BMI (R = 0.6989; P = .0010). The change in T2 value of the anterior part of the MFC cartilage was also significantly correlated with BMI (R = 0.7089; P = .0217). Changes in T2 values did not correlate with marathon time, height, age, or sex in any of the ROIs.
Discussion
The most important finding of this study was that biochemical changes in the knee joint cartilage were seen after running a marathon, as demonstrated by increased T2-relaxation times compared with before the marathon. Specifically, in the PFJ cartilage, higher T2 values were found in both the patellar and the trochlear compartments. In the TFJ cartilage, sub-ROI analysis demonstrated that the anterior parts of the MFC and MTP had a higher percentage elevation of T2 after the marathon. In addition, changes in T2 values in these sub-ROIs after the marathon correlated strongly with body weight and BMI. To the best of our knowledge, this is the first study that has quantitatively evaluated the effects of marathon running on different functional ROIs of the knee articular cartilage and reported a strong relationship between alterations in biochemical composition and BMI in this setting.
The most common knee injury in a marathon is patellofemoral pain syndrome.8 In this study, T2 values increased by 10% in both the medial and lateral compartments of the patellar cartilage, demonstrating an immediate adverse effect of running a marathon on the PFJ cartilage, which is in accordance with previous studies. Luke et al18 found that T2 values increased by 5.0% (premarathon, 31.7 ms; postmarathon, 33.3 ms; P = .010) in the patellar cartilage and increased by 6.1% (premarathon, 32.6 ms; postmarathon, 34.6 ms; P = .008) in the trochlea after participants ran a marathon. The amplitude of elevation was larger in the current study, and the reason may be that we performed MRI within 10 hours after the marathon, whereas in the previous study the scan was performed within 48 hours. A longer resting time may lead to a decreased T2 value in the knee joint cartilage. Increased T2 values in the PFJ cartilage can reveal a deterioration in cartilage quality after marathon running.
At the TFJ cartilage, elevated T2 values were found in the MFC and MTP cartilage, suggesting glycosaminoglycan (GAG) content change or altered collagen fibril arrangement in these regions. Luke et al18 reported the highest T2-value elevation in the medial compartment of the TFJ cartilage after participants ran a marathon, suggesting a higher risk of cartilage degeneration in this region. In a recent study assessing articular cartilage in young professional soccer players, the investigators found that after daily routine soccer activities, the greatest changes in T2 signal were located in the MFC cartilage (elevated by 5.0% compared with 0.3%-4.1% in other regions).25 In the current study, subregion analysis found that the anterior part of the MFC and MTP cartilage contributed most to T2 elevation in medial TFJ cartilage. Kozanek et al14 measured knee joint condylar movement during the stance phase of a gait using a dual fluoroscopic imaging system and found anterior movement of the femur between heel strike and contralateral toe-off, which may explain the greater loading intensity during stance phase in the anterior part of the MTP cartilage. These results may explain why the anterior medial part of the TFJ cartilage had the highest T2-value elevation after the marathon in our study participants.
In the current study, the correlation between T2 elevation and BMI was found in the anterior part of the MFC and MTP cartilage. Dunn et al7 found that the T2 values in the MTP were elevated by 8.1% and 12.0% in patients with mild and severe OA, respectively (in comparison with healthy participants), indicating that T2-relaxation times could reveal the severity of OA. According to the linear regression results of the current study, a runner with a BMI of 23.5 or higher would experience a postmarathon T2 elevation that is comparable with values found in patients with OA (Figure 4), which indicates similar biochemical composition changes. Because this study did not include a histological assessment of knee cartilage, the exact biochemical content within the knee cartilage is unknown. However, it is unlikely that the knee articular cartilage degenerated to severe OA status immediately after the marathon. Several studies have demonstrated that T2 values were correlated with GAG content and collagen fibril arrangement.22,23 That different events led to similar T2-value changes may explain this. In this study, marathon running resulted in changes in the physiologic status of normal knee cartilage such that it was similar to degenerative cartilage.
Figure 4.
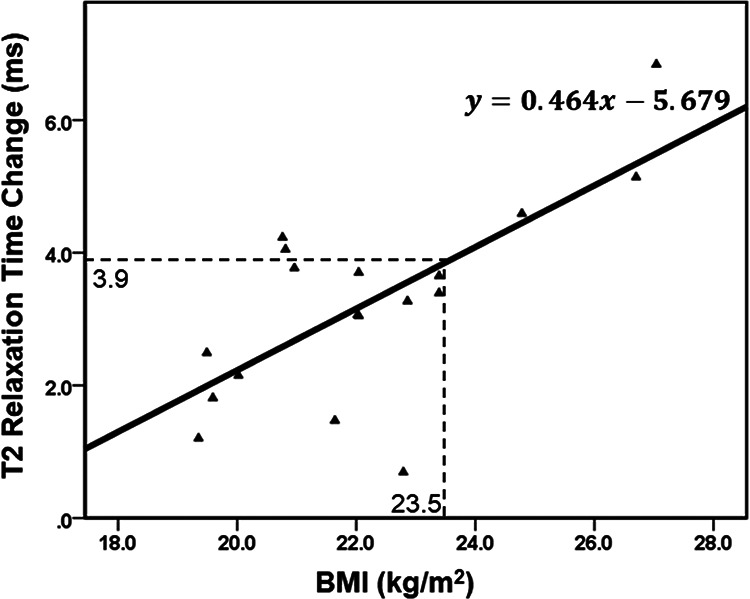
Linear regression curve of T2-relaxation time change against body mass index (BMI). Results show that the change in T2-relaxation time in the anterior part of the medial tibial condyle significantly correlated with BMI.
Higher BMI is associated with higher absolute tibiofemoral compressive forces, as demonstrated by Harding et al.9 Because chondrocyte metabolism is related to the mechanical environment, hyperphysiologic magnitudes of mechanical loading on the cartilage can lead to decreased synthesis of extracellular matrix components, increased production of proinflammatory cytokines, and potentially cell death.5,11 Collins et al6 found that participants with a high BMI had higher tibiofemoral T1ρ relaxation times after a 20-minute treadmill walk. These results suggest that the higher joint loading intensity generated by a higher BMI has a substantial impact on the biochemical composition of knee joint cartilage and may subsequently lead to OA. According to early research on marathon runners, nearly 50% of the adult male marathon participants in the United States were overweight.19 Because BMI correlated strongly with postmarathon T2-value elevation, overweight people (BMI larger than 23.5 according to the current study) are not recommended to engage in marathon running.
T2 mapping has been validated to be a useful tool for the quantification of microstructural changes in the cartilage.26 The fluid within the knee articular cartilage may be pumped out due to repetitive loading during marathon running, which may lead to elevated T2 values. Regional increases in T2-relaxation times in cartilage have been shown to be associated with cartilage matrix damage, particularly the loss of collagen integrity and increase in water content.2 Most studies related to this research have been conducted in patients with OA or other orthopaedic diseases. Liebl et al17 collected 130 knee MRI scans and found that volunteers with higher baseline T2 values were more likely to develop knee OA. This finding suggests that T2 mapping could serve as an early detector of OA. Medial knee OA is a common type of OA due to higher mechanical loading on the medial knee joint. However, similar T2-value alteration was found in our study participants after marathon running, which indicates that marathon running can alter the physiologic status of normal knee cartilage so it is more similar to degenerative cartilage. Morphological MRI and daily activity may also be considered together with T2 mapping for the detection of early OA. Further studies could investigate the relationship between a higher percentage elevation of T2 after a marathon and the onset of OA.
Several limitations of this study should be noted. First, no long-term longitudinal data were included in the current research. Thus, it is unknown whether T2 values will return to baseline levels or remain persistently elevated. A longer term follow-up could show the relationship between T2-relaxation times, repetitive loading, and knee OA. Second, no histological evaluation was included in this research. However, Nishioka et al23 demonstrated that T2-value changes were correlated with GAG and cartilage fibril arrangement. Third, body fat measurements were not included in this study. Marathoners with a high BMI may be muscular without having a particularly high body fat percentage. Another limitation was that the participants’ weight and height were obtained from the runners, which may be a possible source of error.
Conclusion
Marathon running led to an immediate postmarathon elevation of T2-relaxation values within the PFJ and TFJ articular cartilage, suggesting biochemical content alteration. Also, runners with a higher BMI may carry a higher risk of anteromedial tibiofemoral cartilage degeneration compared with runners with lower BMI. To the best of our knowledge, this is the first study evaluating the correlation between changes in T2 value after marathon running and BMI. Further studies should investigate whether these changes are sustained over time to determine whether marathon running may lead to early cartilage degeneration in the knee.
Acknowledgment
The authors thank all those who participated in this study, particularly radiologist Qi Sun (Shanghai Ninth People’s Hospital, Shanghai, China).
Footnotes
Final revision submitted february 18, 2020; revised accepted march 25, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This project was sponsored by the National Natural Science Foundation of China (31771017, 31972924), the Science and Technology Commission of Shanghai Municipality (16441908700), the Innovation Research Plan supported by Shanghai Municipal Education Commission (ZXWF082101), the National Key R&D Program of China (2017YFC0110700, 2018YFF0300504, 2019YFC0120600), and the Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018QNA06, YG2017MS09). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Ethics Committee of Shanghai Sixth People’s Hospital (approval No. 2016-111).
References
- 1. Alentorn-Geli E, Samuelsson K, Musahl V, Green CL, Bhandari M, Karlsson J. The association of recreational and competitive running with hip and knee osteoarthritis: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(6):373–390. [DOI] [PubMed] [Google Scholar]
- 2. Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13(7):76–86. [DOI] [PubMed] [Google Scholar]
- 3. Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19(7):781–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin N Am. 2009;47(4):675–686. [DOI] [PubMed] [Google Scholar]
- 5. Coleman MC, Ramakrishnan PS, Brouillette MJ, Martin JA. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheum. 2016;68(3):662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins AT, Kulvaranon ML, Cutcliffe HC, et al. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res Ther. 2018;20(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fredericson M, Misra AK. Epidemiology and aetiology of marathon running injuries. Sports Med. 2007;37(4-5):437–439. [DOI] [PubMed] [Google Scholar]
- 9. Harding GT, Dunbar MJ, Hubley-Kozey CL, Stanish WD, Astephen Wilson JL. Obesity is associated with higher absolute tibiofemoral contact and muscle forces during gait with and without knee osteoarthritis. Clin Biomech (Bristol, Avon). 2016;31:79–86. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg M, Lethbridge-Cejku M, Scott JW, Reichle R, Plato C, Tobin J. The association of body weight, body fatness and body fat distribution with osteoarthritis of the knee: data from the Baltimore Longitudinal Study of Aging. J Rheumatol. 1995;22(3):488–493. [PubMed] [Google Scholar]
- 11. Honda K, Ohno S, Tanimoto K, et al. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79(9):601–609. [DOI] [PubMed] [Google Scholar]
- 12. Horisberger M, Fortuna R, Valderrabano V, Herzog W. Long-term repetitive mechanical loading of the knee joint by in vivo muscle stimulation accelerates cartilage degeneration and increases chondrocyte death in a rabbit model. Clin Biomech (Bristol, Avon). 2013;28(5):536–543. [DOI] [PubMed] [Google Scholar]
- 13. Kim HA, Lee Y-J, Seong S-C, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27(2):455–462. [PubMed] [Google Scholar]
- 14. Kozanek M, Hosseini A, Liu F, et al. Tibiofemoral kinematics and condylar motion during the stance phase of gait. J Biomech. 2009;42(12):1877–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krampla W, Mayrhofer R, Malcher J, Kristen K, Urban M, Hruby W. MR imaging of the knee in marathon runners before and after competition. Skeletal Radiol. 2001;30(2):72–76. [DOI] [PubMed] [Google Scholar]
- 16. Krampla WW, Newrkla SP, Kroener AH, Hruby WF. Changes on magnetic resonance tomography in the knee joints of marathon runners: a 10-year longitudinal study. Skeletal Radiol. 2008;37(7):619–626. [DOI] [PubMed] [Google Scholar]
- 17. Liebl H, Joseph G, Nevitt MC, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74(7):1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38(11):2273–2280. [DOI] [PubMed] [Google Scholar]
- 19. Milvy P. Statistical analysis of deaths from coronary heart disease anticipated in a cohort of marathon runners. Ann N Y Acad Sci. 1977;301(1):620–626. [DOI] [PubMed] [Google Scholar]
- 20. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskel R. 2004;8(04):355–368. [DOI] [PubMed] [Google Scholar]
- 21. Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18(3):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nieminen MT, Rieppo J, Töyräs J, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46(3):487–493. [DOI] [PubMed] [Google Scholar]
- 23. Nishioka H, Hirose J, Nakamura E, et al. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35(1):147–155. [DOI] [PubMed] [Google Scholar]
- 24. Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. [DOI] [PubMed] [Google Scholar]
- 25. Waldenmeier L, Evers C, Uder M, et al. Using cartilage MRI T2-mapping to analyze early cartilage degeneration in the knee joint of young professional soccer players. Cartilage. 2018;10(3):288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watrin-Pinzano A, Ruaud J-P, Cheli Y, et al. T2 mapping: an efficient MR quantitative technique to evaluate spontaneous cartilage repair in rat patella. Osteoarthritis Cartilage. 2004;12(3):191–200. [DOI] [PubMed] [Google Scholar]