Abstract
Background
Juvéderm Volux (VYC-25L; Allergan plc) is an injectable hyaluronic acid gel designed to restore and create facial volume.
Objective
The aim of this study was to evaluate the safety and effectiveness of Volux for chin retrusion over 18 months and after repeat treatment.
Methods
This prospective, single-blind, controlled study enrolled subjects aged ≥18 years with chin retrusion (glabella-subnasale-pogonion facial angle 145°-165°). Subjects were randomized (3:1) to Volux at study onset or 3 months later (control group), and could receive a single repeat treatment during months 18 to 24. Assessments included mean facial-angle change from baseline, Global Aesthetic Improvement Scale (GAIS) responder rates (improved/much improved), improvements in 3 subject-reported FACE-Q scales, and safety.
Results
Of 132 enrolled subjects, 119 received initial Volux treatment and 89 received repeat treatment. Mean changes (95% confidence interval) in glabella-subnasale-pogonion angle from baseline for treatment and control groups, respectively, were: 1.15° (0.75°, 1.56°) and 1.16° (0.57°, 1.75°) at month 18, and 3.14° (2.68°, 3.61°) and 2.72° (1.78°, 3.66°) 1 month after repeat treatment. Investigators rated 52.5%/60.0% of treated/control subjects at month 18 and 96.9%/100% after retreatment as GAIS responders; subject-reported rates were 62.0%/64.0% and 93.8%/100%. Durable improvements in Satisfaction with Chin, Satisfaction with Lower Face and Jawline, and Psychological Well-Being were reported in 82.1%, 78.2%, and 60.3% of subjects, respectively, at month 18, and 92.3%, 93.8%, and 67.7% of subjects after retreatment. The safety profile was as expected.
Conclusions
Volux injectable gel is a safe, effective, and durable alternative to surgical treatments for increasing chin projection and jaw volume, and results in high patient satisfaction.
Level of Evidence: 2

The chin is an important facial unit that can confer attractiveness or strength to the face.1,2 Attempts to quantify attractiveness and sexually dimorphic traits have often focused on the lines and angles of the chin, jaw, and lower face,1-4 emphasizing the importance of these facial structures as elements of beauty. Notably, the chin is an important component of facial profile that influences ratings of attractiveness.4 Chin contour is contingent upon the shape, size, and position of the mandible and overlying soft tissues.2 A retrusive chin can be the result of disproportionately less growth of the lower third of the face during maturation (eg, following an imbalance of hormone-influenced bone modeling and remodeling), trauma, or aging. Age-related changes such as those occurring with estrogen or androgen deficiency5 can induce resorption of the maxilla and mandible as well as changes in dentition, all of which can further alter chin and jawline appearance.2,6 As chin retrusion, weakness, or imbalance may be considered unattractive or perceived as a sign of weak personality, affected individuals often desire cosmetic correction.4
Many approaches used for chin augmentation (eg, grafts, implants) rely on surgical procedures.2 Biodegradable soft tissue fillers offer a safe and effective alternative to invasive surgery for chin augmentation.6 Juvéderm Volux (Volux [VYC-25L]; Allergan plc, Dublin, Ireland) is a moldable and versatile hyaluronic acid (HA)–based soft tissue filler containing lidocaine that was designed to provide a safe, minimally invasive method to restore and create facial volume.7 The present study evaluated the safety and effectiveness of Volux for creation of facial volume in the chin and jaw. The study met the 3-month primary effectiveness endpoint, showing that the mean change in facial angle from baseline at month 3 was significantly greater in the treatment vs delayed treatment (control) group (difference: 2.51°; P < 0.0001), with treatment effects sustained for up to 12 months.8 This report presents safety, effectiveness, and satisfaction results for Volux through 18 months after treatment and at 1 month after repeat treatment in subjects with chin retrusion.
METHODS
Study Design
Ten investigational sites in Germany (6 sites), France (2 sites), and the Netherlands (2 sites) participated in this prospective, multicenter, randomized, controlled study (clinicaltrials.gov identifier NCT02559908) between March 5, 2015 and August 28, 2017. The design of the study has been previously reported.8 Briefly, subjects were randomly assigned in a 3:1 ratio to treatment with Volux at the beginning of the study (Volux group) or after a 3-month delay (control group). During the 3-month delay, control subjects underwent 3-dimensional facial imaging at month 1 and month 3; after receiving initial treatment at month 3, the controls and the Volux treatment group underwent the same procedures. Subjects could receive an initial treatment followed by a touch-up treatment approximately 30 days later. Subjects could also receive a single repeat treatment between month 18 and month 24 if this was warranted in the investigator’s opinion and desired by the subject. Randomization to the control or treatment groups was stratified by study site; treatment assignments were accessed by the investigator via an automated interactive voice/web response system. Neither the investigators nor the subjects were blinded to treatment; only the image analysis technicians who made facial angle and volumetric measurements from digital images were blinded.
This study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice, and was approved by the ethics committees of each study site (Comité de protection des personnes [Bordeaux, France]; Ethik-Kommission der Universitat Witten/Herdecke [Witten, Germany], and METC Isala [Zwolle, The Netherlands]). Subjects provided written informed consent prior to the initiation of any study-specific procedures.
Subjects
Subject eligibility has been previously described.8 Briefly, subjects (18 years of age or older) had to have chin retrusion (glabella-subnasale-pogonion angle 145°-165°) that was amenable to correction with increased horizontal projection. Subjects could not have: received permanent facial implants in the face or neck; undergone semipermanent soft tissue filler treatment in the chin or jaw within 36 months before enrollment; undergone soft tissue filler injections, fat injections, or surgery in the chin or jaw within 24 months before enrollment; undergone any dental procedure (other than prophylaxis or fillings) during the study; received soft tissue filler injection in the glabellar area or nose within 12 months before enrollment; or undergone mesotherapy or cosmetic resurfacing in the face or neck, or botulinum toxin injections below the subnasale within 6 months before enrollment.
Treatment
Volux was injected via a 27-gauge 13-mm needle into the chin and jaw area to restore and create facial volume. The investigator determined the appropriate injection volume for each treatment based on clinical experience. A maximum volume of 4.0 mL was allowed for the initial and touch-up treatments combined, and up to 4.0 mL could be injected for repeat treatment. No more than 2.0 mL could be injected into any single treatment area. Treatment areas included the pogonion, mentum, prejowl sulci (left and right), and sublabial (mental) crease.
Effectiveness Assessments
The primary effectiveness endpoint was the comparison of mean change from baseline in glabella-subnasale-pogonion angle between the Volux group and the control group at month 3, which has been previously reported.8 Glabella-subnasale-pogonion angle was assessed via 3D facial digital imaging.
The secondary effectiveness measure was the Global Aesthetic Improvement Scale (GAIS) as assessed by both investigator and subject. The GAIS is rated on a 5-point scale ranging from –2 (much worse) to 2 (much improved). A GAIS responder was defined as a response of 1 (improved) or 2 (much improved).
Additional effectiveness endpoints included subject responses on 3 scales of the validated FACE-Q questionnaire (Satisfaction with Chin, Satisfaction with Lower Face and Jawline, and Psychological Well-Being), and investigator ratings of ease of injection and Volux moldability. The Satisfaction with Chin9 and Satisfaction with Lower Face and Jawline10 scales are rated based on 4 responses (very dissatisfied, somewhat dissatisfied, somewhat satisfied, very satisfied). The Psychological Well-Being scale11 is based on 4 responses (definitely agree, somewhat agree, somewhat disagree, definitely disagree). FACE-Q questionnaires were administered by the study staff at each site during scheduled follow-up visits (ie, at months 1, 3, 6, 12, 18, as well as at 1 month after repeat treatment). These questionnaires were not anonymous. Ease of injection ratings were based on an 11-point scale ranging from 0 (difficult) to 10 (easy), and Volux moldability on an 11-point scale ranging from 0 (stiff) to 10 (moldable) after each treatment.
Safety Assessments
Pain was rated by subjects immediately after each treatment on an 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable). Safety endpoints included the incidence of injection site responses (ISRs) as reported by the subject and treatment-emergent adverse events (TEAEs) as reported by the investigator. Subjects recorded the presence, severity, and duration of ISRs in an e-diary for 30 days after each treatment.
Statistics
Analyses of effectiveness endpoints were based on a modified intent-to-treat population, which comprised all subjects who were randomly assigned to the Volux treatment group who received treatment and completed at least 1 follow-up visit; and all subjects who were randomly assigned to the control group and who completed at least 1 follow-up visit. The safety population included all randomized subjects who received at least 1 treatment. Raw scores for responses on the FACE-Q questionnaires were summed to provide total scores, which were transformed with Rasch unidimensional methods to an overall score.12,13 Transformed scores ranged from 0 to 100, where a higher score indicated greater satisfaction/better outcomes. Ranges of transformed scores for each response category for all items were calculated according to threshold estimates. Missing effectiveness data were not imputed. Analyses of other effectiveness endpoints were descriptive.
RESULTS
Subjects
Subject disposition and baseline characteristics are shown in Table 1. A total of 120 subjects were randomized (90 in the Volux group, 30 in the control group); of these, 119 subjects received initial/touch-up Volux treatment (90 Volux, 29 control) and 89 subjects (65 Volux, 24 control) received repeat treatment. The mean age was 46.2 [13.3] years (range, 20-75 years), most subjects were female (92%, n = 110; the remaining 8% were men, n = 10), and the population was predominantly white (85.8%). At baseline, the mean glabella-subnasale-pogonion angles were 160.6° [4.2°] for the Volux group and 161.3° [2.8°] for controls.
Table 1.
Baseline Demographics and Characteristics
| Treatment group (n = 90) | Control group (n = 30) | Total (N = 120) | |
|---|---|---|---|
| Age, years | |||
| Mean [SD] | 46.4 [12.87] | 45.7 [14.67] | 46.2 [13.28] |
| Median (range) | 48.5 (22-75) | 50.0 (20-68) | 49.0 (20-75) |
| Female, n (%) | 81 (90.0) | 29 (96.7) | 110 (91.7) |
| Race, n (%) | |||
| White | 74 (82.2) | 29 (96.7) | 103 (85.8) |
| Asian descent | 14 (15.6) | 1 (3.3) | 15 (12.5) |
| Black | 1 (1.1) | 0 | 1 (0.8) |
| Height (cm) | |||
| Mean [SD] | 166.8 [7.24] | 166.1 [8.11] | 166.6 [7.44] |
| Median (range) | 168.0 (150-189) | 166.0 (154-193) | 167.0 (150-193) |
| Weight (kg) | |||
| Mean [SD] | 62.8 [10.52] | 62.6 [11.73] | 62.7 [10.78] |
| Median (range) | 61.5 (41-92) | 62.5 (44-95) | 62.0 (41-95) |
| Body mass index, kg/m2, mean [SD] | 22.5 [3.0] | 22.6 [3.6] | 22.5 [3.2] |
| Fitzpatrick skin type, n (%) | |||
| I-III | 68 (75.6) | 23 (76.7) | 91 (75.8) |
| IV-VI | 22 (24.4) | 7 (23.3) | 29 (24.2) |
| Baseline glabella-subnasale-pogonion angle, degrees, mean [SD] | 160.6 [4.2] | 161.3 [2.8] | — |
Treatment Administration
The total median injection volume of Volux was smaller for repeat treatments compared to the initial total median injection volumes for the Volux group (2.28 mL; range, 1.0-4.0 mL) and the control group (2.50 mL; range, 1.6-3.0 mL) (Table 2). The distribution of treatment sites (pogonion, mentum, prejowl sulci, and/or sublabial crease, Figure 1) was similar between the initial/touch-up treatment and repeat treatment. On average, investigators rated the ease of injection as 9.0 for repeat treatment (scale, 0-10), and rated the product moldability as 8.2 (scale, 0-10).
Table 2.
Injection Volumes with Initial/Touch-Up and Repeat Volux Treatment (Safety Population; n = 119)
| Median (range) volume of Volux injected, mL | ||||
|---|---|---|---|---|
| Treatment group (after initial/touch-up Volux treatment) | Treatment group (after repeat Volux treatment) | Control group (after initial/touch-up Volux treatment) | Control group (after repeat Volux treatment) | |
| Total volume | 3.43 (1.2-4.1), n = 90 | 3.00 (0.5-5.0), n = 65 | 3.55 (1.7-4.0), n = 29 | 2.50 (0.8-4.0), n = 24 |
| Pogonion | 0.94 (0.1-2.1), n = 90 | 0.70 (0.1-1.5), n = 57 | 0.90 (0.1-1.9), n = 29 | 0.70 (0.1-1.3), n = 21 |
| Mentum | 0.95 (0.1-2.1), n = 85 | 0.75 (0.1-2.0), n = 56 | 1.25 (0.2-2.2), n = 27 | 0.50 (0.1-1.2), n = 21 |
| Prejowl sulcia | 1.00 (0.2–3.0), n = 85 | 1.20 (0.2–2.3), n = 61 | 1.00 (0.2-2.4), n = 29 | 1.00 (0.3-3.4), n = 24 |
| Sublabial crease | 0.45 (0.1-1.2), n = 85 | 0.45 (0.1-1.1), n = 40 | 0.40 (0.1-1.2), n = 23 | 0.55 (0.2-1.0), n = 18 |
aCombined right and left.
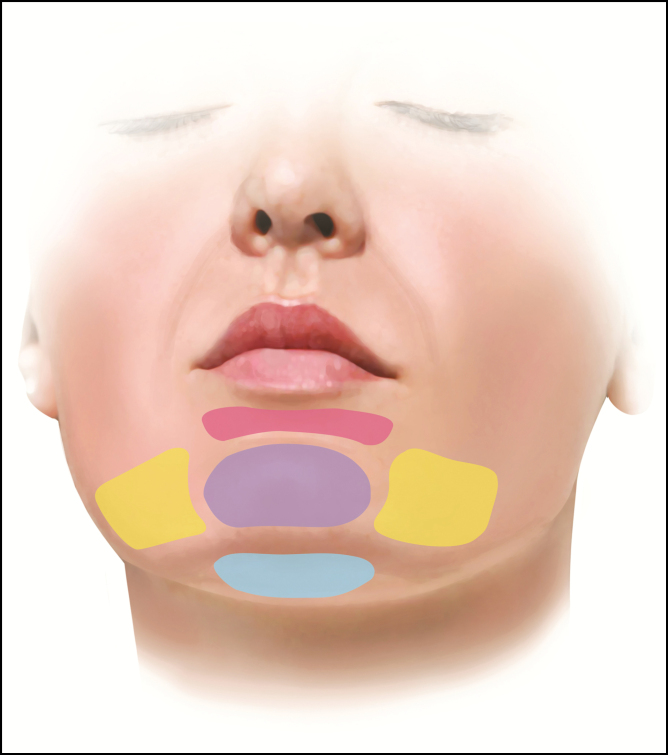
Figure 1.
Schematic representation of treatment sites. Sublabial crease (pink), pogonion (purple), mentum (blue), and prejowl sulci (yellow).
Effectiveness
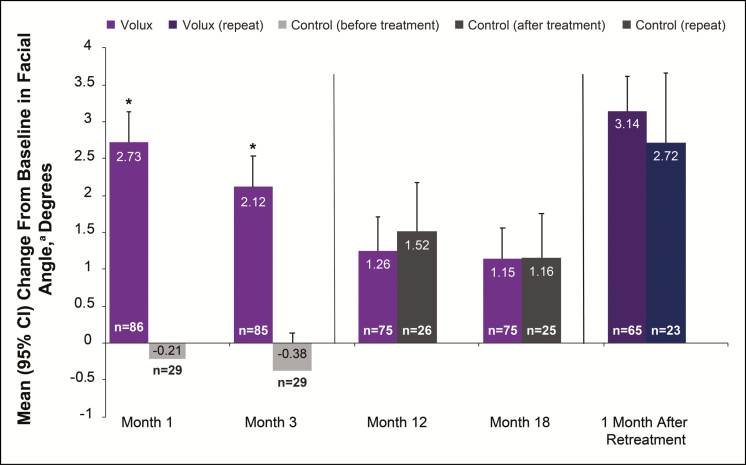
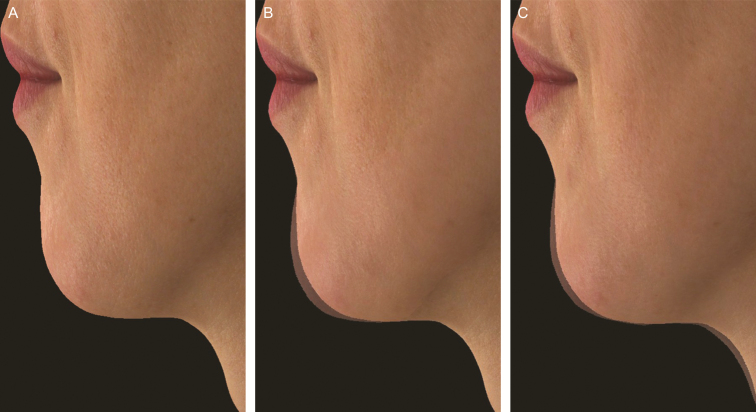
Significant improvements following initial Volux treatment in chin projection were achieved at day 30 and generally maintained through 18 months (Figure 2). At 1 month after repeat treatment, the mean change in glabella-subnasale-pogonion angle was similar to that after initial treatment (mean change 3.14°; 95% confidence interval [CI]: 2.68°, 3.61°) in the Volux group (Figure 2). Representative effects of Volux treatment at month 3 and month 18 silhouetted with the subject’s screening profile are shown in Figures 3 to 5. Of note, most of the subjects who received repeat treatment did so at month 18, leaving only a small, biased sample of subjects beyond month 18 of initial Volux treatment (n < 15). Therefore, effectiveness results are presented through month 18. Figures 3C, 4C, and 5C show the effects of initial Volux treatment at month 18; Figure 5D shows the effect of repeat Volux treatment at 1 month.
Figure 2.
Change from baseline in glabella-subnasale-pogonion angle before and after treatment with Volux. aA positive value indicates an increase in projection of the chin and/or jaw.
Figure 3.
Photographs of a 33-year-old Asian woman demonstrating Volux chin treatment at baseline (A), and silhouetted with her baseline profile at month 3 (B) and month 18 (C). The glabella-subnasale-pogonion angle was 161.8° at screening (A), 164.9° at month 3 (B), and 166.1° at month 18 following initial Volux treatment of 1.70 mL, and 167.3° at month 1 of repeat Volux treatment of 2.20 mL. This subject did not receive top-up treatment.
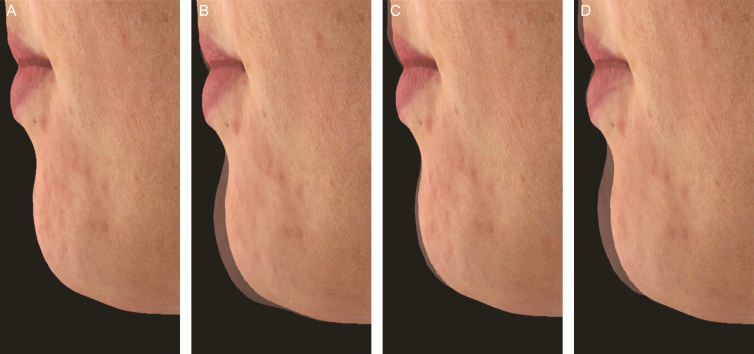
Figure 5.
Photographs of a 27-year-old Caucasian woman demonstrating Volux chin treatment at baseline (A), silhouetted with her baseline profile at month 3 (B) and month 18 (C) following initial treatment, and at month 1 following repeat treatment (D). The glabella-subnasale-pogonion angle was 163.9° at screening (A), 167.6° at month 3 (B), and 165.4° at month 18 following initial and top-up Volux treatments of 2.00 mL (each), and 168.7° at month 1 of repeat Volux treatment of 1.40 mL.
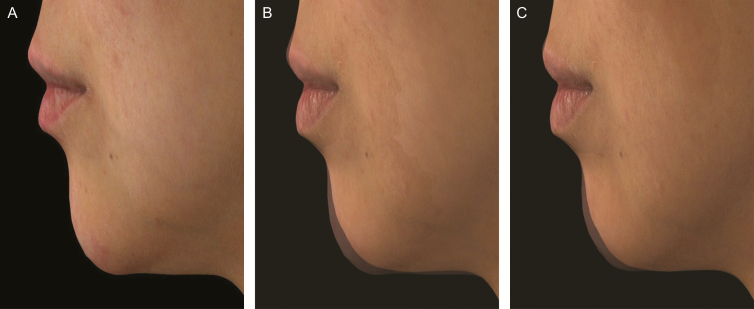
Figure 4.
Photographs of a 43-year-old Caucasian woman demonstrating Volux chin treatment at baseline (A), and silhouetted with her baseline profile at month 3 (B) and month 18 (C). The glabella-subnasale-pogonion angle was 161.5° at screening (A), 163.1° at month 3 (B), and 163.5° at month 18 (C) following initial and top-up Volux treatment of 1.00 and 0.80 mL, respectively. This subject did not receive repeat Volux treatment, and her glabella-subnasale-pogonion angle was 162.8° at month 24 (not shown).
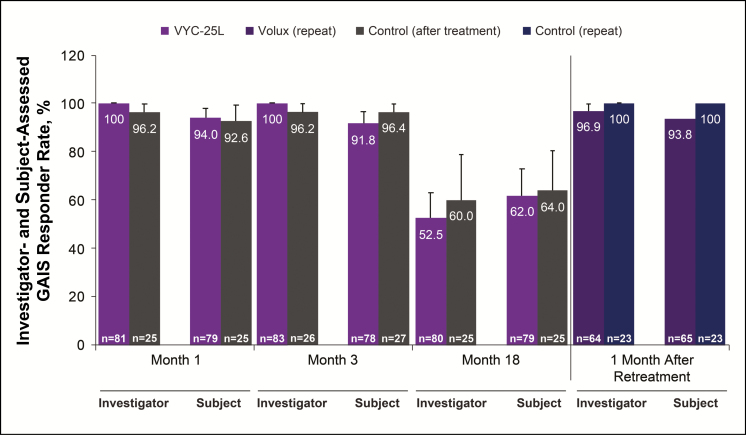
GAIS was assessed at months 1, 3, 6, 9, 12, 15, 18, as well as at 1 month after repeat treatment. The GAIS responder rate gradually decreased from month 3 (100% and 91.8% as scored by the investigator and the subject, respectively) through month 18 (52.5% and 62.0%) following initial Volux treatment. At 1 month after repeat treatment, responder rates returned to the high levels achieved following initial Volux treatment. GAIS responder rates among the control group after treatment were similar to the Volux group at month 18 after initial treatment and at 1 month after repeat treatment (Figure 6).
Figure 6.
Global Aesthetic Improvement Scale (GAIS) responder rate (proportion of subjects rated as “improved” or “much improved”) according to the investigators’ and subjects’ assessments.
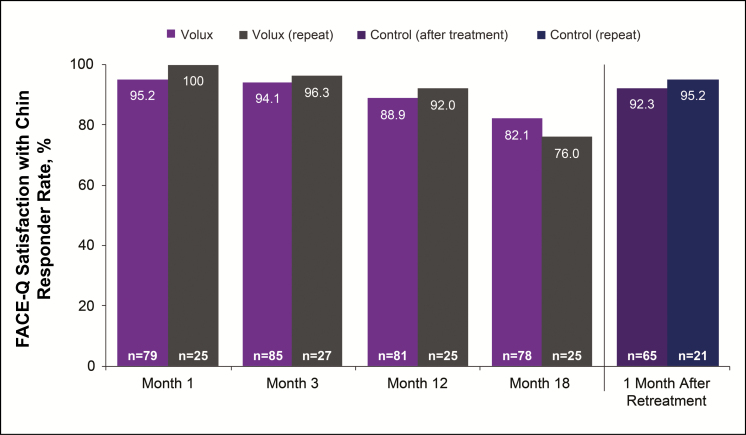
Overall mean (95% CI) Satisfaction with Chin scores improved from 41.4 (95% CI: 38.6, 44.3) at baseline to 55.1 (95% CI: 51.4, 58.8) at month 18 after initial treatment and to 74.2 (95% CI: 69.2, 79.2) after repeat treatment in the Volux group; results were similar in the control group after treatment. The percentage of subjects reporting improvement from baseline in satisfaction with chin in the Volux group was similar at month 12 (88.9%) and month 18 (82.1%). At 1 month after repeat treatment, improvement was reported by 92.3% in the Volux group. Similar results were observed in the control group after treatment (Figure 7).
Figure 7.
Proportion of subjects reporting improvement from baseline on the FACE-Q Satisfaction with Chin scale.
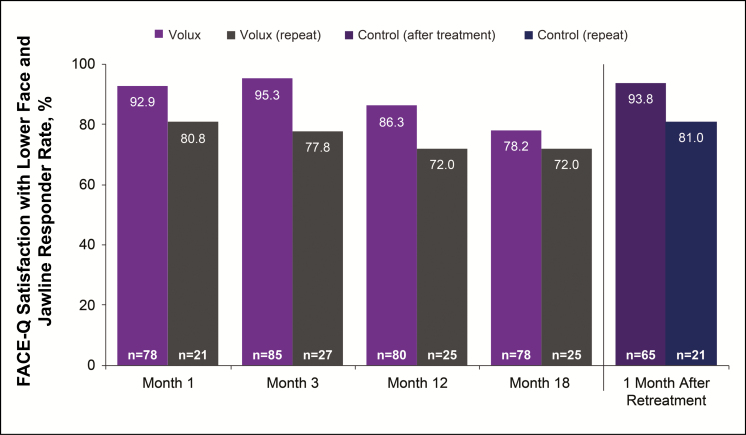
Overall mean Satisfaction with Lower Face and Jawline scores improved from 36.7 (95% CI: 32.6, 40.8) at baseline to 54.5 (95% CI: 49.4, 59.5) at month 18 after initial treatment and to 73.1 (95% CI: 68.6, 77.6) after repeat treatment in the Volux group; results were similar in the control group after treatment. The percentage of subjects reporting improvement from baseline in satisfaction with lower face and jawline in the Volux group declined slightly from month 12 (86.3%) to month 18 (78.2%) after initial treatment. At 1 month after repeat treatment, improvement was reported by 93.8% in the Volux group. Similar results were observed in the control group after treatment (Figure 8).
Figure 8.
Proportion of subjects reporting improvement from baseline on the FACE-Q Satisfaction with Lower Face and Jawline scale.
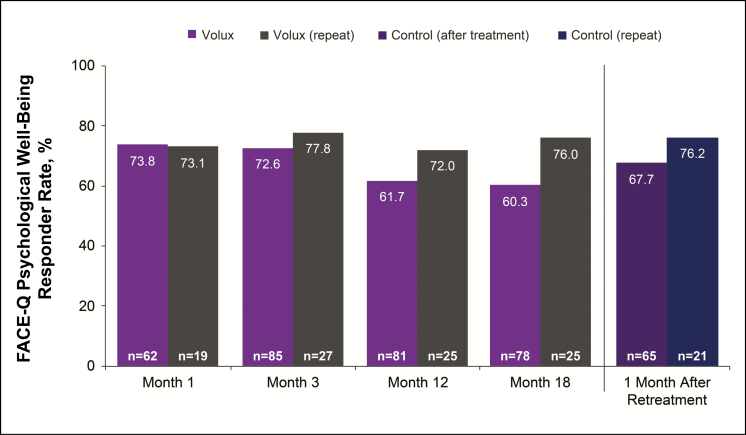
Overall mean Psychological Well-Being scores improved from 65.3 (95% CI: 61.5, 69.1) at baseline to 72.4 (95% CI: 67.8, 77.1) at month 18 after initial treatment and to 79.7 (95% CI: 75.5, 83.9) after repeat treatment in the Volux group; results were similar in the control group after treatment. The percentage of subjects reporting improvement from baseline in psychological well-being in the Volux group was similar at month 12 (61.7%) and month 18 (60.3%) following initial treatment. At 1 month after repeat treatment, improvement was reported by 67.7% in the Volux group. Similar results were observed in the control group after treatment (Figure 9).
Figure 9.
Proportion of subjects reporting improvement from baseline on the FACE-Q Psychological Well-Being scale.
Safety
The mean pain score as assessed by all treated subjects immediately after initial and touch-up treatment (n = 119) was 2.6 [1.8]; almost identical to that after repeat treatment (n = 87; 2.6 [2.2]). Most subjects who received treatment reported at least 1 ISR; 96.6% for initial and touch-up treatment combined and 95.5% for repeat treatment. The most commonly reported ISRs with initial/touch-up and repeat treatments were firmness (95.8% vs 94.4%) and tenderness to touch (95.8% vs 94.4%), respectively (Table 3). After repeat treatment, firmness was most commonly reported to be moderate in severity, whereas ISRs of redness, pain after injection, lumps/bumps, discoloration, and itching were most commonly reported to be mild, similar to that following initial/touch-up treatment (Table 3). Severe ISRs of bruising and firmness occurred at higher-than-expected rates after initial/touch-up treatment, but at typical rates for a HA soft tissue filler after repeat treatment (statistical significance testing was not performed). Most ISRs had a duration of 1 week.
Table 3.
Incidence, Severity, and Duration of ISRs Following Initial/Touch-Up and Repeat Volux Treatment (Safety Population; n = 119)
| Severity, n (%)a | Duration, n (%)b | ||||||
|---|---|---|---|---|---|---|---|
| Any | Mild | Moderate | Severe | 1-7 days | 8-14 days | 15-30 days | |
| All treated subjects (after combined initial/touch-up Volux treatment, n = 119) | |||||||
| Firmness | 114 (95.8) | 22/114 (19.3) | 52/114 (45.6) | 40/114 (35.1) | 67/114 (58.8) | 31/114 (27.2) | 16/114 (14.0) |
| Tenderness to touch | 114 (95.8) | 39/114 (34.2) | 59/114 (51.8) | 16/114 (14.0) | 87/114 (76.3) | 17/114 (14.9) | 10/114 (8.8) |
| Swelling | 109 (91.6) | 35/109 (32.1) | 50/109 (45.9) | 24/109 (22.0) | 91/109 (83.4) | 16/109 (14.7) | 2/109 (1.8) |
| Pain after injection | 108 (90.8) | 46/108 (42.6) | 51/108 (47.2) | 11/108 (10.2) | 97/108 (89.8) | 7/108 (6.5) | 4/108 (3.7) |
| Redness | 108 (90.8) | 51/108 (47.2) | 41/108 (38.0) | 16/108 (14.8) | 99/108 (91.7) | 8/108 (7.4) | 1/108 (0.9) |
| Lumps/bumps | 102 (85.7) | 40/102 (39.2) | 49/102 (48.0) | 13/102 (12.7) | 61/102 (59.8) | 26/102 (25.5) | 15/102 (14.7) |
| Bruising | 101 (84.9) | 23/101 (22.8) | 40/101 (39.6) | 38/101 (37.6) | 62/101 (61.4) | 38/101 (37.6) | 1/101 (1.0) |
| Discolorationc | 62 (52.1) | 38/62 (61.3) | 19/62 (30.6) | 5/62 (8.1) | 58/62 (93.5) | 2/62 (3.2) | 2/62 (3.2) |
| Itching | 57 (47.9) | 48/57 (84.2) | 8/57 (14.0) | 1/57 (1.8) | 50/57 (87.7) | 5/57 (8.8) | 2/57 (3.5) |
| All treated subjects (after repeat Volux treatment, n = 89) | |||||||
| Firmness | 84 (94.4) | 23/84 (27.4) | 39/84 (46.4) | 22/84 (26.2) | 51/84 (60.7) | 23/84 (27.4) | 10/84 (11.9) |
| Tenderness to touch | 84 (94.4) | 39/84 (46.4) | 34/84 (40.5) | 11/84 (13.1) | 62/84 (73.8) | 17/84 (20.2) | 5/84 (6.0) |
| Swelling | 79 (88.8) | 49/79 (62.0) | 20/79 (25.3) | 10/79 (12.7) | 74/79 (93.7) | 4/79 (5.1) | 1/79 (1.3) |
| Pain after injection | 77 (86.5) | 39/77 (50.6) | 29/77 (37.7) | 9/77 (11.7) | 72/77 (93.5) | 2/77 (2.6) | 3/77 (3.9) |
| Redness | 77 (86.5) | 36/77 (46.8) | 29/77 (37.7) | 12/77 (15.6) | 68/77 (88.3) | 7/77 (9.1) | 2/77 (2.6) |
| Lumps/bumps | 72 (80.9) | 38/72 (52.8) | 23/72 (31.9) | 11/72 (15.3) | 44/72 (61.1) | 17/72 (23.6) | 11/72 (15.3) |
| Bruising | 62 (69.7) | 26/62 (41.9) | 25/62 (40.3) | 11/62 (17.7) | 50/62 (80.7) | 10/62 (16.1) | 2/62 (3.2) |
| Discolorationc | 39 (43.8) | 26/39 (66.7) | 12/39 (30.8) | 1/39 (2.6) | 36/39 (92.3) | 3/39 (7.7) | 0/39 (0) |
| Itching | 32 (36.0) | 24/32 (75.0) | 7/32 (21.9) | 1/32 (3.1) | 26/32 (81.2) | 5/32 (15.6) | 1/32 (3.1) |
ISR, injection site response. aPatients reporting data in e-diary. bPatients reporting event. cNot red/bruising.
A total of 53 TEAEs occurred in 18 (20.2%) subjects. All TEAEs were at the injection site. Three TEAEs were reported more than 1 year after initial/touch-up treatments; 2 subjects reported swelling and 1 subject reported pain. All 3 events were of mild or moderate severity, and resolved within 2 days without treatment or sequelae.
The most common TEAEs following repeat treatment were the same as TEAEs following initial/touch-up treatments, although rates were more variable: injection site mass (14.6% vs 21.8%), injection site induration (13.5% vs 12.6%), and injection site pain (7.9% vs 12.6%), respectively (Table 4). Nearly all TEAEs were related to ongoing ISRs. No serious TEAEs (related or unrelated to treatment) were reported, and no subject discontinued the study because of a TEAE after repeat treatment. Four subjects experienced speech disorder (specifically, unclear pronunciation) following initial and touch-up treatments, and 1 subject experienced unclear pronunciation following repeat treatment.8
Table 4.
TEAEs with Onset Prior to or After Repeat Volux Treatment (Safety Population; n = 119)
| TEAE, n (%) | ||||
|---|---|---|---|---|
| Prior to repeat treatment | After repeat treatment | |||
| All treated subjects (n = 119) | Events (n = 98) | All treated subjects (n = 89) | Events (n = 57) | |
| Any TEAE | 46 (38.7) | 98 (100.0) | 18 (20.2) | 53 (100) |
| TEAE at injection site | 43 (36.1) | 91 (92.9) | 18 (20.2) | 53 (100) |
| Injection site mass | 26 (21.8) | 26 (26.5) | 13 (14.6) | 13 (24.5) |
| Injection site induration | 15 (12.6) | 21 (21.4) | 12 (13.5) | 12 (22.6) |
| Injection site pain | 15 (12.6) | 21 (21.4) | 7 (7.9) | 11 (20.8) |
| Injection site swelling | 7 (5.9) | 7 (7.1) | 4 (4.5) | 4 (7.5) |
| Injection site erythema | 6 (5.0) | 6 (6.1) | 4 (4.5) | 4 (7.5) |
| Injection site discoloration | 4 (3.4) | 4 (4.1) | 3 (3.4) | 3 (5.7) |
| Injection site pruritus | 4 (3.4) | 4 (4.1) | 2 (2.2) | 2 (3.8) |
| Speech disorder | 4 (3.4) | 6 (6.1) | 1 (1.1) | 1 (1.9) |
| Headache | 2 (1.7) | 2 (4.4) | 0 | 0 |
| Injection site nodule | 2 (1.7) | 2 (4.4) | 0 | 0 |
| Injection site bruising | 1 (0.8) | 1 (2.2) | 1 (1.1) | 1 (1.9) |
| Presyncope | 1 (0.8) | 1 (2.2) | 0 | 0 |
| Myalgia | 1 (0.8) | 1 (2.2) | 0 | 0 |
| Device dislocation | 1 (0.8) | 1 (2.2) | 0 | 0 |
| Injection site hematoma | 0 | 0 | 1 (1.1) | 1 (1.9) |
| Injection site abscess | 0 | 0 | 1 (1.1) | 1 (1.9) |
TEAE, treatment-emergent adverse event.
DISCUSSION
Chin augmentation has traditionally been achieved by surgically altering the bony projection and, more recently, with adjunctive use of fillers.14,15 Volux is the first HA filler specifically designed to both restore and create volume to be systematically investigated in a controlled, randomized clinical study.8 Chin augmentation is an ideal indication for testing Volux, as Volux can be used to treat not only the loss of fatty and bone tissue caused by aging (ie, restoring volume), but also to treat any deficiency in volume that may have developed throughout life (ie, creating volume). Although there are several HA dermal fillers in the Juvéderm product line, Volux was chosen for this study because it has the highest concentration of HA (25 mg/mL), which was anticipated to provide the strong volumizing and lift properties necessary to sculpt, shape, and contour the high-mobility chin and jaw areas.
The findings demonstrate that Volux is a safe and effective nonsurgical alternative for chin augmentation in subjects with chin retrusion. Physical improvements in glabella-subnasale-pogonion facial angle following Volux treatment lasted beyond 18 months; these facial-angle improvements were similar between initial and repeat Volux treatments. Subjective improvements in GAIS and FACE-Q were durable, as they persisted through 18 months following initial Volux treatment. Repeat treatment of Volux administered 18 months after initial treatment required less injection volume to achieve comparable improvements in facial angle, and investigators found Volux to be easy to inject and moldable.
The safety profile of Volux was similar between initial and repeat treatments, and the rate of severe ISRs was lower for most types of ISRs with repeat treatment compared with initial/touch-up treatment. The mild transient speech disorder (specifically, unclear pronunciation) experienced by 5 subjects following initial/touch-up and repeat Volux treatment was considered to result from, at least in part, filler injection–related swelling; 4 of these subjects experienced unclear pronunciation within the first 3 days of initial Volux administration, as previously reported.8 The final subject experienced unclear pronunciation after repeat Volux treatment, which lasted 82 days. None of these events required treatment, nor were any deemed to have neurologic impact. Instead, myomodulation of the chin muscles following injection-induced swelling may explain these transient speech disorders.16
There are few published studies that focus on HA fillers for chin augmentation. In a full-facial volume restoration study of 60 subjects, 51 received at least 1 HA filler (HAEL; Galderma SA, Lausanne, Switzerland) treatment for chin restoration, and up to another 9 subjects received filler treatment for jawline restoration. Over the 18-month study, at least a 1-grade improvement in the 4-point volume loss scale was observed for the chin (77.8%) and jawline (43.2%) indications, and subjects reported GAIS aesthetic improvements that persisted through 18 months for both the full face and individual indications, although the specific GAIS rates for chin and jawline restoration were not provided. Most subjects had ISRs immediately post-injection, and 6 subjects had investigator-assessed nodule formation in the jawline (5 subjects) and chin (1 subject), most of which were considered mild. In an open-label study of 4 subjects (out of 72) who received subcutaneous injection of HA filler (Restylane SubQ; Q-Med, Uppsala, Sweden) under the mentalis muscle of the chin, all reported a persistence of chin augmentation during the posttreatment observation period of up to 64 weeks. Transient bruising and swelling, which were reported in 15% of all subjects, were considered technique dependent. Although direct comparisons of these findings with our study results are not possible due to the low number of subjects specifically treated in the jaw and chin areas, differences in endpoints, and/or endpoint reporting (eg, GAIS), it does appear that Volux was at least as safe and effective as HAEL or Restylane SubQ, if not more so, providing additional support for the utility of HA fillers as a safe and effective nonsurgical option for chin augmentation.
A limitation of the current study was the small sample size at months 21 and 24 (n < 15), which precluded meaningful analyses of the durability of Volux in this population. Although most subjects elected to undergo a repeat treatment at month 18, subjects without retreatment may not have had appreciable improvements in facial shape, resulting in selection bias at later time points. An additional limitation of the study was the use of a scoring system that was based only on frontal views as previously discussed.8 The results of this study are also limited by the short follow-up duration (1 month) after repeat treatment.
CONCLUSIONS
Volux injectable gel is a safe, effective, and durable alternative to surgical treatments in restoring and creating facial volume, and in sculpting, shaping, and contouring in the chin and jaw area. Repeat treatment required between one-tenth and two-thirds of the injection volume to achieve similar improvement in facial angle as afforded by initial/touch-up treatment. Subjects were highly satisfied with Volux treatment and reported sustained improvements in the augmentation of their chin.
Acknowledgments
The authors thank Julia R. Gage, PhD, and Kristin E. Larsen, PhD, of Peloton Advantage, LLC, an OPEN Health company, for medical writing and editorial support, which was funded by Allergan plc.
Disclosures
Dr Ogilvie has received research funding as an investigator and honoraria as a consultant and trainer from Allergan plc, Galderma, Merz, Evolus, and Revance. Drs Benouaiche, Belhaouari, and Gaymans have received research funding as an investigator and honoraria as a consultant and trainer from Allergan plc. Dr Philipp-Dormston has received research funding as an investigator and honoraria as a consultant and trainer from Allergan plc, Galderma, and Merz. Dr Sattler serves as an investigator for Allergan plc. Ms Harvey and Dr Schumacher are employees of Allergan and may own stock in the company. The opinions expressed in this article are those of the authors. The authors received no honorarium or other form of financial support related to the development of this article.
Funding
This study was funded by Allergan, Inc. Editorial support for this article was provided by Peloton Advantage, LLC (Parsippany, NJ, USA), an OPEN Health company, and was funded by Allergan, Inc. Allergan was responsible for the data analyses. The study design and data interpretation were conducted by Allergan in consultation with the study investigators. Data collection was the responsibility of the study investigators.
REFERENCES
- 1. González-Ulloa M, Stevens E. The role of chin correction in profileplasty. Plast Reconstr Surg. 1968;41(5):477-486. [DOI] [PubMed] [Google Scholar]
- 2. Romo T 3rd, Lanson BG. Chin augmentation. Facial Plast Surg Clin North Am. 2008;16(1):69-77, vi. [DOI] [PubMed] [Google Scholar]
- 3. Saponaro G, Gasparini G, Boniello R, et al. . The rules of attractiveness: a study on the lower facial third. J Craniofac Surg. 2018;29(7):1945-1946. [DOI] [PubMed] [Google Scholar]
- 4. Naini FB, Donaldson AN, McDonald F, Cobourne MT. Assessing the influence of chin prominence on perceived attractiveness in the orthognathic patient, clinician and layperson. Int J Oral Maxillofac Surg. 2012;41(7):839-846. [DOI] [PubMed] [Google Scholar]
- 5. Almeida M, Laurent MR, Dubois V, et al. . Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1S):S4-S9. [DOI] [PubMed] [Google Scholar]
- 7. Eccleston D, Murphy DK. Juvéderm® Volbella™ in the perioral area: a 12-month prospective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogilvie P, Sattler G, Gaymans F, et al. . Safe, effective chin and jaw restoration with VYC-25L hyaluronic acid injectable gel. Dermatol Surg. 2019;45(10):1294-1303. [DOI] [PubMed] [Google Scholar]
- 9. Klassen AF, Cano SJ, Scott A, Snell L, Pusic AL. Measuring patient-reported outcomes in facial aesthetic patients: development of the FACE-Q. Facial Plast Surg. 2010;26(4):303-309. [DOI] [PubMed] [Google Scholar]
- 10. Klassen AF, Cano SJ, Scott AM, Pusic AL. Measuring outcomes that matter to face-lift patients: development and validation of FACE-Q appearance appraisal scales and adverse effects checklist for the lower face and neck. Plast Reconstr Surg. 2014;133(1):21-30. [DOI] [PubMed] [Google Scholar]
- 11. Klassen AF, Cano SJ, Schwitzer JA, Scott AM, Pusic AL. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375-386. [DOI] [PubMed] [Google Scholar]
- 12. Tennant A, Conaghan PG. The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper? Arthritis Rheum. 2007;57(8):1358-1362. [DOI] [PubMed] [Google Scholar]
- 13. Pallant JF, Tennant A. An introduction to the Rasch measurement model: an example using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol. 2007;46(Pt 1):1-18. [DOI] [PubMed] [Google Scholar]
- 14. Hsu AK, Frankel AS. Modification of chin projection and aesthetics with onabotulinumtoxinA injection. JAMA Facial Plast Surg. 2017;19(6):522-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vanaman Wilson MJ, Jones IT, Butterwick K, Fabi SG. Role of nonsurgical chin augmentation in full face rejuvenation: a review and our experience. Dermatol Surg. 2018;44(7):985-993. [DOI] [PubMed] [Google Scholar]
- 16. de Maio M. Myomodulation with injectable fillers: an innovative approach to addressing facial muscle movement. Aesthetic Plast Surg. 2018;42(3):798-814. [DOI] [PMC free article] [PubMed] [Google Scholar]