Abstract
Intracranial electroencephalography (iEEG) has been the mainstay of identifying the seizure onset zone (SOZ), a key diagnostic procedure in addition to neuroimaging when considering epilepsy surgery. In many patients, iEEG has been the basis for resective epilepsy surgery, to date still the most successful treatment for drug-resistant epilepsy. Intracranial EEG determines the location and resectability of the SOZ. Advances in recording and implantation of iEEG provide multiple options in the 21st century. This not only includes the choice between subdural electrodes (SDE) and stereoelectroencephalography (SEEG) but also includes the implantation and recordings from microelectrodes. Before iEEG implantation, especially in magnetic resonance imaging -negative epilepsy, a clear hypothesis for seizure generation and propagation should be based on noninvasive methods. Intracranial EEG implantation should be planned by a multidisciplinary team considering epileptic networks. Recordings from SDE and SEEG have both their advantages and disadvantages. Stereo-EEG seems to have a lower rate of complications that are clinically significant, but has limitations in spatial sampling of the cortical surface. Stereo-EEG can sample deeper areas of the brain including deep sulci and hard to reach areas such as the insula.
To determine the epileptogenic zone, interictal and ictal information should be taken into consideration. Interictal spiking, low frequency slowing, as well as high frequency oscillations may inform about the epileptogenic zone. Ictally, high frequency onsets in the beta/gamma range are usually associated with the SOZ, but specialized recordings with combined macro and microelectrodes may in the future educate us about onset in higher frequency bands. Stimulation of intracranial electrodes triggering habitual seizures can assist in identifying the SOZ. Advanced computational methods such as determining the epileptogenicity index and similar measures may enhance standard clinical interpretation. Improved techniques to record and interpret iEEG may in the future lead to a greater proportion of patients being seizure free after epilepsy surgery.
Keywords: intracranial EEG, epilepsy surgery, stereoencephalography (SEEG), subdural electrodes, seizure onset zone, epileptogenic zone, interictal epileptiform discharges
Introduction
This review is a report from the Epilepsy Specialist Symposium delivered at the AES meeting in 2018. This symposium was meant to educate about the current methods of intracranial electroencephalography (iEEG) as well as advanced and novel analysis methods of iEEG for seizure onset localization using ictal and interictal markers. Faculty delivered outstanding content. They were tasked to identify the seizure onset zone with their respective expertise in a real patient based on iEEG data alone. Relevant data about the patient was anonymously sent to them prior to the symposium. We are proud to report that all the faculty delivered on the task and identified the appropriate abnormality based on their unique data analysis. The entire symposium can be reviewed at https://www.pathlms.com/aes/courses/10686. For details of the case presented and the decision points faculty had to comment on please see supplementary file and video.
Planning an Intracranial EEG Study
1. Indications for chronic intracranial EEG recordings—patient selection, who is a candidate and who is not. (Beate Diehl, MD, PhD)
Epilepsy surgery is the most effective treatment for epilepsy in well-selected drug-resistant patients.1 The decision on surgical candidacy is typically taken in a multidisciplinary team meeting based on all noninvasive information, including history, risk factors for epilepsy, possible etiology, seizure semiology, interictal and ictal video EEG findings, structural and functional imaging, and may be complemented by magnetencephalography, high-density EEG, and ictal single-photon emission computerized tomography (SPECT) if required. These tests are performed to establish surgical candidacy. For a surgical plan we must delineate the so-called epileptogenic zone (EZ)/network, which is the brain region for which resection or destruction or disconnection is both necessary and sufficient to ensure a surgical cure.1,2
The risks and benefits of a possible intervention need to be carefully considered including cognitive side effects. If patients suffer from drug-resistant focal epilepsy and a primary surgical hypothesis exists, but uncertainties about the EZ remain, patients likely require intracranial EEG (iEEG). Recent guidelines for iEEG have formalized indications for iEEG in general3 and stereoelectroencephalography (SEEG) specifically4; the goal is to precisely define the EZ when noninvasive data are inconclusive, to resolve divergence of noninvasive data pointing to two or more regions, and to map eloquent functions. Secondary considerations for iEEG include gaining electrophysiological information of prognostic value, and to explore selective ablation of active regions using thermocoagulation (in some centers only). Conversely, iEEG is not indicated when no clear hypothesis can be formulated or the chances of finding a resectable focus are extremely low, the risks of the operative procedure is too high and/or side effects of potential resection are unacceptable.
Although there is no class 1 or 2 evidence that supports iEEG application in specific clinicopathologic settings,3 some clinical scenarios often require iEEG. These include (a) nonlesional likely extratemporal epilepsy, (b) presence of a lesion, but lesion is either large or deep/small, or there are numerous lesions and one region or lesion needs to be confirmed as the epileptogenic lesion, (c) great proximity of the putative EZ to eloquent cortex, (d) previous failed surgery.3-5 It is important to have a clear discussion with the patient ahead of the iEEG investigation to take into account personalized odds of finding a resectable focus and counselling on risks and benefits of the resection if possible after iEEG. Regarding (a), the presence of a lesion improves the odds of becoming seizure free after a resection by a factor of 2.5,6 and particularly concordance of lesion with EEG improves chances of a good outcome.7 The type of lesion, if present, carries some prognostic information as well. In recent years with the more widespread use of SEEG, patients with certain lesion types considered inoperable before are now being considered as candidates for iEEG. This includes large lesions such as polymicrogyria (PMG). A recent multicenter study has concluded that the extent of the PMG should not deter from exploration8: overall, 63% of all patients explored with SEEG (49 cases) had a resection; 65% became seizure free. Deep small or multiple lesions such as heterotopic gray matter has recently also been subject to study showing that such nodules often are an important part of the epileptogenic network. The evidence is still small, but a significant subset may benefit from SEEG explorations, particularly if there is capability to perform thermocoagulation lesioning.4
Complex lesions such as encephalomalacias following head trauma can be successfully addressed using iEEG as long as the lesion is concordant with other noninvasive data, and there is no evidence that history of head injury, presence of encephalomalacia were independent, univariate prognostic factors for outcome of surgery.7
A common indication for bilateral depth EEG explorations is suspected bitemporal lobe epilepsy (TLE). A recent meta-analysis of 1403 patients with presumed bitemporal TLE on scalp EEG undergoing bilateral iEEG showed that the majority (73% of over 1400 patients reviewed) proved to have unilateral TLE on iEEG, and following resection 58% had Engel class I, 9% class II outcome. Of the 132 patients reported to have true bitemporal TLE on iEEG, 23% had Engel class I, 14% Engel class II outcomes after resection.9
Finally, failed epilepsy surgery cases may be candidates for reevaluation. Patients with lesional epilepsy with congruent electrophysiology data have a 58% chance of becoming seizure free with redo surgery, often based on iEEG results; the overall odds of seizure freedom are 47%.10
Intracranial EEG is often performed to map eloquent cortex and understand the relationship to the EZ, to predict cognitive and motor outcomes following resection. This is particularly critical in extratemporal or neocortical temporal lobe epilepsies when seizure onset and critically important functions may be close. Finally, it is assumed that the added risk and cost of iEEG may allow a number of patients to go forward to resections who otherwise could not reduce risk of deficits after resection, and in a small number also allow for therapeutic interventions such as lesioning. Clear criteria to determine extent of resection based on iEEG findings are lacking, but active research is in the process of assessing the contribution of markers such as high frequency oscillations.
2. Chronic SEEG and subdural electrode recording—Methods, risks, and benefits. (Ashwini Sharan, MD, Barbara Jobst, MD, PhD)
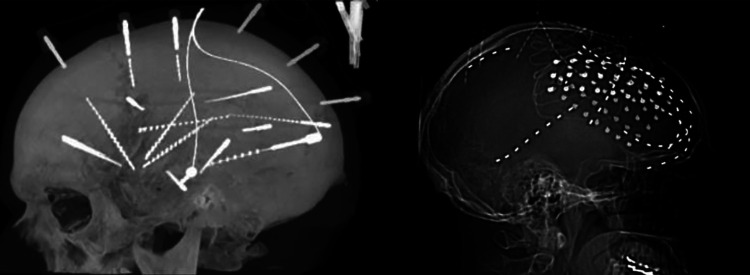
Intracranial EEG (iEEG) can be and strip electrodes or with penetrating SEEG electrodes (Figure 1). Subdural electrodes (SDE) consist of 2 to 5 mm diameter platinum-iridium contacts embedded in Silastic and contacts are usually spaced 10-mm apart. The placement of SDE requires a craniotomy for grid electrodes and burr holes for strip electrodes. Wires may be sutured to the dura to minimize migration or tunneled to the site remote from incision. The skull flap may or may not be replaced. Subdural grid and strip electrodes allow for sampling of large cortical areas with optimal coverage of cortex adjacent to the subdural space. Subdural electrodes allow for functional mapping of large cortical areas. However, SDE do not cover gray matter residing in the sulci and cannot reach many deep structures, and have poor 3-dimensional representation. There is an approximately 10% risk of clinically significant complications with this procedure.11,12
Figure 1.
Stereoelectroencephalography study of temporal lobe epilepsy on the left, grid study with SDE of frontal lobe epilepsy with mesial and lateral coverage to the right.
Stereo-EEG electrodes are penetrating electrodes based on placement with stereotactic techniques. Stereo-EEG electrodes have cylindrical contact 2-mm long with a diameter of 0.8 mm. They are spaced 1.5-8 mm apart and held in place with skull fixation anchor bolts. Trajectories are determined prior to implantation and Stereo-EEG allows for better sampling of epileptic networks as well as precise and accurate mapping of deep cortical areas. Deep cortical areas such as the insula can be easily sampled. Stereo-EEG easily allows for bilateral symmetric implants, which are much more complicated with larger grid implantations. However, limited superficial coverage makes sampling of larger cortical areas challenging and may limit surface functional mapping. Stereo-EEG is usually tolerated better in the immediate postoperative periods compared to grid and strips. Clinical significant complications rate seems 3-times less than with SDE, but high rates of intracranial, most often small and asymptomatic hemorrhages have been reported.11,13 No robust direct comparison of the effectiveness and complications of SDE and SEEG exists.
Identifying the Seizure Onset Zone and the Size of the Resection With Intracranial EEG
3. Interictal features on intracranial EEG to determine the seizure onset zone. (Birgit Frauscher, MD)
When interpreting intracranial EEG (iEEG) studies, it is important to pay careful attention to the interictal EEG, as it provides very useful clues on the epileptogenic zone (EZ) similar to that provided by the ictal EEG. Interictal markers of interest are spikes, high-frequency oscillations (HFOs), and slowing of the EEG background signal.
Spikes are the traditional interictal marker of epilepsy. It is known for many years that there is a correlation between spikes and the EZ, with a high sensitivity but low specificity, when traditionally analyzed. A quantitative iEEG study showed that 56% of patients had a good concordance between spike density and the seizure-onset zone (SOZ). This proportion was higher (75%) in focal cortical dysplasia.14 The specificity of spikes can be further increased, when accounting for spike duration and earliest spike occurrence; spikes are shorter and occur earlier inside than outside the EZ.15,16 Persistence of spikes at seizure onset but outside the SOZ might point to the presence of a second EZ. Absence of spikes in a presumed SOZ should be considered as red flag, that most likely the “true” epileptic generator was missed (this is always an issue in iEEG due to the limited spatial sampling of the cortex).
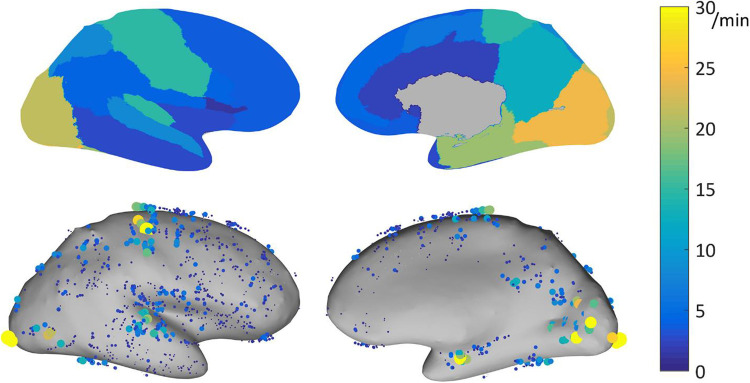
High frequency oscillations are another promising marker of the EZ.17 A meta-analysis found a higher resection ratio in seizure-free versus nonseizure-free patients.18 Using acute postsurgical electrocorticography, presence of postsurgical fast ripples was demonstrated to be indicative of poor surgical outcome.19 A study focusing on normative HFO values in a large data set of 71 patients showed that there are marked interregional variations of HFOs, with highest rates in eloquent cortical areas (Figure 2).20 Normalizing HFO rates for interregional differences might potentially resolve one factor limiting the current use of HFOs in determining the EZ at the individual level.21,22 In fact, a recent multicenter study showed that HFOs did not correctly identify the EZ in 31% of patients; not accounting for region-specific physiological HFO rates might be one explanation.21
Figure 2.
Physiological ripple (80-250 Hz) rate results for bipolar channels recorded with DIXI electrodes, represented on the inflated cortex. Top: 95th percentile of the physiological ripple rate per brain region. Bottom: rate of the individual channels. Each dot represents a channel, the size and color indicate its ripple rate (left: lateral view, right: medial view). Note is made that physiological ripple rates show marked interregional variations, with highest rates in eloquent cortical areas. Reproduced with permission Frauscher et al.20
Abnormal slow wave activity, major alteration of background activity, or electrical silence is often a sign of the lesional area. However, it has to be kept in mind that there are considerable interregional variations, following a caudo-rostral transient with slower posterior frequencies and faster anterior frequencies.23 Alternatively, slowing can be iatrogenic due to electrode insertion or when the electrode is inserted in the vicinity of a resection cavity.
In summary, it is important to carefully analyze the interictal iEEG regarding spikes and background alterations. High frequency oscillations are increasingly recognized to be an interictal marker of the EZ. Prospective well-designed and sampled studies using HFOs for clinical decision making are currently under way.
4. Ictal features used to determine the area of resection—patterns of ictal onset and spread. (Gregory Worrell, MD, PhD, Barbara Jobst, MD, PhD)
Intracranial ictal onset EEG patterns remain the gold standard for identifying the seizure onset zone, planning resective epilepsy surgery and focal electrical stimulation. The epileptogenic zone (EZ) is defined as the brain tissue that needs to be resected or disconnected to render the patient seizure free.24 There are 2 competing concepts for what represents the EZ. Are just focal abnormalities in the cortex responsible for epileptogenicity or does it require neuronal networks to generate epileptic seizures? In temporal lobe epilepsy, an approach with SEEG which can explore the temporal network including the orbitofrontal, cingulate, and insular structures may be appropriate. There is class I evidence that temporal lobe surgery is superior to best medical therapy.25,26 In extratemporal lobe epilepsy, exploration with SEEG or grids and strips can add important additional information including information about the pathological and functional networks and for mapping but overall seizure free outcomes after surgery are less common.27,28 There is evidence that when recording iEEG of a focal region of epileptogenic brain, the most common pattern is a direct current (DC) change or slow-wave if filtered, and concurrent high frequency fast activity. The fast frequency discharge reported at seizure onset, however, can be variable.29-31 The onset pattern can depend on the location within the epileptic network, recording electrode, and amplifiers.
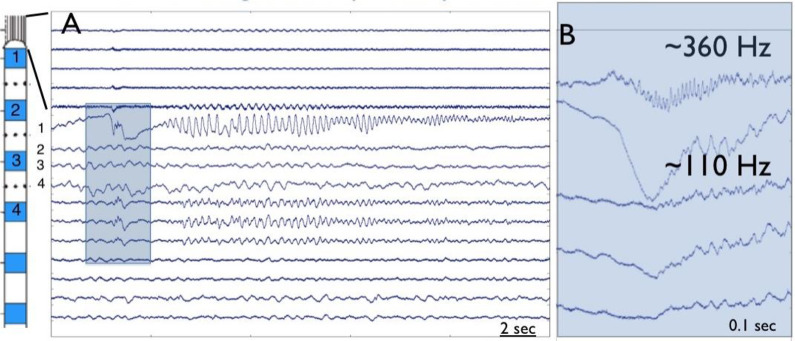
Historically clinical iEEG was recorded with millimeter scale disk electrodes over a limited dynamic range of frequency (∼ 0.5-100 Hz), but recordings using micrometer scale electrodes over a wide dynamic range show high frequency, interictal ripple (200-250 Hz) and fast ripple (250-500 Hz) oscillations.32 Studies with combined macro and microscale electrode combinations (Figure 3) demonstrate that seizure discharges with ictal high frequency oscillations occur on submillimeter spatial scales “microseizures” in addition to high frequency onsets on macroelectrodes.33,34 Lastly, the continued advance in neural recording and analysis capabilities show promise for further mechanistic elucidation of the generation of human focal seizures in the future.35-38
Figure 3.
Ictal high frequency oscillation at the onset of a seizure recorded with macro and microelectrodes (A). High frequency oscillatory activity at onset is detected slightly earlier in the microelectrodes as compared to the macroelectrodes (B).
5. Electrical stimulation as an aid in establishing the area of resection (Philippe Kahane, MD, PhD, Nastasia Tardy, MD, Lorella Minotti, MD)
Despite decades of direct cortical stimulation (DCS) procedures in epilepsy surgery candidates, only a few studies have investigated the relevance of DCS-induced seizures (DCS-S) to aid in delineating the epileptogenic zone. In a recent review of 14 intracranial EEG studies identified in a 30-year period,39 the percentage of patients in whom seizures could be elicited varied from 37% to 100%, with a large predominance of elicited auras; DCS-S were more easily elicited during SEEG procedure than during subdural or electrocorticography recordings, and using 50 Hz DCS than using 1 Hz DCS; still, the concordance rate between DCS-S sites and those overlying the onset of spontaneous seizures (SS), evaluated in 6 studies, varied from 26% to 100%, with the best results obtained for focal cortical dysplasia or temporal lobe epilepsy cases; only one study assessed how the information obtained from DCS might influence seizure outcome, a study which failed to show any differences according to whether the DCS-S sites were included in the resection or not. Overall, the clinical utility of DCS to elicit seizures remains controversial, a controversy which is partly linked to the great variability in the methodologies used across centers, to the differences in the conceptual approach to epilepsy surgery, and to the lack of a consensual definition of DCS-S.
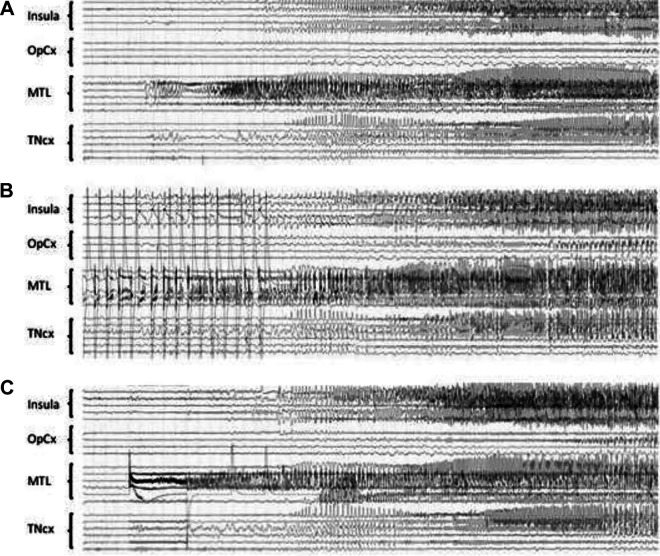
Recently, however, a few studies have provided new information on how DCS-S and SS overlapped, both clinically and electrically, and how DCS-S might help for the surgical decision. DCS-S referred to electro-clinical events that occurred as an immediate result of DCS, and in which both clinical signs and SEEG discharge outlasted the electrical stimulus. In a series of 24 patients comparing 99 DCS-S with 402 SS during SEEG recordings,40 the Grenoble group found a strong electrical (77.4%) and clinical (85.1%) concordance between DCS-S and SS, this correlation being even better when including only the seizures with a complete clinical pattern (86.7% and 95.3%, respectively; see Figure 4). Non habitual seizures were evoked in only very rare instances (9/99 DCS-S), mainly in the temporal lobe and using 50 Hz DCS. Whether clinical signs occurred earlier during DCS-S than during SS was not evaluated, a finding that seems frequent in DCS-S presenting with initial motor semiology.41 In a second SEEG series where 59 (57%)/103 patients had at least one electroclinical DCS-S,42 the groups of Montreal and Grenoble showed that the use of 50 Hz DCS and longer time (>24 hours) since the last seizure was associated with a higher likelihood of seizure elicitation (P < .05). More importantly, they demonstrated that the percentage of patients with DCS-S was higher in the good (Engel class I) versus the poor (class II-III-IV) surgical outcome group (70% vs 47%, P = .020), and that the median percentage of the resected contacts encompassing the DCS-induced seizure-onset zone was higher in the good (63%) versus the poor (33%) surgical outcome group (P = .003). This prognostic value of DCS-S was also found in a third SEEG series by the groups of Marseille and Milan, who showed in a large cohort of 346 patients that 1 Hz DCS-S (obtained in 34% of the cases) represented a positive predictive factor for seizure outcome after surgery.43
Figure 4.
A, Spontaneous seizure, which starts in the mesio-temporal lobe region (MTL) and then spreads to the insula and to the temporal neocortex (TNcx). The supra- and infrasylvian opercular cortex (OpCx) is much less involved. B, 1 Hz DCS (3 mA) of the anterior hippocampus, same patient. The stimulation is stopped (stop) after the onset of the elicited discharge, which is clearly visible between the DCS artefacts. Note the similarity of the shape and extent of the elicited discharge as compared with the spontaneous one. C, Same patient, 50 Hz DCS (2 mA) of the entorhinal cortex. The onset of the elicited discharge is obscured by the DCS artefact, the shape and extent of the elicited discharge is then similar to what was observed spontaneously or by stimulating the hippocampus at 1 Hz. DCS indicates direct cortical stimulation.
Altogether, these findings point out that (i) DCS-S are very similar to habitual spontaneous seizures, both clinically and electrically, which could contribute to significantly reduce the duration of intracranial EEG investigations; (ii) the elicitation of seizures by DCS is a marker of favorable surgical outcome, which suggests that the absence of DCS could be a red flag for an incomplete/incorrect definition of the epileptogenic zone; (iii) the removal of the DCS-S sites and of the DCS-induced seizure-onset zone may contribute to improve the surgical outcome; (iv) both low (1 Hz) and high (50 Hz) DCS should be used, they may provide complimentary information (see Figure 4); (v) all the electrode contacts located within the grey matter should be stimulated to assess the epileptogenicity of all explored cortical areas; (vi) DCS should be performed at the beginning of the invasive investigation, if possible at least 24 hours after the last SS.
6. Computerized methods for determining the area of resection (Fabrice Bartolomei, MD, PhD)
The presurgical assessment of focal epilepsy involves in a large number of cases electrophysiological invasive investigations, among which stereoelectroencephalography (SEEG) is a good method to record EEG activity in different brain regions. The seizure onset zone (SOZ) is increasingly recognized as a network of connected hyperexcitable regions. Thus, the onset of seizures (and early propagation) may appear quite complex in its aspect, involving variable spatiotemporal patterns. In 75% of cases, seizures onset patterns (SOP) involve high frequencies, most often in the high beta or gamma band (20-30 Hz).44
Several computerized methods for quantifying the SOZ have been developed over the past 10 years.30,45-48 Most of them are based on a spectral analysis of SEEG signals obtained in each region and particularly based on the detection of high frequency content (beta-gamma bands). As an example, a method (SPM Gamma index) uses a statistical estimate of the seizure onset gamma power compared to a baseline period using a neuroimaging methodology (SPM).46 The first developed method and the one with the largest published patient population is the Epileptogenicity Index (EI). Epileptogenicity Index mathematically combines the quantification of an energy ratio of fast frequencies (beta/gamma) relative to slow frequencies and the delay of involvement of each region.45 The EI method therefore measures the speed of involvement of each studied brain regions and their ability to generate rapid tonic discharges. Epileptogenicity Index values are normalized in each channel and can be viewed in the magnetic resonance imaging (Figure 5).49 This method was the basis of several studies estimating the number of epileptogenic structures (the majority of focal epilepsies have at least 2 epileptogenic regions) or the extension of the epileptogenic network over time.50 The number of epileptogenic regions is inversely correlated with surgical prognosis. The estimation of EI can be used as a basis for comparisons with interictal biomarkers such as spikes or HFOs.22
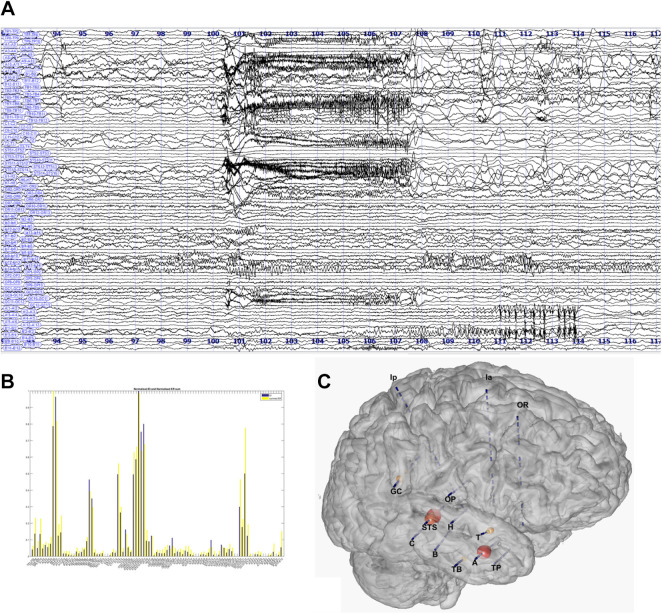
Figure 5.
Estimation of the Epileptogenicity Index in a case of complex pattern of seizure onset. A, SEEG bipolar traces. B, Estimation for each channels of the EI values. C, Representation of the EI values on a 3D MESH of the magnetic resonance imaging and electrodes. EI indicates Epileptogenicity Index.
All these methods are limited by sampling problems and are most often blind to the slower SOP that represent 20% of cases.44 New approaches are currently emerging that combine computational modeling and structural connectivity and can provide hope for better interpretation of SEEG recordings in the future.51
Conclusion
Intracranial EEG remains a mainstay diagnostic test to identify the seizure onset zone or area of resection for epilepsy surgery. Intracranial EEG should only be performed if there is an adequate hypothesis for networks involved in seizures generation (Table 1). Techniques for electrode implantation have advanced over the last 2 decades to assure accurate electrode placement. Interictal EEG should not only be evaluated for interictal epileptiform activity but also be examined for high frequency oscillations and slow frequency activity. Ictal EEG should be analyzed in detail and advanced recording can add further information. Inducing clinical seizures with electrical stimulation can add information to localize the seizure onset zone. Advanced computational analysis of intracranial data can assist with further identification of the seizure onset zone. With all the advanced methods, better identification of the seizure onset zone should be possible in the 21st century.
Table 1.
Clinical Scenarios and the Preferred Method of IEEG.
| Clinical Scenario | Preferred method | Secondary method |
|---|---|---|
| Lesion of hypothetical SOZ near or in eloquent cortex | SDE | SEEG |
| Lesion or hypothetical SOZ has a deep location or is not near eloquent cortex | SEEG | SDE & depth electrodes |
| Need for bilateral explorations & reoperation | SEEG | SDE & depth electrodes |
| Prior failure of SDE to localize SOZ | SEEG | SDE & depth electrodes |
| Need to map epileptogenic network | SEEG | SDE & depth electrodes |
Abbreviations: IEEG, intracranial electroencephalography; SDE, subdural electrodes; SEEG, stereoelectroencephalography; SOZ, seizure onset zone.
Supplemental Material
Supplemental Material, Permission_Figure1.pdf for Intracranial EEG in the 21st Century by Barbara C. Jobst, Fabrice Bartolomei, Beate Diehl, Birgit Frauscher, Philippe Kahane, Lorella Minotti, Ashwini Sharan, Nastasia Tardy, Gregory Worrell and Jean Gotman in Epilepsy Currents
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Barbara C. Jobst  https://orcid.org/0000-0001-9243-2238
https://orcid.org/0000-0001-9243-2238
Jean Gotman  https://orcid.org/0000-0002-9796-5946
https://orcid.org/0000-0002-9796-5946
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13(11):1114–1126. [DOI] [PubMed] [Google Scholar]
- 2. Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124(pt 9):1683–1700. [DOI] [PubMed] [Google Scholar]
- 3. Jayakar P, Gotman J, Harvey AS, et al. Diagnostic utility of invasive EEG for epilepsy surgery: indications, modalities, and techniques. Epilepsia. 2016;57(11):1735–1747. [DOI] [PubMed] [Google Scholar]
- 4. Minotti L, Montavont A, Scholly J, Tyvaert L, Taussig D. Indications and limits of stereoelectroencephalography (SEEG). Neurophysiol Clin. 2018;48(1):15–24. [DOI] [PubMed] [Google Scholar]
- 5. Kovac S, Vakharia VN, Scott C, Diehl B. Invasive epilepsy surgery evaluation. Seizure. 2017;44(3):125–136. [DOI] [PubMed] [Google Scholar]
- 6. Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):31031–31038. [DOI] [PubMed] [Google Scholar]
- 7. West S, Nolan SJ, Cotton J, et al. Surgery for epilepsy. Cochr Data Syst Rev. 2015;2( 7):CD010541. [DOI] [PubMed] [Google Scholar]
- 8. Maillard LG, Tassi L, Bartolomei F, et al. Stereoelectroencephalography and surgical outcome in polymicrogyria-related epilepsy: a multicentric study. Ann Neurol. 2017;82(5):781–794. [DOI] [PubMed] [Google Scholar]
- 9. Aghakhani Y, Liu X, Jette N, Wiebe S. Epilepsy surgery in patients with bilateral temporal lobe seizures: a systematic review. Epilepsia. 2014;55(12):1892–1901. [DOI] [PubMed] [Google Scholar]
- 10. Krucoff MO, Chan AY, Harward SC, et al. Rates and predictors of success and failure in repeat epilepsy surgery: a meta-analysis and systematic review. Epilepsia. 2017;58(12):2133–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt RF, Wu C, Lang MJ, et al. Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia 2016;57(10):1697–1708. [DOI] [PubMed] [Google Scholar]
- 12. Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia. 2013;54(5):828–839. [DOI] [PubMed] [Google Scholar]
- 13. McGovern RA, Ruggieri P, Bulacio J, Najm I, Bingaman WE, Gonzalez-Martinez JA. Risk analysis of hemorrhage in stereo-electroencephalography procedures. Epilepsia. 2019;60(3):571–580. [DOI] [PubMed] [Google Scholar]
- 14. Bartolomei F, Trebuchon A, Bonini F, et al. What is the concordance between the seizure onset zone and the irritative zone? A SEEG quantified study. Clin Neurophysiol. 2016;127(2):1157–1162. [DOI] [PubMed] [Google Scholar]
- 15. Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia 2000;41(4):467–478. [DOI] [PubMed] [Google Scholar]
- 16. Khoo HM, von Ellenrieder N, Zazubovits N, He D, Dubeau F, Gotman J. The spike onset zone: the region where epileptic spikes start and from where they propagate. Neurology. 2018;91(7):e666–e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frauscher B, Bartolomei F, Kobayashi K, et al. High-frequency oscillations: the state of clinical research. Epilepsia. 2017;58(8):1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holler Y, Kutil R, Klaffenbock L, et al. High-frequency oscillations in epilepsy and surgical outcome. A meta-analysis. Front Hum Neurosci. 2015;9:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Klooster MA, Van Klink NEC, Zweiphenning W, et al. Tailoring epilepsy surgery with fast ripples in the intraoperative electrocorticogram. Ann Neurol. 2017;81(5):664–676. [DOI] [PubMed] [Google Scholar]
- 20. Frauscher B, von Ellenrieder N, Zelmann R, et al. High-frequency oscillations in the normal human brain. Ann Neurol. 2018;84(3):374–385. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs J, Wu JY, Perucca P, et al. Removing high-frequency oscillations: a prospective multicenter study on seizure outcome. Neurology. 2018;91(11):e1040–e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roehri N, Pizzo F, Lagarde S, et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann Neurol. 2018;83(1):84–97. [DOI] [PubMed] [Google Scholar]
- 23. Frauscher B, von Ellenrieder N, Zelmann R, et al. Atlas of the normal intracranial electroencephalogram: neurophysiological awake activity in different cortical areas. Brain. 2018;141(4):1130–1144. [DOI] [PubMed] [Google Scholar]
- 24. Luders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord. 2006;8(suppl 2):S1–S9. [PubMed] [Google Scholar]
- 25. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. [DOI] [PubMed] [Google Scholar]
- 26. Engel J, Jr, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(pt 5):1188–1198. [DOI] [PubMed] [Google Scholar]
- 28. Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA. 2015;313(3):285–293. [DOI] [PubMed] [Google Scholar]
- 29. Wetjen NM, Marsh WR, Meyer FB, et al. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neuro. 2009;110(6):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grinenko O, Li J, Mosher JC, et al. A fingerprint of the epileptogenic zone in human epilepsies. Brain. 2018;141(1):117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41(3):297–307. [DOI] [PubMed] [Google Scholar]
- 32. Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100-500 Hz) in human epileptic brain and in kainic acid—treated rats with chronic seizures. Epilepsia. 1999;40(2):127–137. [DOI] [PubMed] [Google Scholar]
- 33. Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133(9):2789–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schevon CA, Ng SK, Cappell J, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25(6):321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jun JJ, Steinmetz NA, Siegle JH, et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551(7679):232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viventi J, Kim DH, Vigeland L, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14(12):1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith EH, Liou JY, Davis TS, et al. The ictal wavefront is the spatiotemporal source of discharges during spontaneous human seizures. Nat Commun. 2016;7:11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinet LE, Fiddyment G, Madsen JR, et al. Human seizures couple across spatial scales through travelling wave dynamics. Nat Commun. 2017;8:14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kovac S, Kahane P, Diehl B. Seizures induced by direct electrical cortical stimulation—mechanisms and clinical considerations. Clin Neurophysiol. 2016;127(1):31–39. [DOI] [PubMed] [Google Scholar]
- 40. Tardy N. Electro-clinical correlation between seizures induced by direct electrical cortical stimulation and spontaneous seizures: relevance to define the epileptogenic zone. MD Thesis. Grenoble-Alpes University; 2017. [Google Scholar]
- 41. McGonigal A, Lagarde S, Trebuchon-Dafonseca A, Roehri N, Bartolomei F. Early onset motor semiology in seizures triggered by cortical stimulation during SEEG. Epilepsy Behav. 2018;88:262–267. [DOI] [PubMed] [Google Scholar]
- 42. Cuello Oderiz C, von Ellenrieder N, Dubeau F, et al. Association of cortical stimulation-induced seizure with surgical outcome in patients with focal drug-resistant epilepsy. JAMA Neurol. 2019;76(9):1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Racilla A, Trebuchon-Da Fonseca A, Lagarde S, et al. Electrical stimulation for seizure induction during SEEG exploration: are they useful or not? Paper presented at: 13th Congress on Epileptology; Vienna, Austria; 2018, pp. 2–18. [Google Scholar]
- 44. Lagarde S, Buzori S, Trebuchon A, et al. The repertoire of seizure onset patterns in human focal epilepsies: determinants and prognostic values. Epilepsia. 2019;60(1):85–95. [DOI] [PubMed] [Google Scholar]
- 45. Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131(pt 7):1818–1830. [DOI] [PubMed] [Google Scholar]
- 46. David O, Blauwblomme T, Job AS, et al. Imaging the seizure onset zone with stereo-electroencephalography. Brain. 2011;134(pt 10):2898–2911. [DOI] [PubMed] [Google Scholar]
- 47. Gnatkovsky V, de Curtis M, Pastori C, et al. Biomarkers of epileptogenic zone defined by quantified stereo-EEG analysis. Epilepsia. 2014;55(2):296–305. [DOI] [PubMed] [Google Scholar]
- 48. Gnatkovsky V, Francione S, Cardinale F, et al. Identification of reproducible ictal patterns based on quantified frequency analysis of intracranial EEG signals. Epilepsia. 2011;52(3):477–488. [DOI] [PubMed] [Google Scholar]
- 49. Medina Villalon S, Paz R, Roehri N, et al. Epi tools, a software suite for presurgical brain mapping in epilepsy: intracerebral EEG. J Neurosci Methods. 2018;303:7–15. [DOI] [PubMed] [Google Scholar]
- 50. Bartolomei F, Lagarde S, Wendling F, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia. 2017;58(7):1131–1147. [DOI] [PubMed] [Google Scholar]
- 51. Proix T, Bartolomei F, Guye M, Jirsa VK. Individual brain structure and modelling predict seizure propagation. Brain. 2017;140(3):641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Permission_Figure1.pdf for Intracranial EEG in the 21st Century by Barbara C. Jobst, Fabrice Bartolomei, Beate Diehl, Birgit Frauscher, Philippe Kahane, Lorella Minotti, Ashwini Sharan, Nastasia Tardy, Gregory Worrell and Jean Gotman in Epilepsy Currents