Abstract
The covalently closed circular RNA has recently been proposed as a pivotal player in tumorigenesis. In the current study, we found that circ-CDYL was notably elevated in multiple myeloma tissue and plasma samples and had good diagnostic and prognostic efficacy. Functional assays showed that circ-CDYL enhanced the viability and DNA synthesis of multiple myeloma cells and inhibited apoptosis. Mechanically, cytoplasmic circ-CDYL was co-localized with miR-1180, and circ-CDYL absorbed miR-1180 to upregulate yes-associated protein (YAP), thereby facilitating multiple myeloma progression. Importantly, we further confirmed the existence of this circ-CDYL/miR-1180/YAP regulatory axis in vivo by using the xenograft tumor model. Taken together, our data demonstrate that circ-CDYL is novel promoter of multiple myeloma, and targeting circ-CDYL and its associated network implicates the therapeutic possibility for multiple myeloma patients.
Impact statement
Multiple myeloma (MM) is an extremely complex and heterogeneous disease, and its pathogenesis is poorly understood. Here, we described an important MM-related circular RNA (circRNA), circ-CDYL. It was remarkably increased in both MM cells and plasma. Depletion of circ-CDYL evidently stunted MM growth. Circ-CDYL could absorb miR-1180 and alleviated the repression of miR-1180 on YAP, leading to increased YAP expression, ultimately triggering MM uncontrolled growth. Therefore, our findings advance the understanding of MM pathogenesis, and also raise the possibility of considering circ-CDYL as a potential therapeutic intervention for MM.
Keywords: Circular RNA, biomarker, multiple myeloma, miRNA sponge
Introduction
Multiple myeloma (MM) is a malignant plasma cell disease derived from B lymphocytes in the bone marrow that develop to the ultimate functional stage.1 MM accounts for 1% of all malignant tumors and is the second most common malignancy of the blood system.2 Despite numerous novel drugs have been discovered for this disease in the past several years, the prognosis is still poor, especially in patients with high-risk MM.3 In-depth research into the mechanisms underlying MM development and progression will facilitate the optimization of the choice of therapeutic regimen and improve clinical outcomes.
It has been well documented that non-coding RNA participates in the vast majority of life processes.4 Circular RNA (circRNA) is a kind of special non-coding RNA that has a closed loop structure.5 Currently, extensive circRNAs have been found in eukaryotes and expressed in the cell- or disease-context-dependent manner.6 Eighty percent of circRNAs are cytoplasmic circRNAs that regulate gene expression via sponging microRNA (miRNA).7 For example, circ-OSBPL10 could sponge miR-136-5p to upregulate WNT2 expression, thereby acting as an oncogenic circRNA in gastric cancer.8 Circ-LDLRAD3 was shown to suppress pancreatic cancer progression by abundantly absorbing miR-137-3p and increasing PTN expression.9 Circ-ANKS1B was identified to be a metastasis promoter in breast cancer via concurrently sponging miR-148 and miR-152.10 These studies suggest that the circRNA-centric non-coding regulatory network is functional in cancer cell biology.
Recently, a novel circRNA, circ-CDYL, has been proposed as an new biomarker in mantle cell lymphoma.11 However, the molecular functions and biological roles of circ-CDYLin MM remain unknown. Herein, we found that circ-CDYL was also overexpressed in MM tissue and plasma samples, and further investigated the mechanism underlying its carcinogenic effect.
Materials and methods
MM samples
The bone marrow tissues and peripheral blood specimens of 72 MM patients and 13 healthy donors (volunteers) were collected from Affiliated Shengjing Hospital of China Medical University. And multiple myeloma cells in the bone marrow were sorted. All enrolled patients were followed up every three months, and the informed consent of each patient was obtained prior to this study. All processes were in accordance with the policy of the ethics committee of Affiliated Shengjing Hospital of China Medical University.
Cell culture and transfection
Two MM cell lines (MM1.S and NCI-H929) purchased from ATCC (Manassas, VA, USA) were grown in a special cell incubator. The cells were tested for mycoplasma contamination before use. Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) reagent was applied to conduct cell transfection as per manufacturer’s manual. To generate stable circ-CDYL knock down cell lines, the pLKO.Tet.On shRNA (Addgene, Cambridge, UK) vector was packed with lentivirus and infected into MM1.S and NCI-H929 cells in the presence of polybrene. After infection, 0.5 μg/mL puromycin was applied for two weeks to select stable cells.
qRT-PCR assay
TRIzol reagent was applied to extract total RNA, followed by generation of first-strand cDNA by using PrimeScript™ RT reagent Kit (TaKaRa, Dalian, China). Then, cDNA amplification and quantification were performed by using TB Green Premix Ex Taq Kit (TaKaRa) based on the standard protocols. The primer sequences: circ-CDYL: forward: 5′-ACCCACTAGTGCCTCAGGTG-3′, reserve: 5′-AGCCTT TCCACCGAACCAAA-3′. YAP:: forward: 5′-CAGACAG TGGACTAAGCATGAG-3′, reserve: 5′-CAGGGTGCTTTG GTTGATAGTA-3′. GAPDH: forward: 5′-ACCCAGAAGA CTGTGGATGG-3′, reserve: 5′-TTCAGCTCAGGGATGAC CTT-3′.
CCK-8 and EdU assays
For CCK-8 assay, the circ-CDYL-silenced cells were plated onto 96-well plates and cultured for 24 h, 48 h, and 72 h, followed by treatment with CCK-8 solution (Abmole Bioscience, PA, USA) for 1 h. Lastly, the absorbance at 450 nm was detected and analyzed. In addition, EdU assay was carried out by using Cell-LightTM EdU Detection Kit purchased from RiboBio Company (Guangzhou, China).
Cell apoptosis assay
Cell apoptosis was conducted by using Annexin V PE/7-AAD Detection Kit as per standard protocols (BD Biosciences, San Jose, CA, USA). In brief, 1 × 106 cells were collected and treated with 500 μL binding buffer, followed by in dark incubation with 5 μL PE and 5 μL 7-ADD for 15 min and analysis of apoptotic cells.
RNA pull-down assay
The circ-CDYL probe labeled with biotin (5′-ACGGGAAAGGTTGAAAGGATT-3′) was purchased commercially from GenePharma (Shanghai, China). Then, the above probe was incubated with MM1.S and NCI-H929 cell lysates at 25°C for 2 h. The streptavidin-coupled dynabeads (Invitrogen) were added into above lysates and incubated at 25°C for another 2 h. The complex was washed and eluted for qRT-PCR.
Luciferase reporter assay
The wild-type or mutant full length of circ-CDYL and 3ʹ-UTR of YAP were synthesized and cloned into pmirGLO REPORT vector, followed by co-transfection with miR-1180 mimics into MM1.S and NCI-H929 cells using Lipofectamine™ 2000 (Invitrogen). The commercial luciferase kit (Promega, Madison, WI, USA) was applied to determine the luciferase activity.
Western blot
Total protein was collected using RIPA buffer supplemented with proteasome inhibitors, and added with loading dye, followed by electrophoresis on SDS-PAGE gel, transfer, and blockade. After that, the membrane was incubated with anti-YAP (#14074, CST) primary antibody and anti-rabbit secondary antibody. Lastly, the membrane was developed using chemiluminescence solution in darkroom.
Xenograft tumor model
The xenografted mice were established by subcutaneously injecting control or circ-CDYL-depleted MM1.S cells into BALB/c nude mice. The mice were grown under specific condition with approval from the Animal Ethics Committee of Affiliated Shengjing Hospital of China Medical University. After five weeks, all mice were euthanized and tumors were dissected. Immunohistochemistry (IHC) staining was conducted by using DAB IHC Detection Kit (#ab236466, Abcam) in accordance with the standard manual. The antibodies used for IHC staining in this study were anti-Ki-67 (#ab833, Abcam) and anti-YAP (#14074, CST).
Statistical analysis
The difference between the two groups was compared by Student’s t-test, while the differences between the multiple groups were compared by the analysis of variance (ANOVA). All results were calculated using the SPSS software. P value less than 0.05 was statistically significant.
Results
Circ-CDYLis frequently overexpressed in MM
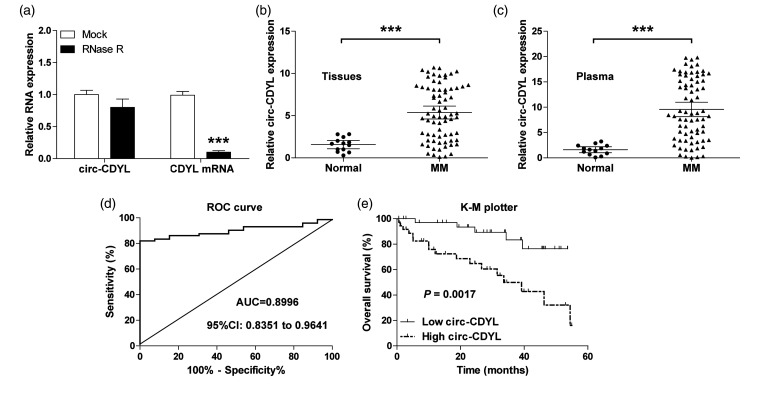
First, we assessed the characteristic of circ-CDYL, and found that circ-CDYL, but not its linear isoform, was highly resistant to RNase R (Figure 1(a)). We then tested circ-CDYL expression in MM tissues, as shown in Figure 1(b), and circ-CDYL was remarkably upregulated in MM tissues in comparison with normal tissues. High circ-CDYL was closely linked to advanced international staging system (ISS) and Durie-Salmon (DS) stage (Table 1). Importantly, a significantly upregulation of circ-CDYL was also observed in MM plasma in comparison to normal plasma (Figure 1(c)), and the area under the curve (AUC) value was 0.8996 (95%CI: 0.8351 to 0.9641) (Figure 1(d)), suggesting that plasma circ-CDYL level is an excellent biomarker for the diagnosis of MM. In addition, high circ-CDYL was positively linked to shorter survival time, as demonstrated by the Kaplan–Meier plotter (Figure 1(e)).
Figure 1.
The expression characteristic of circ-CDYL in MM. (a) qRT-PCR analysis of circ-CDYL and CDYL mRNA expression after treatment with RNase R. (b, c) qRT-PCR analysis of circ-CDYL expression in normal (n = 13) and MM (n = 72) tissue and plasma samples. (d). ROC curve of MM patients based on plasma circ-CDYL expression. (e) The survival curve of MM patients based on median circ-CDYL expression in MM tissues. ***P < 0.001.
Table 1.
Difference in the circ-CDYL expression in multiple myeloma patients grouped by clinicopathological characteristics.
| Clinicopathological characteristics | Total (n=72) |
Circ-CDYL expression |
P value | |
|---|---|---|---|---|
| Low (n=36) | High (n=36) | |||
| Age (years) | ||||
| ≤42 | 33 | 17 | 16 | 0.813 |
| >42 | 39 | 19 | 20 | |
| Gender | ||||
| Male | 31 | 16 | 15 | 0.812 |
| Female | 41 | 20 | 21 | |
| M protein | ||||
| IgG | 22 | 11 | 11 | 0.961 |
| IgA | 21 | 11 | 10 | |
| Light chain | 29 | 14 | 15 | |
| ISS stage | ||||
| I | 11 | 10 | 1 | 0.000 |
| II | 20 | 16 | 4 | |
| III | 41 | 10 | 31 | |
| DS stage | ||||
| I | 15 | 13 | 2 | 0.000 |
| II | 19 | 10 | 9 | |
| III | 38 | 13 | 25 | |
| Hypercalcemia | ||||
| No | 48 | 26 | 22 | 0.317 |
| Yes | 24 | 10 | 14 | |
| Bone disease | ||||
| No | 32 | 19 | 13 | 0.155 |
| Yes | 40 | 17 | 23 | |
| Cytogenetic abnormality | ||||
| No | 51 | 27 | 24 | 0.437 |
| Yes | 21 | 9 | 12 | |
ISS: international staging system; DS: Durie-Salmon.
Depletion of circ-CDYL inhibits MM cell proliferation
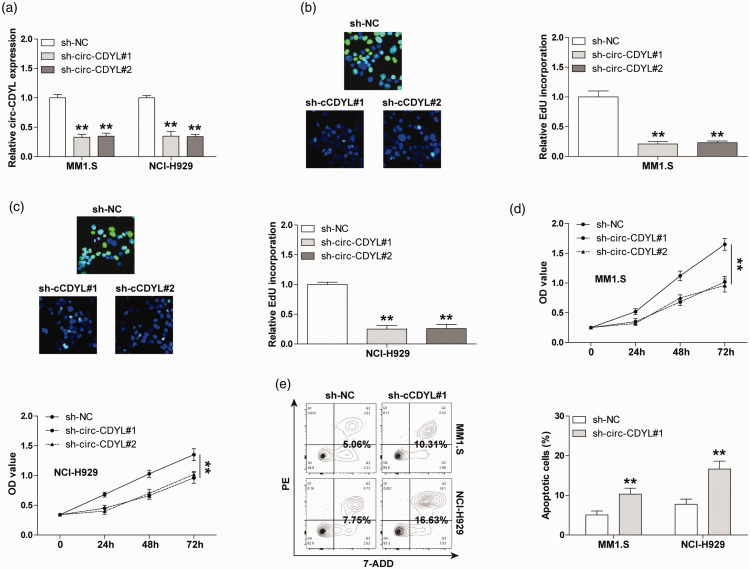
To clarify the biological function of circ-CDYL in MM, we generated stable circ-CDYL-silenced MM1.S and NCI-H929 cell lines (Figure 2(a)). The EdU staining results displayed that circ-CDYL knockdown substantially reduced DNA synthesis rate (Figure 2(b) and (c)). And compared with control groups, the cell activities of circ-CDYL-depleted groups were significantly weakened (Figure 2(d)), as demonstrated by the CCK-8 assay. We also evaluated the number of apoptotic cells after altering circ-CDYL expression. The results of flow cytometry showed that the knockdown of circ-CDYL notably increased the cell apoptosis both in MM1.S and NCI-H929 cells (Figure 2(e)). These phenotypic assays suggest that circ-CDYL is a promoter of MM aggressive progression.
Figure 2.
The effect of circ-CDYL on MM cell phenotype. (a) qRT-PCR analysis confirming the knockdown efficiency of circ-CDYL in MM cells. (b, c) EdU assay detecting DNA synthesis rate in MM cells after circ-CDYL depletion. (d) CCK-8 assay detecting cell viability of MM cells after circ-CDYL depletion. (e) Flow cytometry detecting the number of apoptotic MM cells after circ-CDYL depletion. **P < 0.01. (A color version of this figure is available in the online journal.)
Circ-CDYL absorbs miR-1180 in MM cells
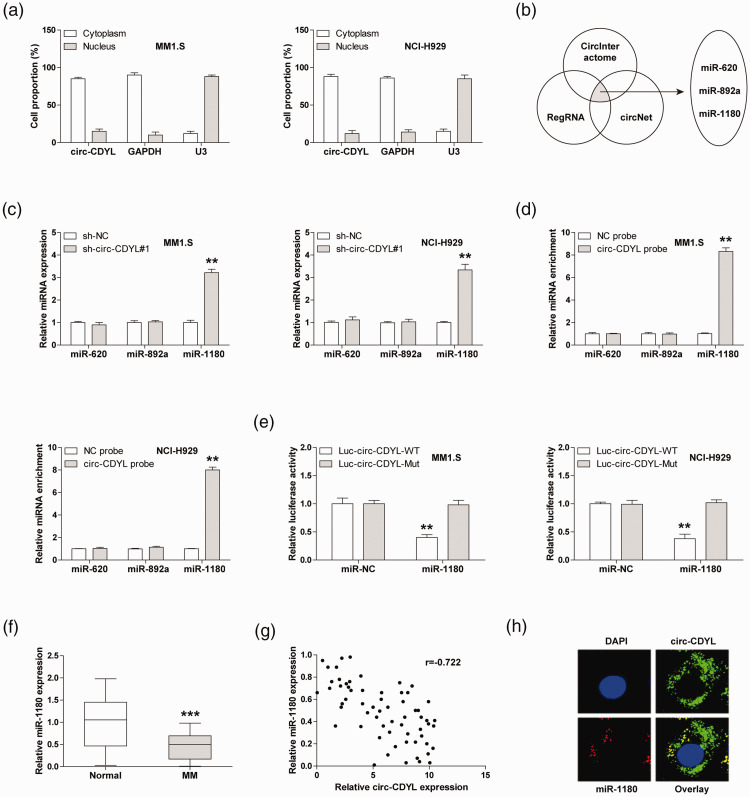
To determine the underlying mechanism by which circ-CDYL functions, we first tested the localization of circ-CDYL in MM cells. The qRT-PCR results revealed that circ-CDYL was a cytoplasmic circRNA (Figure 3(a)). In light of the ‘miRNA sponge’ role of cytoplasmic circRNA, we predicted the miRNAs that may be bound by circ-CDYL by using three online tools, and the overlapping results showed that miR-620, miR-892a, and miR-1180 were simultaneously predicted by the above tools (Figure 3(b)). We then performed qRT-PCR assay in MM1.S and NCI-H929 cells, and the results displayed that only miR-1180 expression was significantly increased after circ-CDYL knockdown (Figure 3(c)). As displayed in Figure 3(d), circ-CDYL probes could abundantly enriche miR-1180, but not miR-620 and miR-892a. Consistently, overexpression of miR-1180 dramatically reduced the luciferase activity of wild-type circ-CDYL reporter (Figure 3(e). Besides, miR-1180 was significantly reduced in MM tissues (Figure 3(f)), and a negative link was observed between the circ-CDYL and miR-1180 expression (Figure 3(g)). Importantly, circ-CDYL was co-located with miR-1180 in MM cells, as shown by the FISH assay (Figure 3(h)). The above findings suggest that miR-1180 is the downstream target of circ-CDYL in MM cells.
Figure 3.
Circ-CDYL sponges miR-1180 in MM cells. (a) qRT-PCR analysis detecting the subcellular localization of circ-CDYL in MM cells. (b) The overlapping miRNAs that are bound by circ-CDYL predicted in the indicated three online tools. (c) qRT-PCR analysis of three miRNA levels in circ-CDYL-depleted MM cells. (d) RNA pull-down detecting the circ-CDYL-bound miRNAs using biotin-labeled probes. (e) The luciferase activity of MM cells co-transfected with wild-type or mutant circ-CDYL reporter and control or miR-1180 mimics. (f) qRT-PCR analysis of miR-1180 expression in MM tissues. (g) The correlation between circ-CDYL and miR-1180 expression in MM tissues. (h) FISH assay detecting the co-location of circ-CDYL and miR-1180 in MM cells. **P < 0.01, ***P < 0.001. (A color version of this figure is available in the online journal.)
Identification of circ-CDYL/miR-1180/Yap in MM cells
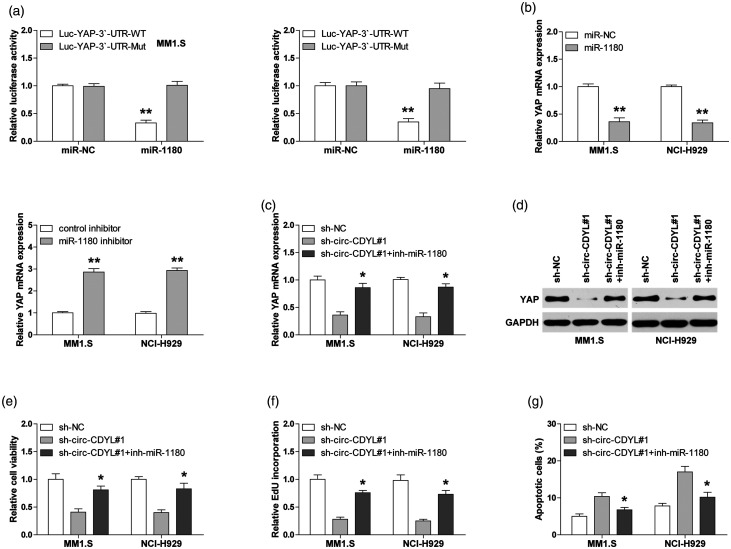
Next, we used the online miRWalk database to identify the downstream target of miR-1180, and found that YAP, the well-known oncogene, may be bound by miR-1180. To test this possibility, we first conducted the luciferase reporter assay in MM1.S and NCI-H929 cells. As demonstrated in Figure 4(a), overexpression of miR-1180 significantly decreased the luciferase activity of wild-type YAP 3ʹ-UTR reporter, while this effect was not observed in the mutant reporter. Consistently, reintroduction of miR-1180 evidently reduced YAP expression, and silencing of miR-1180 resulted in the opposite effect (Figure 4(b)). Importantly, both YAP mRNA and protein levels were reduced in circ-CDYL-depleted cells, and this repression was evidently blocked after miR-1180 knockdown (Figure 4(c) and (d)). Moreover, the diminished malignant phenotypes of MM1.S and NCI-H929 cells were partly rescued after silencing of miR-1180 (Figure 4(e) and (f)).
Figure 4.
Circ-CDYL regulates miR-1180/YAP axis in MM cells. (a) The luciferase activity of MM cells co-transfected with wild-type or mutant YAP 3ʹ-UTR reporter and control or miR-1180 mimics. (b) qRT-PCR analysis of YAP mRNA expression in MM cells transfected with miR-1180 mimics or inhibitors. (c, d) qRT-PCR and Western blot analysis of YAP expression in circ-CDYL-silenced MM cells transfected with miR-1180 inhibitors. (e–g) The CCK-8, EdU, and apoptosis assays in circ-CDYL-silenced MM cells transfected with miR-1180 inhibitors. *P < 0.05, **P < 0.01.
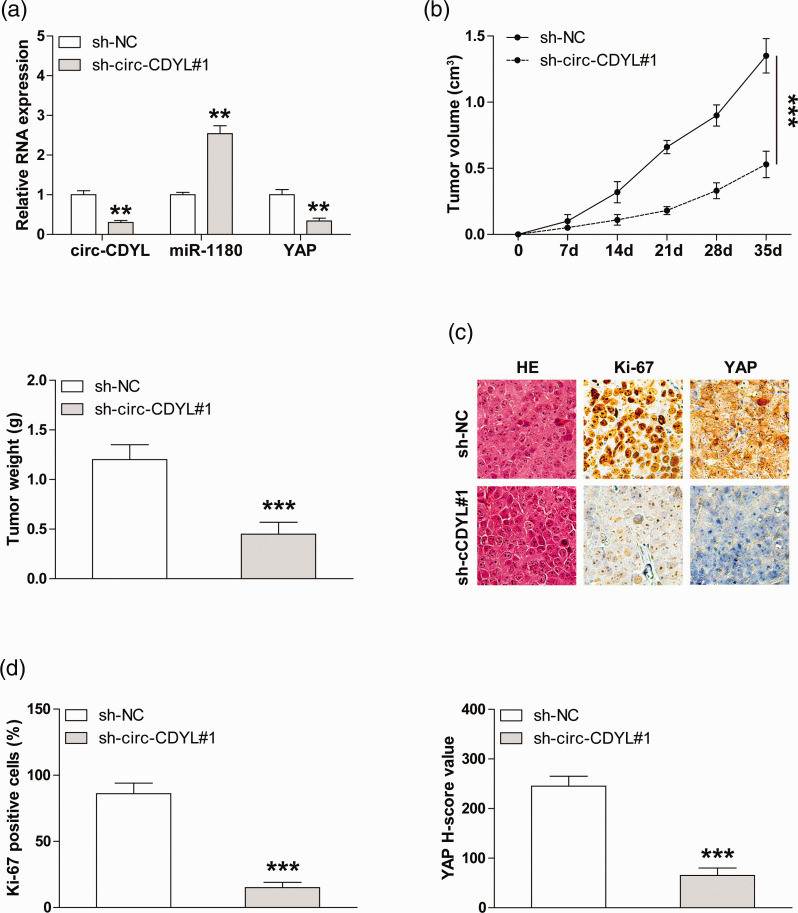
Knockdown of circ-CDYL retards MM in vivo growth
We further established the xenograft model by the subcutaneous injection of MM1.S cells, and the qRT-PCR results indicated that the circ-CDYL and YAP expression was significantly decreased, whereas the miR-1180 expression was significantly increased in circ-CDYL-depleted group in comparison to control group (Figure 5(a)). And the tumor volume and the weight of circ-CDYL-silenced group were lower than those of the control group (Figure 5(b)). Likewise, less Ki-67 and YAP-positive cells were observed after the knockdown of circ-CDYL, as illustrated by the IHC staining (Figure 5(c) and (d)). These data indicate that the circ-CDYL/miR-1180/YAP regulatory axis also exists in vivo.
Figure 5.
The effect of circ-CDYL on MM cell growth in xenografted nude mice. (a) qRT-PCR analysis of circ-CDYL, miR-1180, and YAP levels in circ-CDYL-silenced subcutaneous tumors. (b) The volume and weight of subcutaneous tumors in control and circ-CDYL-depleted groups (n = 5 in each group). (c, d) IHC staining of Ki-67 and YAP in in control and circ-CDYL-depleted groups. **P < 0.01, ***P < 0.001. (A color version of this figure is available in the online journal.)
Discussion
Currently, the field of non-coding RNA is attracting great attention, especially circRNA. A larger number of studies suggest that circRNA plays a fundamental role in carcinogenesis and aggressive progression.12 However, to the best of our knowledge, only one study has reported a link between circRNA and MM, in which circ_0000190 was decreased in MM and inhibited MM progression by modulating miR-767-5p/MAPK4 pathway.13 Here, we described another important circRNA, circ-CDYL, which was significantly upregulated in MM. Knockdown of circ-CDYL evidently stunted MM cell growth. Further mechanism analyses revealed that miR-1180 was a functional target of circ-CDYL, in which circ-CDYL alleviated the repression of miR-1180 on YAP, leading to increased YAP expression, ultimately triggering MM uncontrolled growth. Therefore, our data advance the understanding of the biological function of circRNA in MM.
Non-invasive biomarkers are especially important when early diagnosis of MM is needed to improve survival.14 Emerging evidence suggests that the circulating transcriptome represents a rich source of potential cancer biomarkers.15 Due to the high stability of circRNA, it is better suited as a cancer biomarker than linear RNA. For instance, plasma circ-PTGES3,16 circ-VAPA,17 circ-YWHAZ/circ-BNC2,18 and circ-LDLRAD319 were proposed as effective diagnostic biomarkers of liver cancer, colorectal cancer, lung cancer, and pancreatic cancer, respectively. Here, circ-CDYL was significantly upregulated in MM plasma, and the AUC value was 0.8996, implicating that plasma circ-CDYL may be a novel non-invasive diagnostic biomarker of MM. This notion needs to be further confirmed by the large-scale MM specimens. Besides, unlike upregulation of circCDYL shown in our data and in hepatocellular carcinoma,20 Sun et al.21 showed that circ-CDYL was downregulated in bladder cancer, and this may be due to the specific expression patterns of circRNA in different tissues and developmental stages.21
It has been shown that circRNA functions in a variety of ways, the best known function is “miRNA sponge,” in which circRNA is able to effectively sponge miRNA to reduce the inhibition of miRNA on its target gene.22 Numerous studies have reported that circRNA participated in cancer stemness, occurrence, metastasis, and drug resistance by sponging miRNA.23,24 Intriguingly, circRNA sponged different miRNAs in different contexts. For example, circ-HIPK3 could sponge miR-558 in bladder cancer,25 but could only sponge miR-7 in colorectal cancer26 and miR-654 in glioma.27 Likewise, in our study, we found that circ-CDYL was capable to sponge miR-1180, but not miR-892a and miR-328-3p as reported by Wei et al.20 (data not shown). miR-1180 was shown as a tumor suppressor in pancreatic cancer28 and bladder cancer,29 whereas as an oncogene in hepatocellular carcinoma30 and ovarian cancer.31 However, its role in MM is unknown. Herein, miR-1180 was shown to be dramatically decreased in MM and was inhibited by circ-CDYL, and silencing of miR-1180 significantly rescued the decreased aggressive phenotype induced by circ-CDYL depletion, suggesting that miR-1180 is a tumor-inhibiting factor in MM. Further, our results revealed that YAP was the direct downstream target gene of miR-1180. YAP, the key effector of the Hippo signaling pathway, is frequently hyperactivated in multiple cancers, including MM.32 YAP expression is tightly controlled by various regulators and dysregulation of YAP can trigger tumorigenesis, chemoresistance, and metastasis.33 In our study, YAP was reduced in circ-CDYL knockdown cells, and miR-1180 silencing rescued this effect, indicating that the regulatory axis of circ-CDYL/miR-1180/YAP does exist in MM cells. Therefore, these results demonstrate that circ-CDYL functions in a miRNA-dependent pattern in MM, and also enrich our knowledge of the regulatory mechanism of YAP.
Collectively, our findings conceivably show that circ-CDYL is a carcinogenic circRNA in MM, which promotes MM growth by the regulation of miR-1180/YAP pathway. And our data also raise the possibility of considering circ-CDYL as a potential therapeutic intervention for the MM patients.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; F Chen participated in the design of the study, conducted the experiments and drafted the manuscript. XH Wang, S Fu, SK Wang and Y Fu collected and analyzed the data. ZG Liu and JH Zhang designed the study, revised the manuscript. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research was supported by grants from National Natural Science Foundation of China (Grant No. 81300420) and the Liaoning Province Natural Science Foundation of China (Grant No. 20170540996).
ORCID iD
Zhuogang Liu https://orcid.org/0000-0003-3279-3829
References
- 1.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet 2015; 385:2197–208 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 3.Pawlyn C, Morgan GJ. Evolutionary biology of high-risk multiple myeloma. Nat Rev Cancer 2017; 17:543–56 [DOI] [PubMed] [Google Scholar]
- 4.Kaikkonen MU, Adelman K. Emerging roles of non-coding RNA transcription. Trends Biochem Sci 2018; 43:654–67 [DOI] [PubMed] [Google Scholar]
- 5.Wilusz JE. A 360 degrees view of circular RNAs: from biogenesis to functions. Wires RNA 2018; 9:e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Ohman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 2015; 58:870–85 [DOI] [PubMed] [Google Scholar]
- 7.Panda AC. Circular RNAs act as miRNA sponges. Adv Exp Med Biol 2018; 1087:67–79 [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Zhang X, Li Z, Wang W, Li B, Huang X, Sun G, Xu J, Li Q, Xu Z, Xia Y, Wang L, Zhang Q, Li Q, Zhang L, Chen J, Wu Y, Cao J, Xu P, Zhang D, Xu H, Xu Z. Circular RNA profile identifies circOSBPL10 as an oncogenic factor and prognostic marker in gastric cancer. Oncogene 2019; 38:6985–7001 [DOI] [PubMed] [Google Scholar]
- 9.Yao J, Zhang C, Chen Y, Gao S. Downregulation of circular RNA circ-LDLRAD3 suppresses pancreatic cancer progression through miR-137-3p/PTN axis. Life Sci 2019; 239:116871. [DOI] [PubMed] [Google Scholar]
- 10.Zeng K, He B, Yang BB, Xu T, Chen X, Xu M, Liu X, Sun H, Pan Y, Wang S. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer 2018; 17:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei M, Wang Y, Wang Q, Liu Y, Song W, Zhang M. CircCDYL serves as a new biomarker in mantle cell lymphoma and promotes cell proliferation. Cancer Manag Res 2019; 11:10215–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach DH, Lee SK, Sood AK. Circular RNAs in cancer. Mol Ther Nucleic Acids 2019; 16:118–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Zhang L, Wu J, Khadka B, Fang Z, Gu J, Tang B, Xiao R, Pan G, Liu J. CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway. J Exp Clin Cancer Res 2019; 38:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad N, Haider S, Jagannathan S, Anaissie E, Driscoll JJ. MicroRNA theragnostics for the clinical management of multiple myeloma. Leukemia 2014; 28:732–8 [DOI] [PubMed] [Google Scholar]
- 15.Sole C, Arnaiz E, Manterola L, Otaegui D, Lawrie CH. The circulating transcriptome as a source of cancer liquid biopsy biomarkers. Semin Cancer Biol 2019; 58:100–8 [DOI] [PubMed] [Google Scholar]
- 16.Zhu K, Zhan H, Peng Y, Yang L, Gao Q, Jia H, Dai Z, Tang Z, Fan J, Zhou J. Plasma hsa_circ_0027089 is a diagnostic biomarker for hepatitis B virus-related hepatocellular carcinoma. Carcinogenesis 2019; ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XN, Wang ZJ, Ye CX, Zhao BC, Huang XX, Yang L. Circular RNA circVAPA is up-regulated and exerts oncogenic properties by sponging miR-101 in colorectal cancer. Biomed Pharmacother 2019; 112:108611. [DOI] [PubMed] [Google Scholar]
- 18.Liu XX, Yang YE, Liu X, Zhang MY, Li R, Yin YH, Qu YQ. A two-circular RNA signature as a noninvasive diagnostic biomarker for lung adenocarcinoma. J Transl Med 2019; 17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang F, Liu DY, Guo JT, Ge N, Zhu P, Liu X, Wang S, Wang GX, Sun SY. Circular RNA circ-LDLRAD3 as a biomarker in diagnosis of pancreatic cancer. World J Gastroenterol 2017; 23:8345–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye X, Zhang H, Yang P, Cui X, Ren Y, Xin X, Li H, Wang R, Wang W, Jiang F, Liu S, Ding J, Zhang B, Li L, Wang H. A noncoding regulatory RNAs network driven by Circ-CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology 2020; 71:130–47 [DOI] [PubMed] [Google Scholar]
- 21.Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta 2016; 1859:163–8 [DOI] [PubMed] [Google Scholar]
- 22.Rong D, Sun H, Li Z, Liu S, Dong C, Fu K, Tang W, Cao H. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 2017; 8:73271–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y, Wei F, Guo C, Wu X, Li X, Li Y, Li G, Zeng Z, Xiong W. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer 2018; 17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang Y, Li X, Wu Z, Yang D, Zhou Y, Wang H, Liao Q, Wang W. Circular RNAs in cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol Cancer 2019; 18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zheng F, Xiao X, Xie F, Tao D, Huang C, Liu D, Wang M, Wang L, Zeng F, Jiang G. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep 2017; 18:1646–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis 2018; 9:417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Jin P, Huang Y, Zhu P, Zou Y, Shao T, Wang O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun 2018; 503:1570–4 [DOI] [PubMed] [Google Scholar]
- 28.Gu L, Zhang J, Shi M, Peng C. The effects of miRNA-1180 on suppression of pancreatic cancer. Am J Transl Res 2017; 9:2798–806 [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Q, Wang C, Chen Z, Li F, Hu J, Ye Z. The suppressive effects of miR-1180-5p on the proliferation and tumorigenicity of bladder cancer cells. Histol Histopathol 2017; 32:77–86 [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Zhu HQ, Ma CQ, Li HG, Liu FF, Chang H, Lu J. MiR-1180 promoted the proliferation of hepatocellular carcinoma cells by repressing TNIP2 expression. Biomed Pharmacother 2016; 79:315–20 [DOI] [PubMed] [Google Scholar]
- 31.Hu J, Zhao W, Huang Y, Wang Z, Jiang T, Wang L. MiR-1180 from bone marrow MSCs promotes cell proliferation and glycolysis in ovarian cancer cells via SFRP1/Wnt pathway. Cancer Cell Int 2019; 19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Z, Tian Y, Cao C, Niu G. The emerging role of Yap/TAZ in tumor immunity. Mol Cancer Res 2019; 17:1777–86 [DOI] [PubMed] [Google Scholar]
- 33.Yan F, Qian M, He Q, Zhu H, Yang B. The posttranslational modifications of hippo-YAP pathway in cancer. Biochim Biophys Acta Gen 2020; 1864:129397. [DOI] [PubMed] [Google Scholar]