Abstract
The recent outbreak of coronavirus disease (COVID 19), spreading from China all around the world in early 2020, has led scientists to investigate the immuno-mediated mechanisms underlying the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) infection. Depending on the amount of cytokines released as the result of the immunological activation induced by SARS-CoV2, three major clinical phenotypes can be identified: “mild”,symbolized as a “drizzle” of cytokines, severe as a “storm”, and critical as a “hurricane”. In patients with mild symptoms, the release of pro-inflammatory cytokines is balanced to obtain a defense response against the virus which is often self-limiting and overcomes without tissue damage. In severe phenotype, resembling a “cytokine-release syndrome”, SARS-CoV2 causes the lysis of the immune-mediators leading to a cytokine storm able to induce lung epithelium damage and acute respiratory distress syndrome. In critical patients, the immune response may become uncontrolled, thus the cytokine burst resembles a form of secondary hemophagocytic lymphohistiocytosis which may result in a multi organ failure. In addition to the standard of care, an immune-modulatory therapy tailored to each one of the different phenotypes should be used in order to prevent or reduce the release of cytokines responsible for organ damage and disease progression.
Keywords: COVID-19, SARS-CoV2, CRS, HLH/MAS, cytokines
Impact statement
This minireview contributes to providing a point of view regarding the immunological mechanisms beyond COVID-19 infection. In particular, based on the clinical evidences and on the cases reported since the SARS-CoV2 pandemic diffused throughout the world, we suggest to classify patients into three main clinical phenotypes according to the amount and pattern of cytokines released during the infection. Thus, by evaluating the degree of immunological activation provoked by SARS-CoV2, this article contributes in understanding better how to choose the correct clinical approach with immuno-modulatory therapies which, in turn, may help in curbing the hyper-inflammatory condition of patients affected.
Introduction
Coronaviruses include a family of viruses able to cause a variety of respiratory diseases in humans from common cold to severe pneumonia.1 The case experienced from the recent worldwide outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) developed from Wuhan, China, in early 2020, has led scientists to explore the characteristics of SARS-CoV2 induced immuno-mediated damage.
The clinical picture of patients with SARS-CoV2 is characterized by fever, cough and fatigue at onset and quite commonly loss of smell and taste2; other symptoms may include sputum production with mild dyspnea, headache, and diarrhea.3 As reported in several cases, the respiratory condition may get worse in a medium estimated time of four days4 and progress to a severe pneumonia which presents with multiple peripheral ground-glass opacities in sub-pleural regions of both lungs at chest computed-tomography (CT) scan.5 Acute respiratory distress syndrome (ARDS) is the main cause of referral to intensive care unit (ICU) where patients require high-flow oxygen delivery and often invasive mechanical ventilation.6–8 Major laboratory abnormalities in patients with coronavirus disease-19 (COVID-19) are lymphocytopenia (up to 80% of the cases), increase in C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimer, transaminases and ferritin8,9 with usually normal procalcitonin.
Lymphocytopenia is also a prominent feature of severely affected patients with SARS-CoV and Middle East Respiratory Syndrome Coronavirus (MERS) infections. Lymphocytes express the angiotensin-converting enzyme (ACE) 2 and a decrease in lymphocyte count can be attributed to the necrosis or apoptosis of cells caused by invasive viral particle.10 Notably, the decrease in CD4+ and CD8+ T cells, and regulatory T cells may be responsible for a high inflammatory response resulting in a cytokine storm and in an immune response leading to tissue damage.11
The high increase in liver enzymes and ferritin may be due to the hepatic injury and the hyper-inflammation status as it is observed in many autoinflammatory conditions.12
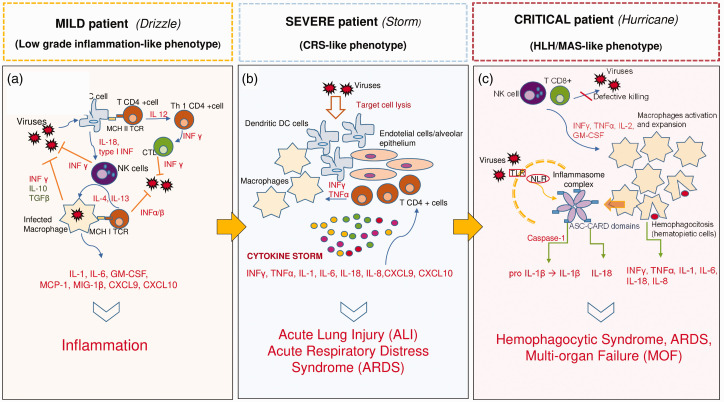
Based on the severity of the immunological damage induced by SARS-CoV2, three major phenotypes of COVID-19 can be identified: “mild,” “severe,” and “critical”. What is crucial in determining the disease severity is the amount of cytokines released following the host immune response to SARS-CoV2 which could vary from a “drizzle” of cytokines (mild-COVID-19), or a “storm” (Cytokine Release Syndrome, CRS-severe-COVID-19), to a “hurricane” (Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome, HLH/MAS-critical-COVID-19) (Figure 1).
Figure 1.
The drizzle, the storm, and the hurricane: three different phenotypes of disease severity for COVID-19. The three main clinical phenotypes are represented. (a) Normal immunological response against viral infection. The clinical picture is typical of mild and self-limiting SARS-CoV2 infection. (b) The cytokine release syndrome (CRS) caused by the lysis of the immune cells may worse the clinical picture and lead to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). This immunological condition is typical of severe COVID-19 patients. (c) Critical patients present with a phenotype similar to a secondary Hemophagocytic Lymphohistyocitosis. The inflammasome is constantly triggered and produces large amounts of pro-inflammatory mediators which lead to life-threatening manifestations and multi organ failure (MOF). CRS: cytokine release syndrome; HLH: hemophagocyic lymphohistyocitosis; MAS: macrophage activation syndrome; MOF: multi organ failure; NLR: nod-like receptor; TLR: toll-like receptor. (A color version of this figure is available in the online journal.)
The “mild” phenotype – The drizzle
Regarding SARS-CoV2 infection, there are anecdotal reports in the literature delineating the variety of the symptoms in COVID-19 patients. The symptoms can range from mild, such as fatigue and low fever, to critical manifestations such as ARDS.13 Beyond the exposure to low or high viral load of SARS-CoV2, the symptoms heterogeneity in individuals can partially be explained by the significant changes occurring in the immune system during childhood and adulthood; in young people, the immunological memory is continuously evolving and the protection provided by the immune response increases overtime. Conversely, in elderly people, the ability to form new immunological memory is decreased, and are thus more susceptible to infections due to an impaired and weak immunological response.14 Moreover, COVID-19 elderly patients may often have comorbidities such as cardiovascular disorders, diabetes, or hypertension which can worsen the course of the disease.15,16 Further studies are required to assess why clinical features of COVID-19 are different by age; however, it is known that children represent a small proportion of COVID-19 cases and their symptoms are often mild.17
It has been reported that men are more commonly and severely affected by COVID-19 than female. One possible explanation is that men are more frequently smokers than female.18 However, whether or not smoking is a predisposing factor for COVID-19 occurrence and severity is still controversial.19 Notably, new emerging evidences suggest that the modulation of Transmembrane Protease Serine (TMPRSS) 2 exerted by androgens may increase the entrance of virus in alveolar lung cells via ACE2, resulting in a more severe infection in men.20 Hence, multiple factors may influence the development of the immune response, including differences in genotypes, hormonal setting, and environmental factors.21,22
In an adult without particular comorbidities affected by COVID-19, the development of the immune response against the virus and the balance between pro- and anti-inflammatory mediators can contribute in limiting the organ damage and in determining a proper viral clearance. This leads to a milder disease, as it occurs with “low pathogenic” coronaviruses or other common viral infections.23
Normally, viral invasion causes the recruitment of different inflammatory cell such as macrophages, neutrophils, and natural killer cells (NK cells) which are the main producers of pro-inflammatory cytokines. These cytokines include type I Interferons (IFN), INF-γ, tumor necrosis factor (TNF)-α, Interleukin (IL)-1, IL-6, IL-2 and represent the first defense against pathogens. Pathogen-associated molecular patterns (PAMPs) are recognized by different pattern-recognition receptors, i.e. toll-like receptors (TLRs), nod-like receptors (NLRs) and retinoic-acid inducible gene 1receptors (RIGs-I), located on the immune host cell surfaces. The adaptive immune cells are subsequently activated and give their contribution directly attacking virus-infected cells or by releasing other pro-inflammatory cytokines, which in turn enhances the immunological response.24 Usually, the response is self-limiting and the infection resolves without tissue damage. This is because the production of anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β contributes in curbing the damage after viral invasion.25
Immuno-modulatory treatment in “mild” patients
For subjects with mild symptoms, including COVID-19 patients who do not require hospitalization, antimalarials drugs such as chloroquine (CQ) and hydroxychloroquine (HCQ), have been suggested since they exert various immunomodulatory and anti-inflammatory activities.26 In vitro studies showed that CQ was able to block COVID-19 infection at low-micromolar concentrations. Other reports demonstrated that CQ exerts antiviral activities by increasing endosomal pH required for virus/cell fusion, as well as interfering with the glycosylation of cellular receptors of SARS-CoV2.27,28 Antimalarials are now recommended in the current version of the Guidelines for the Prevention, Diagnosis, and Treatment of Pneumonia Caused by COVID-19 issued by the National Health Commission of the People’s Republic of China and are currently administered in association with antiviral drugs.29 However, it is still debated if anti-malarials should be used in the prevention of disease progression in patients with mild symptoms or if they are indicated as prophylaxis for the healthcare personnel.30,31 Colchicine has recently been proposed as therapeutic option, based on its capability of inhibiting inflammasome and hampering microtubule polymerization in immune cells; however, further study is required in order to prove the efficacy and safety of this drug in COVID-19.32
COVID-19 patients hospitalized with mild or mild-to-severe symptoms, requiring low flow oxygen and presenting with low levels of inflammatory biomarkers (especially CRP, D-dimer and ferritin), could beneficiate from IL-1 and IL-6 blockade with anakinra or tocilizumab, respectively, administered subcutaneously, in order to prevent the further release of cytokines that might worsen the clinical condition. To date, at the best of our knowledge, there is only one phase II randomized clinical trial (RCT) registered at clinicaltrials.gov based on the use of anakinra in the prevention or treatment of the severe side effects caused by chimeric-antigen receptor 1 (CAR)T cell therapy.33 In this trial, the first subgroup of patients is treated with the IL-1 receptor antagonist (IL-1ra) given subcutaneously every 12 h (starting on day 2 after T cell infusion or after two consecutive documented fevers of ≥38.5°C) and continued until day 10 in the absence of fever or until the resolution of fever (defined as <38.5°C). The second subgroup receive subcutaneous anakinra 100 mg every 24 h from day 0 (CAR-T cell infusion) until day 6. If participants experience fever or neurotoxicity between days 0 and 6, the dose of subcutaneous anakinra is escalated to 100 mg every 12 h likewise the first subgroup. Therefore, a similar regimen should be used in patients with mild COVID-19 who are at risk of developing a CRS. Figure 1(a) summarizes the mechanisms of viral invasion leading to the inflammatory process.
The “severe” phenotype – The storm
Severe COVID-19 patients are the immunological phenotype closely resembling a form of classical CRS. This syndrome can be observed as a result of CAR-T cell therapy or when B and T cells are targeted by various monoclonal antibodies in leukemia or lymphomas. This mechanism provokes the lysis of the immune cells with a consequent release of a great amount of pro-inflammatory cytokines.34 Similarly, the dysregulation of the immune response provoked by SARS-CoV2 may lead to the lysis of macrophages, dendritic cells, B and T cells and to the development of a “cytokine storm”.
Once macrophages are activated by INF-γ, they dramatically contribute in enhancing the massive release of IL-6, IL-1, IL-18, IL-8, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), chemokine C-C motif ligand (CCL) 5, chemokine C-X-C motif ligand (CXCL) 9, and CXCL10.34,35 It has been shown that high levels of pro-inflammatory cytokines and chemokines were correlated with poor COVID-19 outcomes as reported after the 2003 SARS outbreak.36,37 When inflammatory cytokines-producing cells are activated, a virus-induced damage involving predominantly the alveolar epithelium of the lungs occurs.38 In particular, INF-γ enhances the immune-mediated damage contributing to the pathogenesis of acute lung injury (ALI).39,40 Studies carried out in patients with ARDS identified different phenotypes depending on the biomarkers found in the serum: ARDS patients with high expression of angiopoietin, plasminogen activator inhibitor 1, INF-γ and IL-6 had a poorer outcome and a higher mortality compared to ARDS subjects without inflammatory markers.41 Among pro-inflammatory cytokines, IL-6 seems to be a pivotal mediator of CRS as it is able to activate directly T cells and enhancing the cytokine storm. Therefore, IL-6 receptor (IL-6R) blockade with tocilizumab has proved to be effective in the rapid recovering of CRS symptoms, as recently reported.42 A number of studies have been carried out on the therapy of CRS to date.34 Beyond IL-6, also the above-mentioned IL-1 plays a central role in CRS, as it seems to precede the IL-6 release by 24 h. The blockade of IL-1 receptor at the time of CAR-T infusion was proved to prevent CRS without affecting CAR-T expansion or leukemic clearance.43 Thus, it was supposed that IL-1 inhibition could prevent or delay the CRS burst. In addition, anakinra administration was able to prevent the cytokines-induced neurotoxicity, while tocilizumab administration did not improve neurological symptoms.34,42,44–46
Immuno-modulatory treatment in “severe” patients
Tocilizumab was approved by European Medicine Agency (EMA) in June 2018 for severe CRS.47 In severe patients affected with COVID-19, tocilizumab 8 mg/kg given intravenously seems to be effective in reducing the consequences of CRS triggered by SARS-CoV2 and in improving the clinical conditions already after 12 h from the first administration. A retrospective study carried out at the beginning of March 2020 in China in 21 COVID-19 patients, reported a clinical improvement in 18 out of 21 subjects within 5 days with resolution of fever, amelioration of chest CT scan, and a dramatic decrease in flow oxygen delivery.48 There are several clinical trials using tocilizumab currently ongoing worldwide49–51 and on 3 March 2020, antiIL-6R therapy was included in the New Guidelines for the Prevention, Diagnosis, and Treatment of Pneumonia Caused by COVID-19 issued by the National Health Commission of the People’s Republic of China.29 On 19 March 2020 the National Cancer Institute of Naples (Italy) promoted the TOCIVID-19 trial, a phase II multicenter, single-arm, open-label study including 330 patients. One-month mortality rate is the primary endpoint.52 Of note, a phase II randomized clinical trial with sarilumab, a fully human monoclonal antibody against the IL-6R is ongoing in hospitalized patients with COVID-19.53 Figure 1(b) summarizes the mechanisms of CRS development.
The “critical” phenotype – The hurricane
Occasionally, CRS may become severe and uncontrolled. The clinical phenotype of a severe CRS closely resembles a form of secondary HLH or MAS. This syndrome can sometimes occur as a consequence of some autoinflammatory and autoimmune diseases such as Adult-onset Still’s Disease (AOSD), systemic Juvenile Idiopathic Arthritis (sJIA), or Systemic Lupus Erythematosus (SLE).54,55 The clinical features of severe CRS may overlap with HLH/MAS (e.g. high fever, hyper-ferritinemia, increased cytokines levels, elevated transaminases, D-dimer, triglycerides, and lactate dehydrogenase) or become more severe, suggesting the importance of macrophages in the pathogenesis of both the diseases. In this context, it is worth mentioning IL-18 as another pivotal mediator of the onset of HLH/MAS.56 Interestingly, IL-18 is highly expressed in alveolar macrophages, T-CD8 lymphocytes, and alveolar epithelial cells of patients affected with chronic obstructive pulmonary disease (COPD).57 In addition, mice exposed to cigarette smoke developed a severe lung inflammation with alveolar remodeling, fibrosis, and high levels of IL-18 in specimens of alveolar macrophages. Notably, large amounts of IL-18 were found in serum and lungs of ARDS patients and were correlated with a poor disease outcome.58,59 The activation of IL-18 from the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome induced by seasonal influenza viruses seems to be protective in early defense against the virus. On the contrary, coronaviruses and avian influenza viruses (H5N1 and H7N9) were proved to activate NLRP3 strongly and persistently leading to the release of an excess of IL-18 and INF-γ.60,61 In addition, the inflammasome activation in response to various pathogens may induce pyroptosis, a type of programmed cell death characterized by cell swelling, blebs formation, and the final release of cellular contents.62 Therefore, it seems that SARS-CoV2 might enhance the cytokine release by inducing the pyroptosis process. In addition, pyroptosis has been proposed as one of the mechanisms by which lymphopenia may occur in COVID-19 patients,63 despite the depletion of lymphocytes might be the direct effect of the virus on lymphocytes or the effect of various cytokines, mainly IL-6.64,65
Immuno-modulatory treatment in “critical” patients
Therapy with intravenous (IV) tocilizumab is currently undertaken also in critical COVID-19 patients. However, IV anakinra should be considered in critical patients not eligible to tocilizumab or if at high risk of sepsis, based on the good safety profile and the relatively short half-life of IL-1ra.66 Indeed, anakinra was investigated in a series of clinical trials for the treatment of patients with severe sepsis in the past.67,68 Since in these trials anakinra was effective in the management of MAS, especially in patients at risk of fatal multi organ failure (MOF), its administration in critical COVID-19 patients at risk of sepsis seems to be appropriate. In a randomized, placebo-controlled study from Shakoory et al. carried out in 2016, patients were randomly assigned to receive either placebo or anakinra, administered IV at the dosage of 2 mg/kg/h for 72 consecutive hours. The results showed that the 28-day mortality was significantly lower in MAS patients who received anakinra than in those who received placebo (34.6% vs. 64.7%, P = 0.0006), corresponding to a 47% decrease in mortality associated with anakinra given continuously.67 This confirms the central role played by IL-1 in the pathogenesis of MAS and suggests that a similar treatment could be considered in critical COVID-19 patients admitted to ICU.
Since a rapid worsening towards HLH/MAS may occur in COVID-19, laboratory work up should be daily assessed in critical patients. If MAS is suspected, a bone marrow biopsy should be performed if feasible, in order to obtain a histological confirmation of hemophagocytosis. Therapy with the HLH-2004 protocol69 may be appropriate in these subjects. However, etoposide may be responsible for severe cytopenias70 and the treatment should be reserved to selected cases, after excluding potential non-COVID-19 infections (bacterial/fungal). Considering the damage provoked on the lung epithelium, neutralizing INF-γ might be useful in controlling severe lung inflammation.39 In November 2018, emapalumab, a fully human immunoglobulin G1 monoclonal antibody directed against INF-γ, received the first approval for the treatment of pediatric and adult patients with primary HLH.71,72 In Italy, a phase II/III randomized, open-label, multicenter study is currently ongoing in which the efficacy and safety of emapalumab versus anakinra IV in COVID-19 are compared.73 Hence, this treatment should be taken into account as a “rescue therapy” for refractory critical cases. However, a particular attention must be paid to fungal and bacterial infections.
Taking into consideration the above-mentioned pathways involved into the development of MAS, IL-18 administration might also find a rationale in critical COVID-19 management. In 2018, a phase II clinical trial with tadekinig alpha, the IL-18 binding protein (BP) showed a favorable safety profile and was associated with early signs of efficacy in patients with AOSD.74 Thus, despite the need of further studies, IL-18 BP therapy should be considered especially in COVID-19 patients with pulmonary comorbidities, such as COPD, who are at high risk of developing ARDS. Finally, recent evidences in murine models suggested the efficacy of Janus Kinases Inhibitors (JAKi) in refractory and particularly aggressive forms of HLH. Indeed, the JAK-STAT pathway inhibition may lead to a prompt decrease in pro-inflammatory cytokines and a reversal of the organ damage within few days.75 In this regard, a recorded clinical trial with ruxolitinib on secondary HLH is currently ongoing.76 Thus, despite still not available in current clinical practice, the above-mentioned “rescue therapies” should be considered in COVID-19 critical patients admitted to ICU. Figure 1(c) summarizes the mechanisms of HLH/MAS development.
In conclusion, we are still at the onset of understanding which treatment is the best option in COVID-19 patients. It is well established that SARS-CoV2 may provoke a massive release of cytokines which determine an immunological damage on lungs, especially in the severe forms; thus, immuno-modulation including anti-cytokines drugs should be considered as a treatment in association to the standard of care in order to control the inflammation and prevent a pulmonary clinical worsening. However, as different “degrees” of immunological activation may characterize SARS-CoV2 infection, each patient should be carefully assessed with the aim of properly tailoring the treatment to the single case.
Authors’ contributions
All authors participated in the review of the manuscript. SB wrote the manuscript and prepared the figure. MF and PS drafted and revised the manuscript. AD critically evaluated, wrote and revised the manuscript. All the authors approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
S Bindoli https://orcid.org/0000-0002-9409-3329
References
- 1.Al-Ahmari A. Is secondary hemophagocytic lymphohistiocytosis behind the high fatality rate in Middle East respiratory syndrome corona virus? J Appl Hematol 2015; 6:1–5 [Google Scholar]
- 2.Gautier JF, Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity 2020; 5:848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmunity 2020; 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020; 295:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO/2019-nCoV/clinical/2020.4. Clinical management of severe acute respiratory infection(SARI) when COVID-19 disease is suspected. Interim guidance March, 13th 2020
- 7.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020. doi: 10.1002/jmv.25748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol 2020. doi: 10.1002/jmv.25722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395(10229):1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020; 127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian D-S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset still’s disease. Nat Rev Rheumatol 2018; 14:603–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong X, Cao YY, Lu XX, Zhang JJ, Du H, Yan YQ, Akdis CA, Gao YD. Eleven faces of coronavirus disease 2019. Allergy 2020. doi: 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015; 282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18):1708-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SH, Kim HW, Kang JM, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr 2020; 63:125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020; 8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in covid-19. N Engl J Med 2020. doi: 10.1056/NEJMoa2007621 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discovery 2020. doi: 10.1158/2159-8290.CD-20-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol 2010; 87:385–95 [DOI] [PubMed] [Google Scholar]
- 22.Doria A, Sarzi-Puttini P, Shoenfeld Y. Infections, rheumatism and autoimmunity: the conflicting relationship between humans and their environment. Autoimmun Rev 2008; 8:1–4 [DOI] [PubMed] [Google Scholar]
- 23.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke A, Ferraccioli GF, Herrmann M, Romani L, Schulze C, Zampieri S, Doria A. Induction and protection of autoimmune rheumatic diseases. The role of infections. Clin Exp Rheumatol 2008; 26:S1–7 [PubMed] [Google Scholar]
- 25.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 2010; 10:514–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu C, Lu L, Wan JP, Wen C. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr Med Chem 2017; 24:2241–9 [DOI] [PubMed] [Google Scholar]
- 27.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases?. Lancet Infect Dis 2003; 3:722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza a H5N1 virus infection in an animal model. Cell Res 2013; 23:300–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed 12 May 2020)
- 30.Hydroxychloroquine Chemoprophylaxis in healthcare personnel in contact with COVID-19 patients (PHYDRA Trial), https://ClinicalTrials.gov/show/NCT04318015 (accessed 12 May 2020)
- 31.Chloroquine/Hydroxychloroquine Prevention of coronavirus disease (COVID-19) in the healthcare setting, https://ClinicalTrials.gov/show/NCT04303507 (accessed 12 May 2020)
- 32.Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N, Oikonomou E, Kaoukis A, Kossyvakis C, Raisakis K, Fountoulaki K, Comis M, Tsiachris D, Sarri E, Theodorakis A, Martinez-Dolz L, Sanz-Sanchez J, Reimers B, Stefanini GG, Cleman M, Filippou D, Olympios CD, Pyrgakis VN, Goudevenos J, Hahalis G, Kolettis TM, Iliodromitis E, Tousoulis D, Stefanadis C. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol 202. doi: 10.1016/j.hjc.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A study of Anakinra to prevent or treat severe side effects for patients receiving CAR-T cell therapy, https://ClinicalTrials.gov/show/NCT04148430 (accessed 12 May 2020)
- 34.Garcia Borrega J, Gödel P, Rüger MA, Onur ÖA, Shimabukuro-Vornhagen A, Kochanek M, Böll B. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. Hemasphere 2019; 3:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020. doi: 10.1016/j.jpha.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reghunathan R, Jayapal M, Hsu LY, Chng HH, Tai D, Leung BP, Melendez AJ. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol 2005; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, Ye L, Xu S, Sun R, Wang Y, Lou J. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004; 72:4410–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholls J, Dong XP, Jiang G, Peiris M. SARS: clinical virology and pathogenesis. Respirology 2003; 8(Suppl):S6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mock JR, Tune MK, Dial CF, Torres-Castillo J, Hagan RS, Doerschuk CM. Effects of IFN-γ on immune cell kinetics during the resolution of acute lung injury. Physiol Rep 2020; 8:e14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 2014; 306:L709–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, Artigas A, Martin-Loeches I, Hoogendijk AJ, van der Poll T, Horn J, Juffermans N, Calfee CS, Schultz MJ. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017; 72:876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018; 24:739–48 [DOI] [PubMed] [Google Scholar]
- 43.Rooney C, Sauer T. Modeling cytokine release syndrome. Nat Med 2018; 24:705–6 [DOI] [PubMed] [Google Scholar]
- 44.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018; 24:731–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124:188–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turtle CJ, Hay KA, Gust J, Hanafi L-A, Li D, Liles WC, Wurfel M, Harju-Baker S, Myerson D, Gonzalez-Cuyar L, Yeung CC, Riddell SR, Maloney DG. Cytokine release syndrome (CRS) and neurotoxicity (NT) after CD19-specific chimeric antigen receptor- (CAR-) modified T cells. J Clin Oncol 2017; 35:3020 [Google Scholar]
- 47.https://www.ema.europa.eu/en/medicines/human/EPAR/roactemra) (accessed 12 May 2020)
- 48.Xu XL, Hm Li TT, Sun W, Wang DS, Fu BQ, Zhou YG, Zheng XH, Yang Y, Li XY, Zhang XH, Pan AJ, Wei HM. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS May 19, 2020 117 (20) 10970-10975. doi.org/10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia, https://ClinicalTrials.gov/show/NCT04320615 (accessed 12 May 2020)
- 50.Favipiravir combined with tocilizumab in the treatment of corona virus disease, https://ClinicalTrials.gov/show/NCT04310228 (2019, accessed 12 May 2020)
- 51.Tocilizumab vs CRRT in management of cytokine release syndrome (CRS) in COVID-19, https://ClinicalTrials.gov/show/NCT04306705 (accessed 12 May 2020)
- 52.Tocilizumab in COVID-19 pneumonia (TOCIVID-19), https://ClinicalTrials.gov/show/NCT04317092 (accessed 12 May 2020)
- 53.Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19, https://ClinicalTrials.gov/show/NCT04315298 (accessed 12 May 2020)
- 54.Gilboa M, Bornstein G, Ben-Zvi I, Grossman C. Macrophage activation syndrome complicating rheumatic diseases in adults: case-based review. Rheumatol Int 2020; 40:663–69 [DOI] [PubMed] [Google Scholar]
- 55.Deane S, Selmi C, Teuber SS, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol 2010; 153:109–20 [DOI] [PubMed] [Google Scholar]
- 56.Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 2018; 281:138–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J 2008; 31:287–97 [DOI] [PubMed] [Google Scholar]
- 58.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012; 185:1225–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makabe H, Kojika M, Takahashi G, Matsumoto N, Shibata S, Suzuki Y, Inoue Y, Endo S. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J Anesth 2012; 26:658–63 [DOI] [PubMed] [Google Scholar]
- 60.McAuley JL, Tate MD, MacKenzie-Kludas CJ, Pinar A, Zeng W, Stutz A, Latz E, Brown LE, Mansell A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog 2013; 9:e1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 2005; 75:185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kesavardhana S, Kanneganti TD. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol 2017; 29:201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ming Y. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV Infection. 29 January 2020. Available at SSRN: https://ssrn.com/abstract=3527420 or 10.2139/ssrn.3527420 [DOI]
- 64.Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Ng MH, Yu LM, Hui DS, Tam JS, Cheng G, Sung JJ. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 2003; 326:1358–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang WK, Chen SY, Liu IJ, Kao CL, Chen HL, Chiang BL, Wang JT, Sheng WH, Hsueh PR, Yang CF, Yang PC, Chang SC. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis 2004; 39:1071–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castaneda S, Blanco R, Gonzalez-Gay MA. Adult-onset still’s disease: advances in the treatment. Best Pract Res Clin Rheumatol 2016; 30:222–38 [DOI] [PubMed] [Google Scholar]
- 67.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016; 44:275–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Opal SM, Fisher CJ, Jr., Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit Care Med 1997; 25:1115–24 [DOI] [PubMed] [Google Scholar]
- 69.La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019; 133:2465–77 [DOI] [PubMed] [Google Scholar]
- 70.Joel SP, Shah R, Slevin ML. Etoposide dosage and pharmacodynamics. Cancer Chemother Pharmacol 1994; 34:S69–75 [DOI] [PubMed] [Google Scholar]
- 71.Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood 2019; 134:1783–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A study to evaluate the efficacy, safety and pharmacokinetics of emapalumab in adult patients with HLH, https://ClinicalTrials.gov/show/NCT03985423 (accessed 12 May 2020)
- 73.Efficacy and safety of Emapalumab and Anakinra in reducing hyperinflammation and respiratory distress in patients with COVID-19 infection, https://ClinicalTrials.gov/show/NCT04324021 (accessed 12 May 2020)
- 74.Gabay C, Fautrel B, Rech J, Spertini F, Feist E, Kötter I, Hachulla E, Morel J, Schaeverbeke T, Hamidou MA, Martin T, Hellmich B, Lamprecht P, Schulze-Koops H, Courvoisier DS, Sleight A, Schiffrin EJ. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset still’s disease. Ann Rheum Dis 2018; 77:840–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosee L. P. Alleviating the storm: ruxolitinib in HLH. Blood 2016; 127:1626–7 [DOI] [PubMed] [Google Scholar]
- 76.A pilot study of ruxolitinib in secondary hemophagocytic syndrome, https://ClinicalTrials.gov/show/NCT02400463 (accessed 13 May 2020)