Abstract
Orbital venous malformation results from the aberrant angiogenesis in the orbit; however, the detailed molecular mechanism is still not clear. In this study, tissue samples from 27 patients with orbital venous malformation were collected and subjected to whole exome sequencing. Melanocortin 4 receptor was the gene with highest incidence (7/27) of mutation identified in this series. A total of four types of mutations were found in the coding region and the 5ʹ-untranslated region of melanocortin 4 receptor. All these mutations resulted in the upregulation of melanocortin 4 receptor expression. In vitro assays using human umbilical vein endothelial cells demonstrated that the endothelial properties including cell proliferation, cell cycle, cell migration, and tube formation are positively correlated with the expression level of melanocortin 4 receptor. Melanocortin 4 receptor mutations resulted in increased cAMP production in a cell-based assay. By RNA sequencing technique, melanocortin 4 receptor was found to modulate the downstream genes of PI3K/AKT/mTOR pathway, including p21, cyclin B1, ITGA10, and ITGA11, which are known to regulate the endothelial properties. These data demonstrated that mutations in melanocortin 4 receptor modulate the downstream signaling pathway, facilitate the angiogenic activity of endothelial cells, and therefore is one potential mechanism of orbital venous malformation pathogenesis.
Impact statement
The detailed molecular mechanism of orbital venous malformation (OVM) is still not clear. Using whole exome sequencing, 4 types of melanocortin 4 receptor (MC4R) mutation were detected in 7 of 27 patients with OVM, and all types of MC4R mutations resulted in the upregulation of MC4R expression. In vitro study indicated that MC4R has impacts on the proliferation, cell cycle, migration, and tube formation of the endothelial cells. Moreover, MC4R mutations altered the downstream signaling, including cAMP concentration and the expression levels of several PI3K/AKT/mTOR downstream genes, including p21, cyclin B1, ITGA10, and ITGA11. MC4R mutations may lead to the pathogenesis of OVM through modulating the downstream signaling to alter the angiogenic activity of endothelial cells.
Keywords: Orbital venous malformation, melanocortin 4 receptor, endothelial cells
Introduction
Orbital venous malformation (OVM) is one of the most common forms of vascular lesions in the orbit, which is caused by the aberrant angiogenesis during embryonic development1 and may lead to vision dysfunction and appearance defect.1,2 Multiple treatment approaches for the OVM, including laser therapy, radiotherapy, sclerotherapy, embolization and surgical resection, have been recommended by the International Society for the Study of Vascular Anomalies (ISSVA)3 as well as other review literatures.1,4,5 The diagnosis and management of OVM have been improved by the advances in the angiography, surgical instruments, and multidisciplinary collaboration6; however, it is still challenging due to no standard treatment available up to the present time.
Recently, the molecular genetics of several types of venous malformations has been investigated. Mutations occurred in TIE2, the receptor for angiopoietin, have been identified in the inherited multiple cutaneous and mucosal venous malformations.7,8 A mutation located in the kinase domain of TIE2 activates the signaling pathways mediated by STAT and other proteins, leads to aberrant cell cycle, survival, adhesion, and migration of the endothelial cells.9–13 The mutations in TIE2 are further found in sporadic venous malformations.14 Moreover, mutations in another molecule, PI3KCA, have demonstrated that promoting the downstream signaling for cell proliferation of the endothelial cells and impairing normal vasculogenesis in the embryonic development therefore lead to sporadic venous malformations.15 Aberrant TIE2 and PI3KCA in venous malformations were found to enhance the activity of PI3K/AKT/mTOR pathway, resulting in the promotion of angiogenesis.16 Emerging studies have revealed the molecular pathogenesis of other types of venous malformations, such as glomulin for glomuvenous malformation,17,18 KRIT1, malcavernin and PDCD10 for cerebral cavernous malformation,19 ELMO2 for familial intraosseous vascular malformation,20 and MAP3K3 for verrucous venous malformation.21 In addition to genetic changes, the epigenetic mechanism is suggested to be involved in the pathogenesis of arteriovenous malformations.22 On the other hand, the molecular basis of OVM is currently not clear.
Melanocortin 4 receptor (MC4R) is a G protein-coupled receptor, which has been primarily found to be associated with obesity.23,24 Biochemical analysis suggests that endoplasmic reticulum-targeted agonist for MC4R may be a potential therapeutic target by stabilizing the active form of MC4R.25 In this present study, we investigated the MC4R mutations in patients with OVM, and the potential role of MC4R in OVM pathogenesis.
Materials and methods
Patients
Tissue samples were collected from 27 patients with OVM who received surgical resection in Tianjin Medical University Eye Hospital, China between December 2016 and August 2018. Patients’ demographics including age, gender, and body mass index (BMI) were recorded. The inclusion criteria were: (1) the lesions were classified as the venous malformations according to the 2014 ISSVA classification3 by histopathological examination post-operatively, and (2) the lesions were only found in the orbit. Patients with other types of vascular malformations or with other vascular malformations that are not in the orbit were excluded. This study was approved by the institutional review board of Tianjin Medical University Eye Hospital, China (IRB number: 2017KY-11). Written informed consents were obtained from all patients as well as their guardians for the patients aged < 18 years. After consulting the institutional review board of Tianjin Medical University Eye Hospital, China, a clinical trial registration was not needed according to the objectives and the methodology of this study.
Whole exome sequencing
Tissue samples collected from patients were stored in liquid nitrogen until use. Genomic DNA was extracted from patients’ tissues, and the exome sequences were efficiently enriched from 1.0 µg genomic DNA using SureSelect Human All Exon V5 kit (Agilent, Santa Clara, CA, USA) according to the manufacturer’s protocol. DNA libraries were sequenced on Hiseq 4000 system (Illumina Inc., San Diego, CA, USA) for paired-end 150 bp reads. Valid sequencing data were mapped to the reference genome (UCSC hg19) by Burrows-Wheeler Aligner software26 to obtain the original mapping results in BAM format. SAMtools27 and Picard (http://broadinstitute.github.io/picard) were utilized to sort files for generation of the final BAM file. Reads that aligned to the exon regions were collected for mutation identification and subsequent analysis. The information on single nucleotide polymorphism, insertion, and deletion was obtained and compared to the open data of the 1000 Genome Project (http://www.internationalgenome.org/). Four programs, including SIFT,28 PolyPhen-2,29 MutationTaster,30 and CADD,31 were utilized for estimating the deleterious potential of the genetic variants. A total of 12 genes were identified as pathogenic candidates in at least two of the four prediction programs (unpublished data). The gene with highest incidence of mutation among these 27 patients was further functionally characterized.
Immunoblotting and immunohistochemistry
Antibodies against the following proteins were used in immunoblotting and immunohistochemistry: MC4R (1:1000 dilution; ab24233), β-tubulin (1:1000 dilution; ab15568), vWF (1:1000 dilution; ab6994), and GAPDH (1:5000 dilution; ab128915) from Abcam (Cambridge, UK)
For immunoblotting, tissue samples were stored in liquid nitrogen until use. Protein lysates were extracted by RIPA buffer (150 mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0) and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. After transferred onto the polyvinylidene difluoride membranes, proteins were detected by anti-MC4R, anti-β-tubulin, and anti-GAPDH antibodies as indicated in each figure. Samples from patients without MC4R mutations were used as a control.
For immunohistochemistry, tissue samples were fixed in 4% paraformaldehyde and embedded in the paraffin. Tissue sections were cut with 4-µm thickness on a microtome. Tris/EDTA buffer (pH 9.0) was used for antigen unmasking. MC4R and vWF were detected by the antibodies described above. Samples from patients without MC4R mutations were used as a control.
Endothelial cell assays
Human umbilical vein endothelial cells (HUVECs) were chosen to characterize the functions of MC4R in vitro because of its endothelial origin and the endogenous expression of MC4R (Figure 2(a)). To generate HUVECs harboring the stable MC4R knockdown (MC4R-KD), HUVEC cells were infected with a recombinant lentivirus expressing MC4R-targeting short hairpin RNA and selected by puromycin. The MC4R-KD cells were utilized to characterize the endothelial cell properties affected by low MC4R expression. HUVECs infected with a recombinant lentivirus expressing non-specific short hairpin RNA were used for control (MC4R-NC) cells.
Figure 2.
Increased protein amount for MC4R with mutations at positions 103 (V→I), 166 (V→I), and 178 (T→M) in HEK293T cells. Wildtype and mutant MC4Rs were ectopically expressed in HEK293T cells. Relative expression level of MC4R was quantified and calculated. GAPDH was used as an internal control. An asterisk indicated significant difference compared to MC4R WT group. *P < 0.05; **P < 0.01; ***P < 0.001.
The proliferation activity was measured by MTT assay, and the different phases of cell cycle were determined by propidium iodide staining and flow cytometry. Transwell assay, scratch assay, and tube formation were conducted to examine the endothelial activity as previously described.32 For transwell assay, cells were plated onto a fibronectin-coated transwell with 3-µm pore diameter. After 24 h, cells in lower chamber were quantified by Countess II FL Automated Cell Counter (Thermo Fisher Scientific Inc., Waltham, MA, USA). For scratch assay, cells were grown to near confluence, and a wound was created by a pipet tip. Cell migration toward the denuded area was observed after 24 h. For tube formation, cells were plated onto Matrigel, and the capillary-like structures were observed after 24 h by a light microscope.
cAMP measurement assay
Lentiviruses expressing wildtype and mutant MC4Rs were generated by Sangon Biotech (Shanghai) Co., Ltd, China. Briefly, plasmids used in lentivirus production, including pCMV-dR8.91 and pCMV-VSV-G6363, and 293 T cells were used in virus package. After purified by ultracentrifugation (82,700g at 4°C for 2 h), the titer of each lentivirus was determined by quantitative PCR.
293T cells were transduced by each lentivirus recombinants (MOI = 10) for protein expression. After 72 h, cells were treated with 0.1 µM of α-msh (Calbiochem in EMD Millipore, Billerica, MA, USA) for 0.5 h. Intracellular cAMP concentration was measured by a cAMP ELISA kit purchased from TianJin Saierbio Inc., China.
RNA sequencing
Total RNA was extracted by RNeasy Mini Kit (Qiagen, Germantown, MD, USA). Isolated RNA from MC4R-NC and MC4R-KD cells was used for the construction of whole transcriptome library using Ion AmpliSeq Transcriptome Human Gene Expression Kit (Thermo Fisher Scientific, Waltham, MA, USA), and then subjected to the Ion S5 System (Thermo Fisher Scientific) under standard conditions. Data were analyzed by Ion Reporter Software (Thermo Fisher Scientific) with default parameters.
Quantitative RT-PCR
Tissue samples from patients were stored in liquid nitrogen until use. Total RNA was extracted from patients’ tissues and HUVEC cells by RNeasy Mini Kit (Qiagen), and cDNA was prepared using SuperScript III reverse transcriptase (Thermo Fisher Scientific). Quantitative RT-PCR was conducted using Power SYBR GREEN PCR Master Mix (Thermo Fisher Scientific) and Applied Biosystems™ 7500 Real-Time PCR System (Thermo Fisher Scientific) according to the user manuals. Primer sets for detecting the mRNA expression level of MC4R, p21, cyclin B1, ITGA10, ITGA11, and β-actin are shown in Table S1. Relative fold expression (target gene/β-actin) was calculated by the 2−ΔΔCT method.33 Samples from patients without MC4R mutations and MC4R-NC cells were used as a control.
Statistical analyses
Statistical comparisons for all data sets were conducted with a nonparametric two-tailed Mann–Whitney test. The level of significant differences and the number of replicates were indicated in the figures.
Results
Upregulation of MC4R expression in patients with orbital venous malformation
A total of 27 patients were enrolled into this study, and 59.3% (16/27) were men. Mean age of them was 35.1 ±17.6 years, while the mean BMI was 22.1 ± 2.96 kg/m2. MC4R, the gene with the highest incidence of mutation in this study, was detected in 7 of 27 patients (Table 1). Three independent mutations occurring in the transmembrane regions were found in three patients. On the other hand, four patients bear the same mutation located in the 5ʹ-untranslated region (5ʹ-UTR).
Table 1.
MC4R mutations identified in this study.
| Site | Region | Amino acid change | Frequency |
|---|---|---|---|
| NC_000018.10:g.60371817G>A | Coding; TM | T187M | 1/27 |
| NC_000018.10:g.60371854C>T | Coding; TM | V166I | 1/27 |
| NC_000018.10:g.60372043C>T | Coding; TM | V103I | 1/27 |
| NC_000018.10:g.60372565G>A | 5'-UTR | None | 4/27 |
TM: transmembrane region; T: threonine; M: methionine; V: valine; I: isoleucine; UTR: untranslated region.
The expression level of MC4R was first investigated in patients’ tissues. Patients harboring the 5ʹ-UTR mutation in MC4R exhibited 8.6- to 53-fold increments of MC4R mRNA level (Figure 1(a)). In addition, the protein level of MC4R detected by Western blotting and immunohistochemistry was significantly increased in these four patients (Figure 1(b) and (c)). The upregulated MC4R protein expression was also seen in patients bearing other MC4R mutations identified in this study (Figure S1). Moreover, MC4R was co-localized with vWF, an endothelial cell marker, in tissues from patients with MC4R mutation (Figure 1(d)).
Figure 1.
Upregulation of MC4R expression in patients with orbital venous malformation. (a,b) The mRNA (a) and protein (b) expression level of MC4R were detected in orbital venous malformation patients with MC4R wildtype and mutation in 5ʹ-UTR. (c) MC4R was detected in the tissue sections from orbital venous malformation patients with MC4R wildtype and mutation in 5ʹ-UTR by immunohistochemistry. (d) MC4R and vWF were co-stained in the tissue sections from orbital venous malformation patients with MC4R mutation in 5ʹ-UTR. DAPI was used to indicate the cell nucleus. ***P < 0.001 as compared with the wildtype controls. Scale bar, 10 µm.
Effects of MC4R mutations on the levels of protein expression were further studied. Wildtype and mutant MC4Rs were expressed in HEK293T cells via the lentiviral system. Compared to the control, the wildtype MC4R construct did not significantly lead to the increment of MC4R proteins; in contrast, all examined mutant MC4R constructs resulted in obvious accumulation (25–70% increment) of MC4R protein (Figure 2), consistent with the results observed in patients’ tissues (Figure 1). These data suggested that MC4R mutations identified in this study lead to upregulation of MC4R expression in the endothelial cells of the lesion in patients with OVM.
Aberrant cell proliferation and cell cycle in MC4R-knockdown HUVEC cells
To further characterize the role of MC4R in the pathogenesis of OVM, MC4R-KD cells were generated (Figure 3(a)). The cell proliferation decreased around 50% in MC4R-KD cells compared to that in MC4R-NC cells (Figure 3(b)). Moreover, MC4R was involved in maintaining cell cycle. MC4R-KD cells showed G2/M arrest compared to MC4R-NC cells (Figure 3(c)). These data indicated that MC4R modulates cell proliferation and cell cycle of endothelial cells.
Figure 3.
Aberrant proliferation rate and cell cycle in MC4R-knockdown HUVEC cells. (a) Total cell lysates from control (Lane 1) and MC4R-knockdown (Lane 2) HUVEC cells were immunoblotted with antibodies against human MC4R and β-tubulin. (b) Cell proliferation of control (MC4R-NC) and MC4R-knockdown (MC4R-KD) HUVEC cells was measured according to MTT assay. N = 5. ***P < 0.001 as compared with the control cells. (c) Cell cycle of MC4R-NC and MC4R-KD HUVEC cells was analyzed by PI staining. N = 3.
Impaired cell migration and tube formation in MC4R-knockdown HUVEC cells
The role of MC4R in endothelial property was subsequently examined by several in vitro angiogenesis models. Cell migration examined by Transwell assay was impaired under MC4R-knockdown condition (Figure 4(a)). Using in vitro scratch assay, cell migration toward the denuded area was attenuated in MC4R-KD cells (Figure 4(b)). Moreover, reduced tube formation was observed in MC4R-KD cells (Figure 4(c)). These data demonstrated that MC4R plays a positively regulatory role in the angiogenic potential of endothelial cells.
Figure 4.
Impaired cell migration and tube formation in MC4R-knockdown HUVEC cells. (a) Migration of control (MC4R-NC) and MC4R-knockdown (MC4R-KD) HUVEC cells was measured with a Transwell assay. N = 3. ***P < 0.001 as compared with the control cells. (b) Migration of MC4R-NC and MC4R-KD HUVEC cells was performed with an in vitro scratch assay. Images were acquired at 0 and 24 h in the assay. The lines define the area lacking cells. N = 3. (c) Tube formation of MC4R-NC and MC4R-KD HUVEC cells was performed with Matrigel. Images were acquired at 6 h in the assay. N = 3.
Altered signal transduction dictated by mutant MC4Rs
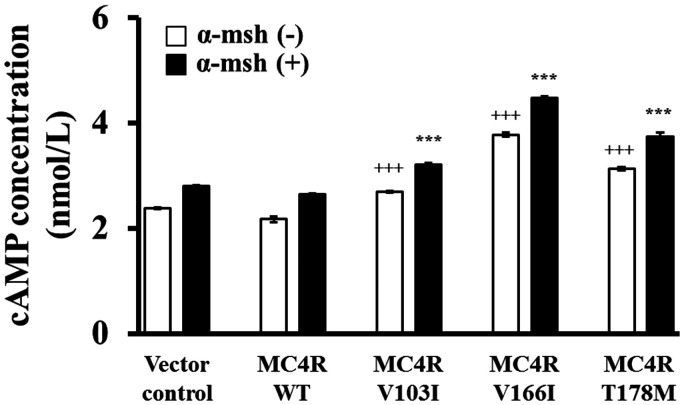
To investigate the detailed molecular mechanism for mutant MC4Rs, we first measured the intracellular cAMP level, a known secondary massager in MC4R signaling pathway, in cells transduced with lentiviruses containing various mutant MC4Rs. Mutant MC4Rs significantly resulted in higher cAMP levels compared to vector control and wildtype MC4R, and cAMP levels were further elevated in the presence of α-msh, a ligand for MC4R. Compared to the wildtype MC4R, the increment of the cAMP level was around 20–70% among the examined mutant MC4Rs, despite of the presence of α-msh (Figure 5).
Figure 5.
cAMP production for wildtype and mutant MC4Rs. HEK293T cells were transfected with each construct as indicated, and cAMP concentration was measured in the absence or presence of α-msh. +++P < 0.001 as compared with MC4R WT group without α-msh treatment; +++P < 0.001 as compared with MC4R WT group with α-msh treatment.
PI3K/AKT/mTOR pathway is crucial for angiogenesis and is affected in venous malformation patients bearing aberrant TIE2 and/or PI3KCA.16 We therefore focused whether MC4R modulates this signaling pathway. RNA sequencing technique was utilized to analyze the expression level of PI3K/AKT/mTOR downstream genes in MC4R-KD cells (Figure 6(a)). Among the PI3K/AKT/mTOR downstream genes, the expression level of p21, cyclin B1, ITGA10 and ITGA11, was further validated by quantitative RT-PCR. The mRNA levels of these examined genes were all decreased around 30–60% (Figure 6(b)). Also, the low MC4R expression was confirmed in MC4R-KD cells (Figure 6(b)). These data showed that MC4R is involved in the regulation of PI3K/AKT/mTOR downstream genes.
Figure 6.
Downregulation of PI3K/AKT/mTOR downstream genes in MC4R-knockdown HUVEC cells. (a) RNA expression profile of control (MC4R-NC) and MC4R-knockdown (MC4R-KD) HUVEC cells was analyzed by RNA sequencing. Expression levels of PI3K/AKT/mTOR downstream genes in MC4R-NC and MC4R-KD HUVEC cells were shown as a heatmap. Red, white, and blue colors represent high, median, and low expression, respectively. (b) The mRNA levels of MC4R as well as several PI3K/AKT/mTOR downstream genes, including p21, cyclin B1, ITGA10 and ITGA11, were detected in MC4R-NC and MC4R-KD HUVEC cells. N = 3. **P < 0.01; ***P < 0.001 as compared with the control cells.
Discussion
Using whole exome sequencing, 4 types of MC4R mutations were detected in 7 of 27 patients with OVM. All types of MC4R mutations resulted in upregulation of MC4R protein expression. The functions of MC4R in endothelial cells were investigated using HUVEC cells under MC4R-knockdown condition. MC4R was important for the property of endothelial cells, including proliferation, cell cycle, migration, and tube formation. Moreover, downstream signaling was altered by mutant MC4Rs. cAMP concentration and the expression level of several PI3K/AKT/mTOR downstream genes, including p21, cyclin B1, ITGA10, and ITGA11, were changed by aberrant MC4R expression. In this present study, we demonstrated that MC4R mutations may lead to the pathogenesis of OVM through modulating the downstream signaling to alter the angiogenic activity of endothelial cells.
MC4R has been reported to involve in a variety of physiological function, including energy homeostasis, cachexia, cardiovascular function, glucose/lipid homeostasis, and reproduction function.23 Among these diverse roles, the association between MC4R and obesity has been extensively reported. More than 150 distinct naturally occurring mutations have been identified in patients from different ethnic origins, which are scattered in the MC4R. All the MC4R mutations identified in this present study have been previously found in patients with obesity.23 One recent study has reported the association between MC4R mutations and binge eating disorder in patients with obesity.34 It is interesting that T187M and V166I as well as the point mutation in 5ʹ-UTR have been reported in obesity patients; on the other hand, V103I has protective effects on obesity, suggesting that the involvement of MC4R in obesity and OVM may undergo differential signaling pathways. Moreover, the codominance of MC4R and other genes in obesity has been suggested because not all people harboring the pathogenic MC4R mutations are obese35; this concept could probably be applied to OVM. On the other hand, we did not find any reported MC4R-mutated obese patients harboring OVMs; probably the presence of OVMs was not investigated in the MC4R-mutated obese patients in these literatures.
Although no animal models responsible for MC4R overexpression have been reported up-to-date, one animal study has demonstrated that MC4R involved in angiogenic balance and vasorelaxation in pregnant rats.36 Another study showed that MC4R agonist led to insulin-induced AKT phosphorylation both in vitro and in vivo.37 In this present study, we demonstrated that MC4R modulates angiogenesis possibly through the production of cAMP and the PI3K/AKT/mTOR signaling pathway in the endothelial cells. Mutant MC4Rs identified in this study elevated the basal level of cAMP but did not alter the sensitivity or responsiveness toward α-msh. Moreover, we showed that the expression levels of several PI3K/AKT/mTOR downstream genes related to endothelial cell property were modulated by MC4R. P21 is a well-known inhibitor of cell cycle which blocks both G1/S and G2/M transitions,38 and cyclin B1 in partnership with Cdk1 is the key component for mitosis.39 MC4R may promote cell proliferation and cell cycle through AKT-mediated p21 and cyclin B1 pathway. On the other hand, ITGA10 and ITGA11 belong to the integrin family which is crucial for cell migration, wound healing, and tissue remodeling.40 MC4R may facilitate the endothelial function through the integrin signaling.
In conclusion, four types of MC4R mutations were identified in OVM patients in this present study. Each of them resulted in higher MC4R expression in endothelial. By a series of angiogenesis assays in an endothelial cell line, MC4R was found to modulate the cAMP production and the PI3K/AKT/mTOR signaling pathway and therefore facilitate the angiogenic activity of endothelial cells.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220919056 for Mutations in MC4R facilitate the angiogenic activity in patients with orbital venous malformation by Xiao-Ming Huang, Wan-Chen Yang, Yang Liu, Dong-Run Tang, Tong Wu and Feng-Yuan Sun in Experimental Biology and Medicine
Authors’ contributions
Xiaoming Huang is responsible for study concepts, literature research, experimental studies, data analysis, statistical analysis and manuscript preparation. Wanchen Yang is responsible for data acquisition, and manuscript preparation. Yang Liu is responsible for sequence analysis and statistical analysis. Dongrun Tang is responsible for manuscript editing and manuscript review. Tong Wu is responsible for study design and manuscript editing. Fengyuan Sun is responsible for the integrity of the entire study.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
This study was approved by the institutional review board of Tianjin Medical University Eye Hospital, China (IRB number: 2017KY-11).
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Feng-Yuan Sun https://orcid.org/0000-0002-6554-491X
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Li T, Jia R, Fan X. Classification and treatment of orbital venous malformations: an updated review. Front Med 2019; 13:547–55 [DOI] [PubMed] [Google Scholar]
- 2.Jia R, Xu S, Huang X, Song X, Pan H, Zhang L, He F, Lin M, Ge S, Fan X. Pingyangmycin as first-line treatment for low-flow orbital or periorbital venous malformations: evaluation of 33 consecutive patients. JAMA Ophthalmol 2014; 132:942–8 [DOI] [PubMed] [Google Scholar]
- 3.ISSVA. ISSVA classifiaction for vascular anomalies, wwwissvaorg (2014, accessed 1 April 2020)
- 4.Arat YO, Mawad ME, Boniuk M. Orbital venous malformations: current multidisciplinary treatment approach. Arch Ophthalmol 2004; 122:1151–8 [DOI] [PubMed] [Google Scholar]
- 5.Benoiton LA, Kenneth C, Frederica S, Trevor F, TT. Management of orbital and periorbital venous malformation. Front Surg 2017; 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundar G. Vascular lesions of the orbit: conceptual approach and recent advances. Indian J Ophthalmol 2018; 66:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vikkula M, Boon LM, Carraway KL, Calvert JT, 3rd, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 1996; 87:1181–90 [DOI] [PubMed] [Google Scholar]
- 8.Calvert JT, Riney TJ, Kontos CD, Cha EH, Prieto VG, Shea CR, Berg JN, Nevin NC, Simpson SA, Pasyk KA, Speer MC, Peters KG, Marchuk DA. Allelic and locus heterogeneity in inherited venous malformations. Hum Mol Genet 1999; 8:1279–89 [DOI] [PubMed] [Google Scholar]
- 9.Korpelainen EI, Karkkainen M, Gunji Y, Vikkula M, Alitalo K. Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene 1999; 18:1–8 [DOI] [PubMed] [Google Scholar]
- 10.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998; 273:18514–21 [DOI] [PubMed] [Google Scholar]
- 11.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem 1999; 274:30896–905 [DOI] [PubMed] [Google Scholar]
- 12.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-Kinase/akt signal transduction pathway. Circ Res 2000; 86:24–9 [DOI] [PubMed] [Google Scholar]
- 13.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the akt/survivin pathway. J Biol Chem 2000; 275:9102–5 [DOI] [PubMed] [Google Scholar]
- 14.Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M. Variable somatic TIE2 mutations in half of sporadic venous malformations. Mol Syndromol 2013; 4:179–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castel P, Carmona FJ, Grego-Bessa J, Berger MF, Viale A, Anderson KV, Bague S, Scaltriti M, Antonescu CR, Baselga E, Baselga J. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci Transl Med 2016; 8:332ra42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011; 4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boon LM, Brouillard P, Irrthum A, Karttunen L, Warman ML, Rudolph R, Mulliken JB, Olsen BR, Vikkula M. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet 1999; 65:125–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouillard P, Boon LM, Mulliken JB, Enjolras O, Ghassibe M, Warman ML, Tan OT, Olsen BR, Vikkula M. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet 2002; 70:866–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalcanti DD, Kalani MY, Martirosyan NL, Eales J, Spetzler RF, Preul MC. Cerebral cavernous malformations: from genes to proteins to disease. J Neurosurg 2012; 116:122–32 [DOI] [PubMed] [Google Scholar]
- 20.Cetinkaya A, Xiong JR, Vargel I, Kosemehmetoglu K, Canter HI, Gerdan OF, Longo N, Alzahrani A, Camps MP, Taskiran EZ, Laupheimer S, Botto LD, Paramalingam E, Gormez Z, Uz E, Yuksel B, Ruacan S, Sagiroglu MS, Takahashi T, Reversade B, Akarsu NA. Loss-of-Function mutations in ELMO2 cause intraosseous vascular malformation by impeding RAC1 signaling. Am J Hum Genet 2016; 99:299–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couto JA, Vivero MP, Kozakewich HP, Taghinia AH, Mulliken JB, Warman ML, Greene AK. A somatic MAP3K3 mutation is associated with verrucous venous malformation. Am J Hum Genet 2015; 96:480–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas JM, Surendran S, Abraham M, Rajavelu A, Kartha CC. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenet 2018; 10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 2010; 31:506–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolby V, Collen A, Lundqvist A, Cronet P. Overexpression and functional characterisation of the human melanocortin 4 receptor in Sf9 cells. Protein Expr Purif 2004; 37:455–61 [DOI] [PubMed] [Google Scholar]
- 25.Granell S, Molden BM, Baldini G. Exposure of MC4R to agonist in the endoplasmic reticulum stabilizes an active conformation of the receptor that does not desensitize. Proc Natl Acad Sci USA 2013; 110:E4733–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009; 25:1754–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics 2009; 25:2078–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res 2003; 31:3812–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet 2013; 76:7.20.1–7.20.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010; 7:575–6 [DOI] [PubMed] [Google Scholar]
- 31.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014; 46:310–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res 2007; 74:172–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402–8 [DOI] [PubMed] [Google Scholar]
- 34.Qasim A, Mayhew AJ, Ehtesham S, Alyass A, Volckmar AL, Herpertz S, Hinney A, Hebebrand J, Meyre D. Gain-of-function variants in the melanocortin 4 receptor gene confer susceptibility to binge eating disorder in subjects with obesity: a systematic review and meta-analysis. Obes Rev 2019; 20:13–21 [DOI] [PubMed] [Google Scholar]
- 35.O’Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: human obesity-lessons from monogenic disorders. Endocrinology 2003; 144:3757–64 [DOI] [PubMed] [Google Scholar]
- 36.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 2013; 1: e00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides 2009; 30:1098–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016; 42:63–71 [DOI] [PubMed] [Google Scholar]
- 39.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 2010; 18:533–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeltz C, Gullberg D. The integrin-collagen connection – a glue for tissue repair? J Cell Sci 2016; 129:653–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220919056 for Mutations in MC4R facilitate the angiogenic activity in patients with orbital venous malformation by Xiao-Ming Huang, Wan-Chen Yang, Yang Liu, Dong-Run Tang, Tong Wu and Feng-Yuan Sun in Experimental Biology and Medicine