Abstract
Objective. To implement an opioid buyback program after ambulatory surgery.
Methods. We performed a prospective cohort study of 578 opioid-naïve patients prescribed opioids after ambulatory surgery at a rural US Veterans Affairs (VA) hospital from 2017 to 2018. We reimbursed $5 per unused opioid pill ($50 limit) returned to our VA for proper disposal. We tracked the number of participants, number of unused opioid pills returned, surgeon prescribing, and refill requests.
Results. Out of 578 eligible patients, 171 (29.6%) returned 2136.5 unused opioid pills. Information shared with surgeons after 6 months led to a 27% decrease in opioid prescribing without an increase in refills.
Conclusions. With this opioid buyback program, rural patients had a safe and convenient place to dispose of unused opioids. Surgeons used information about returns to adjust opioid prescribing after common ambulatory surgeries without an increase in refill requests.
Public Health Implications. Although providers prescribe within state opioid guidelines, there will be variations in patient use after ambulatory surgery. An opioid buyback program helped our patients and surgeons decrease unused prescription opioids available for diversion in our rural communities.
More than 4.7 million veterans live in rural communities.1 While there are many benefits to rural living, there are also challenges. One such challenge is prescription opioid diversion, which can lead to opioid abuse. Prescription opioid diversion has 2 major components: lack of disposal in rural areas and overprescription.
A recent study in California showed that only 19% of pharmacies provided correct opioid disposal information.2 Rural patients are challenged by geographic isolation because rural patients often have to travel hours to find a disposal site, and drug take-back days are offered only twice a year in specific locations. What remains unknown is how to motivate patients to properly dispose of unused opioid pills rather than keep them unsecured in the home. Surgical literature reports that less than 10% of surgery patients properly store or dispose of unused opioids.3,4
In 2000, the Joint Commission on Accreditation of Healthcare Organizations introduced the Pain Management Standards,5,6 which placed priority on pain as the fifth vital sign and recommended aggressive treatment to clinicians, including opioids. Pain management needs after same-day surgery are difficult to predict, and there is a wide range of opioid prescribing and use after similar procedures.7–14 If patients are not provided with adequate pain medication, they may suffer in pain, which can result in a delay of return to normal activity.15,16 If patients do not use all of the opioid pain medications, these unused opioid pills are available for misuse or abuse if they remain in the home.17–25 Studies show that surgeons overprescribe by as much as 70% after elective surgeries,4,10–12,18,26–35 so a measure of actual patient use can help determine appropriate prescribing ranges.36
More than 75% of surgeries in the US Veterans Affairs (VA) system are ambulatory37 or same-day surgery, which means that every year, approximately 320 000 veterans manage their postoperative pain at home. Opioids are commonly prescribed after ambulatory surgery because they are effective in managing acute pain.38,39 Electronic opioid prescriptions are not allowed within the VA system, which forces patients to return to the VA for more opioids if they need more than were prescribed. Many live far away, especially in rural environments, so the need to return for refills could cause financial as well as emotional hardship if there is a need for opioid pain medications beyond what is prescribed at the time of discharge.
This prospective study evaluated if we can simultaneously motivate and educate patients about proper drug disposal while measuring actual opioid use by using a small monetary reimbursement in a rural environment.
METHODS
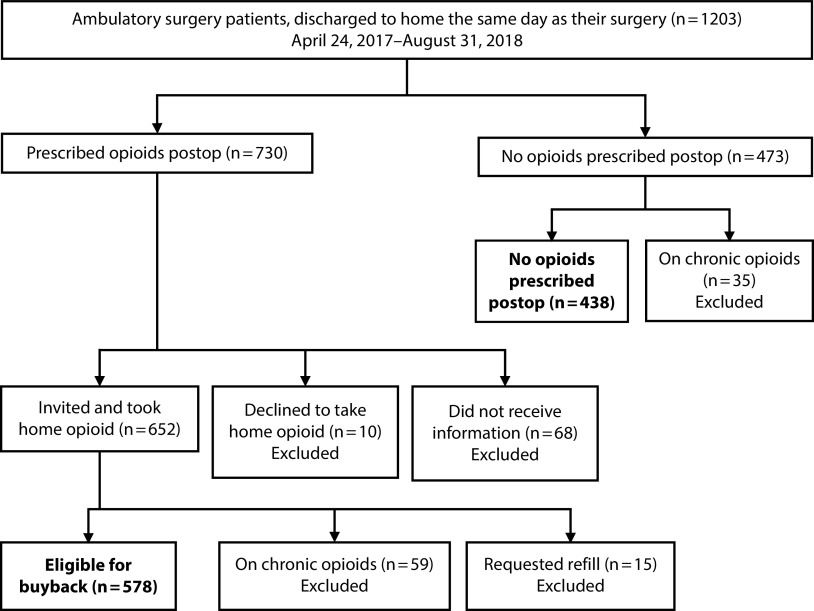
We screened all patients who had ambulatory surgery between April 24, 2017, and August 31, 2018, at a single rural VA institution (level II surgical complexity center) for inclusion. This eligible patient population was aged 18 years or older of any gender, race, or ethnicity. We included all surgical specialties and procedures if opioids were routinely prescribed after ambulatory surgery and included demographic information on patients who had the same procedure but were not prescribed opioids. We excluded procedures in which opioids are not routinely prescribed (pain procedures, ophthalmology procedures, and routine cystoscopy). We excluded patients who required a postoperative opioid refill and those on chronic opioids from participation in the buyback. We only included outpatients (discharged to home on the same day as the procedure). We excluded patients discharged to a nursing home or back to a rehabilitation center (Figure 1).
FIGURE 1—
Inclusion and Exclusion Criteria for Opioid Buyback Eligibility After Ambulatory Surgery: Rural US Veterans Affairs Hospital, 2017 to 2018
All patients received written standard specialty specific discharge instructions, including how to manage the expected postoperative pain.
Buyback Intervention
The VA is unique in that medications are dispensed on site when providers enter medications into our electronic medical record (Computerized Patient Record System). After surgery, the patient leaves the recovery room with filled prescriptions. Potential study participants received an informational letter printed on pink paper in the bag (Figure A, available as a supplement to the online version of this article at http://www.ajph.org) with the dispensed opioid medication after ambulatory surgery as well as a pink reminder sticker on the bottle that says “Return unused pills for $$. See pink info sheet.”
Nurses also reviewed the buyback program with patients as part of the discharge instructions. The letter educated the patients about the need for proper disposal of unused opioid pain medications because of the risk to communities and families of keeping unused opioids in the home. To motivate this behavior, we offered reimbursement of $5 per pill with a limit of $50 if patients returned their unused opioid pain medication within 60 days of surgery to our VA outpatient pharmacy for proper disposal, Monday through Friday from 9 am to 3:30 pm, excluding federal holidays. This medication disposal receptacle could be used for any unused medication. Pharmacists counted and confirmed that the opioid medications returned were what was dispensed. We queried a multistate prescription drug monitoring program (PDMP) to confirm that no duplicate opioid prescriptions were obtained outside of the VA.
Surgeon Education Intervention
After 6 months, data were compiled and information on what the patients were willing to return through the opioid buyback program was shared with each surgical specialty service that performed ambulatory surgery and wrote prescriptions for postoperative opioids. The proportion of patients returning unused opioids for procedures based on Current Procedural Terminology (CPT) code (American Medical Association, Chicago, IL) was given to each of the surgical specialties along with how many pills were unused and how many pills were prescribed. There are some procedures that are performed by more than 1 specialty, and these were shared by CPT code to each specialty. By December 31, 2017, surgeons in each specialty reached a specialty-level consensus with recommendations to adjust and standardize opioid prescribing by procedure after common ambulatory surgeries. We used opioid pain medication refill requests within 30 days of surgery as a proxy for insufficient postoperative pain management with an agreement that if there was an increase in refill requests, we would share that information with surgeons in a timely manner.
Data Collected
We collected demographic data including age, gender, date of index surgery, and CPT codes for the day of the procedure. If multiple CPT codes were assigned to the same procedure, we recorded the CPT code with the highest work relative value unit. If patients had multiple qualifying surgeries during the study period, they were counted as separate participants if more than 30 days elapsed between their surgeries.
Opioid data.
We only included opioid pain medications with a Drug Enforcement Agency (DEA) schedule II, III, or IV40 if they were prescribed in a pill form after surgery. The opioid prescription was converted to milligram morphine equivalents (MMEs) using the Center for Medicare and Medicaid Services conversion table to adjust for differences in surgeon preference for oral opioids.41 To determine eligibility, we collected information from the multistate PDMP and Computerized Patient Record System for preoperative opioid use in the 12 months before the index surgery.
Preoperative opioid use classification.
We defined chronic opioid use as greater than 90 days of opioids prescribed in the previous 12 months and we excluded these patients from the buyback program. If there was at least 1 opioid prescription in the 6 months before the index operation, we assigned the “acute use” classification, and we included these patients in the buyback group. If no opioids were prescribed in the 12 months before the index operation, we classified the patient as opioid naïve and included them the buyback group.
We identified opioid refills in Computerized Patient Record System and the multistate PDMP. We defined refills as any additional opioid written in the 1 to 30 days after the index operation, not including the discharge opioid prescription. The multistate PDMP includes refills from any provider at the VA or outside provider, including emergency department care.
Expected pain.
On July 1, 2017, our state adopted opioid limits for adults aged 18 years old or older. This included categories of expected pain with recommendations for types of procedures to be classified as minor pain, moderate pain, severe pain, and extreme pain.42 We adopted the same approach to classification of expected pain postoperatively based on the procedure performed. For example, we classified open inguinal hernia repair as expected to have moderate postoperative pain with the state recommended range of 72 to 120 MMEs (approximately 10–16 oxycodone 5-milligram pills).
The primary outcome was the number of patients who participated in the opioid buyback and the number of opioid pills returned as a proxy for actual need or use after ambulatory surgery. Other outcomes examined included changes in prescribing with feedback given to surgeons based on the buyback information.
Data analysis.
We compared outcomes in the cohort of opioid buyback participants to nonparticipants. We analyzed outcomes with χ2 analysis or Fisher exact test for proportions and the student t test for continuous variables. We defined statistical significance at a P level of less than .05, and all tests were 2-sided with normal distributions. We used SPSS version 25 (IBM, Somers, NY) for all statistical analyses.
RESULTS
Overall, 1203 patients from April 24, 2017, through August 31, 2018, had ambulatory surgery with a likely requirement for postoperative opioid analgesia to manage pain. Of those, 730 (60.7%) were prescribed opioids, and 473 were not prescribed an opioid. Excluded were 94 patients we identified as already prescribed chronic opioids: 59 prescribed an opioid and 35 not prescribed an opioid. We excluded 15 patients prescribed an opioid who requested a refill; no patients by PDMP check in the no-opioid group subsequently requested an opioid. We also excluded an additional 68 who were prescribed an opioid but did not receive the opioid buyback information and 10 who declined to take the opioid home even after receiving the buyback information. The final eligible-for-buyback population was 578 patients (Figure 1).
Buyback Intervention
Overall, 171 (29.6%) eligible patients returned unused opioid pills after ambulatory surgery as part of this program, resulting in 2136.5 unused opioid pills returned for proper disposal (range 3–49 pills returned; Table 1). We also emptied our medication disposal receptacle every 4 to 5 weeks and destroyed 754.2 pounds of unused medications to include the returned unused opioids and other unwanted or expired medications. Patients who returned unused opioids were older (64.9 years vs 59.0 years; P < .001) and more likely to be opioid naïve (94.7% vs 82.4%; P < .001) compared with those who did not participate in the buyback. This veteran population was mostly male in both the returned opioids and the no-participation groups.
TABLE 1—
Patient Characteristics for Eligible Ambulatory Surgery Patients Who Returned Unused Opioids and Did Not Return Unused Opioids: Rural US Veterans Affairs Hospital, 2017 to 2018
| Returned Unused Opioids (n = 171) | Did Not Return (n = 407) | P | |
| Mean age, y | 64.9 | 59.0 | < .001 |
| Male, % | 91.8 | 92.9 | .73 |
| Preoperative opioid status, % | < .001 | ||
| Opioid naive | 94.7 | 82.4 | |
| Acute exposure (at least 1 opioid in previous 6 mo) | 5.3 | 17.6 | |
| Expected pain, % | .9 | ||
| Minor | 3.5 | 3.7 | |
| Moderate | 75.3 | 72.8 | |
| Severe | 21.2 | 23.5 | |
| Prescriber level, % | .7 | ||
| Attending | 12.9 | 15.4 | |
| Resident | 63.5 | 59.3 | |
| Physician assistant/nurse practitioner | 23.5 | 25.2 | |
| Average MME prescribed | 117.7 | 122.9 | .56 |
| Minor pain | 65.0 | 85.7 | .38 |
| Moderate pain | 90.9 | 99.7 | .2 |
| Severe pain | 218.9 | 201.3 | .5 |
Note. MME = milligram morphine equivalent.
The expected intensity of postoperative pain had similar distributions between the group that returned unused opioids and the group that did not return, and the case mix was also similar in each of these groups (Table 1). There was no difference between the 2 groups in who prescribed the postoperative medications with surgery residents in both groups prescribing the majority of postoperative opioids. The average MME was similar between the 2 groups overall and for the expected postoperative pain groups.
Patients who were not prescribed postoperative opioids and were not on chronic opioids already were in the same age range (average 63.0 years). The vast majority who were not prescribed opioids (91.6%) underwent surgeries with expected minor or moderate pain. None of these patients later requested an opioid prescription during this study period.
Although we found that older patients participated more in the buyback, this result varied by select CPT codes. For the cohort (return, no return, and no opioid prescribed; n = 1016), the average age was 62 years. After carpal tunnel release surgery (CPT 64721; n = 37) those aged 62 years or older were more likely to return unused opioids (P < .007). However, after inguinal hernia repair (CPT 49505, 49520, 49525, 49650, 49651; n = 111), there was no difference (P = .89).
Surgeon Education Intervention
After the surgeon education intervention, surgeons prescribed opioids to fewer patients after the same procedures (62.3% in 2017 vs 50.5% in 2018; P < .001; Table 2). For example, after carpal tunnel release (CPT 64721), the rate of opioid prescriptions decreased from 76% in 2017 to 28% in 2018. Similarly, 79% of vasectomy patients (CPT 55250) in 2017 were prescribed opioids, which decreased to 33% in 2018. The average MMEs prescribed decreased from 136.3 in 2017 to 99.8 in 2018 (P < .001). In 2017, 61.6% of patients were prescribed more than 10 opioid pills after ambulatory surgery. In 2018, only 34.3% of patients were prescribed more than 10 opioid pills (P < .001). The attending surgeons and case mix remained the same during this time period.
TABLE 2—
Surgical Provider Opioid Prescribing Data Following Ambulatory Surgery Before and After Surgeon Education Intervention for Eligible Patients: Rural US Veterans Affairs Hospital, 2017 to 2018
| 2017 | 2018 | P | |
| Overall (n = 1016), no. | 549 | 467 | |
| Postoperative prescription, no. (%) | < .001 | ||
| No opioids | 207 (37.7) | 231 (49.5) | |
| Prescribed postoperative opioid | 342 (62.3) | 236 (50.5) | |
| Prescribed MME, average | 136.3 | 99.8 | < .001 |
| More than 10 pills prescribed, no. (%) | 211 (61.6) | 81 (34.3) | < .001 |
| Returned unused opioids, no. (%) | 97 (28.4) | 74 (31.4) | .46 |
| Amount of opioids returned, no. (%) | .21 | ||
| Returned all/used none | 56 (57.7) | 52 (70.3) | |
| Returned ≥ 50% prescribed/used < 50% | 29 (29.9) | 17 (23.0) | |
| Returned < 50% prescribed/used ≥ 50% | 12 (12.4) | 5 (6.8) | |
| Returned more than 10 pills | 42 (43.3) | 16 (20.5) | .003 |
| Total funds disbursed, sum, $ | 4290 | 3185 |
Participation in the opioid buyback slightly increased from 2017 to 2018 (28.4% vs 31.4%; P = .5). The number of buyback participants who returned more than 10 pills decreased from 43.3% in 2017 to 20.5% in 2018 (P = .003). We also found that 63.2% of patients who participated in the buyback did not use any opioids after surgery and returned all of the opioid pills that were prescribed with no statistical difference from 2017 to 2018. Overall, we paid out $4290 in 2017 and $3185 in 2018 to patients.
The refill requests remained the same before and after the surgeon intervention with 7 refill requests in 2017 and 8 refill requests in 2018, including 1 in 2018 in which the patient reported the postoperative opioid was stolen by a family member. Refill requests were not limited to a single specialty or provider during this time period.
DISCUSSION
To our knowledge, this is the first opioid buyback program successfully implemented. We used a small monetary incentive to motivate patients to return 2136.5 unused opioid pills so they could not be diverted for misuse or abuse in our rural communities. In our rural location, it can be challenging to find an accessible drug disposal location that accepts controlled substances, and transportation is a potential barrier for our patient population to use community disposals. According to our online state disposal locator, one larger town with a state college where our patients frequently come from has 3 police or sheriff station disposal sites and 1 hospital disposal site within 15 miles. But they have limited hours and require a special trip to use these sites. Our VA patients return 2 to 4 weeks after surgery for follow up, so no special trip is needed to participate in the buyback program. If a VA patient does not have a working vehicle or cannot drive or find a driver, VA transportation can bring patients for appointments. Based on income, some VA patients qualify for Travel Pay for appointments.
After ambulatory surgery, almost 1 in 3 of our patients returned unused opioids through the opioid buyback program. No opioids were used for postoperative pain management by almost two thirds of those who participated in the opioid buyback, many returning the bottles with the tamper-proof seal unbroken. Previous studies that included inpatient and outpatient surgery showed 61.5% of opioids went unused,12 and we had a return of unused opioids from about half that percentage through our opioid buyback.
In addition, 43.3% returned more than 10 unused opioid pills in 2017. This feedback was given to surgeons resulting in only 20.5% returning more than 10 unused opioid pills in 2018. This is in direct correlation to surgeons decreasing their prescribing of 10 pills or more by almost half from 61.6% in 2017 to 34.3% in 2018 when opioids were prescribed after ambulatory surgery.
Surgeons decreased prescribing by 27% after the feedback about returns. Oxycodone 5 milligrams was the most commonly prescribed medication after surgery, so to put the MME decrease in perspective, this is a decrease from 18 oxycodone 5-milligram pills per patient to 13 oxycodone 5-milligram pills. In addition, surgeons were prescribing opioids to almost two thirds of patients in 2017, but they were more selective in 2018 with only half the patients prescribed opioids with a similar case mix.
There are many strengths in our analysis. The VA dispenses the opioid pain medications as written by the surgeons after ambulatory surgery. This allowed us to confirm that the patients actually received the opioid pain medication. In our program, pharmacists counted and confirmed the number and type of pill that were returned so we were able to accurately provide information on what patients were using to provide feedback to surgeons. Rather than rely on survey information or patient recall, we were able to count and confirm what patients returned as a proxy for postoperative opioid needs to manage pain. The information on what patients were returning through the opioid buyback program by CPT code was given to the surgeons; there was a measurable decrease in prescribing without an increase in refill requests.
There are many potential implications for our data. Surgeons were interested to know what their colleagues were prescribing for the same procedures and adjusted prescribing toward the lowest prescriber. In our facility, providing information to the recovery room nurses about opioid use after ambulatory surgery helped with their discharge education with the patients. Patients were reminded and encouraged to use nonopioid pain management techniques including acetaminophen and nonsteroidal anti-inflammatory drugs, and things like ice. We did not ask specialties to alter their usual practice as nonopioid pain management varies by specialty. For example, some specialties prohibit nonsteroidal anti-inflammatory medications after surgery. The recovery room nurses called the patients 24 to 48 hours after surgery and provided feedback to providers if pain was a significant problem. Although this is our experience, further analysis of the effect of an opioid buyback in our facility as well as other facilities will give us a broader understanding of what effective perioperative pain management can be with the lowest range of opioid pain pill prescribing and use after ambulatory surgery.
State legislation and proposed guidelines are helpful for identifying ranges for surgeons to prescribe opioids after common surgeries.43–50 For example, our state allows up to 70 tablets of 5 milligrams of Vicodin for bone fracture surgery with expected extreme pain. A surgeon might prescribe 2 tablets every 4 hours (12 per day) and a prescription of 50 tablets is well within the guidelines. But pain is an individual problem, so there may be unused opioids even staying within proposed guidelines attributable to variation in patient use. An opioid buyback program motivates patients to remove unused opioids from their homes for proper disposal, and we are thankful that the patient who had 49 unused opioid pills returned them for proper disposal through our program.
An opioid buyback program may ultimately be a cost-effective tool to help curb the opioid epidemic as the United States spent $78 billion for opioid addiction and treatment in 2013.51,52 An added benefit for our rural population is that we also provide a safe and convenient place for disposal of all unused or expired medications for our patients, not just unused opioids.
Limitations
There are several limitations to this study. First, we do not know if patients used the information provided and were motivated instead to bring unused opioids to local drug take-back days sponsored by the DEA in April and October at select locations around the country. If so, our findings may underestimate the true amount of returned opioids. Second, several declined the reimbursement so we do not know if other patients did not want the reimbursement and disposed of the medications directly into the receptacle for destruction when they came to the pharmacy. Controlled substances can only be accepted in secured medication receptacles. When full, the liners in the receptacles are removed and sealed by our VA police and shipped for incineration. We are not permitted to examine the contents before shipping. Third, since we capped the reimbursement at $50 (10 pills), we do not know if patients are returning the maximum of 10 but keeping the other unused opioid pills, so we may still be overestimating postoperative opioid needs. Fourth, we cannot account for changes in provider practice or patient preferences attributable to an increase in education for providers as well as media attention regarding the opioid crisis. And last, we do not know if our findings would be generalizable to other VA or non-VA facilities or to other rural or urban locations.
Our success led to expansion to a second rural VA in 2019 and institutional review board approval at a third rural VA location to start in 2020. In addition, we are retrospectively reviewing records from 2017 to 2018 to see if there are patient characteristics (such as previous substance abuse or mental health issues) that might lead a patient to participate in the opioid buyback. We are continuing the opioid buyback program at our VA and, since June 2019, partnered with social scientists to see if sending out a reminder card would increase our participation rate.
Conclusions
This opioid buyback program after ambulatory surgery shows that a small monetary incentive motivates patients to return unused opioids for proper disposal so they are no longer available for misuse or abuse in our rural communities. Information on returns provided to surgeons can help optimize opioid prescribing for common ambulatory surgeries. There will always be patient variability in opioid pain medication use after ambulatory surgery, and this program can be a useful tool to keep unused prescription opioids out of our communities.
ACKNOWLEDGMENTS
This work was funded by the VHA Innovators Network and the US Department of Veterans Affairs Office of Rural Health.
We would like to thank the reviewers for their attention and constructive comments.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
HUMAN PARTICIPANT PROTECTION
This study protocol was reviewed and approved by the Veterans Institutional Review Board of Northern New England (VINNE#1019235) and granted an alteration of consent process and authorization to replace written consent with the informational letter provided with each opioid prescription.
Footnotes
REFERENCES
- 1.US Department of Veterans Affairs. Rural veterans. Available at: https://www.ruralhealth.va.gov/aboutus/ruralvets.asp. Accessed March 4, 2020.
- 2.Selekman RE, Gaither TW, Kornberg Z, Liaw A, Copp HL. Unwanted medication disposal: audit of California pharmacy advice. Ann Intern Med. 2020;172(9):632–634. doi: 10.7326/M19-2409. [DOI] [PubMed] [Google Scholar]
- 3.Hasak JM, Roth-Bettlach CL, Santosa KB, Larson EL, Stroud J, Mackinnon SE. Empowering post-surgical patients to improve opioid disposal: a before and after quality improvement study. J Am Coll Surg. 2018;226(3):235–240. doi: 10.1016/j.jamcollsurg.2017.11.023. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicket MC, Long JJ, Pronovost PJ, Alexander G, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066–1071. doi: 10.1001/jamasurg.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Veterans Affairs. Pain as the 5th vital sign tool kit. 2000. Available at: http://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Accessed October 5, 2016.
- 6.Phillips DM. Joint Commission on Accreditation of Healthcare Organizations: JCAHO pain management standards are unveiled. JAMA. 2000;284(4):428–429. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- 7.Waljee JF, Zhong L, Hou H, Sears E, Brummett C, Chung KC. The use of opioid analgesics following common upper extremity surgical procedures: a national, population-based study. Plast Reconstr Surg. 2016;137(2):355e–364e. doi: 10.1097/01.prs.0000475788.52446.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SP, Chung KC, Zhong L et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947–957. doi: 10.1016/j.jhsa.2016.07.113. e3. [DOI] [PubMed] [Google Scholar]
- 9.Thiels CA, Ubl DS, Yost KJ et al. Results of a prospective, multicenter initiative aimed at developing opioid-prescribing guidelines after surgery. Ann Surg. 2018;268(3):457–468. doi: 10.1097/SLA.0000000000002919. [DOI] [PubMed] [Google Scholar]
- 10.Hill MV, McMahon ML, Stucke RS, Barth RJ. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709–714. doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 11.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267(3):468–472. doi: 10.1097/SLA.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 12.Thiels CA, Anderson SS, Ubl DS et al. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266(4):564–573. doi: 10.1097/SLA.0000000000002365. [DOI] [PubMed] [Google Scholar]
- 13.Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180–188. doi: 10.2106/JBJS.17.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekhri S, Arora NS, Cottrell H et al. Probability of opioid prescription refilling after surgery: does initial prescription dose matter? Ann Surg. 2018;268(2):271–276. doi: 10.1097/SLA.0000000000002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North Am. 2005;23(1):185–202. doi: 10.1016/j.atc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen DK, Amid PK, Chen DC. Groin pain after inguinal hernia repair. Adv Surg. 2016;50(1):203–220. doi: 10.1016/j.yasu.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551–555. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 18.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11(1):e0147972. doi: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg AE, Chesney TR, Srikandarajah S, Acuna SA, McLeod RS. Opioid use after discharge in postoperative patients: a systematic review. Ann Surg. 2018;267(6):1056–1062. doi: 10.1097/SLA.0000000000002591. [DOI] [PubMed] [Google Scholar]
- 20.Harris K, Curtis J, Larsen B et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317–321. doi: 10.1001/jamadermatol.2013.1871. [DOI] [PubMed] [Google Scholar]
- 21.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 22.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425–430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 23.Brat GA, Agniel D, Beam A et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi: 10.1136/bmj.j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brummett CM, Waljee J, Goesling J et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1733–1740. doi: 10.1213/ANE.0000000000002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauley CE, Anderson G, Haynes AB, Menendez M, Bateman BT, Ladha K. Predictors of in-hospital postoperative opioid overdose after major elective operations: a nationally representative cohort study. Ann Surg. 2017;265(4):702–708. doi: 10.1097/SLA.0000000000001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emerson JB, Danilack VA, Kulkarni A, Brousseau EC, Matteson KA. Outpatent opioid use after cesarean section. Oral presentation at: American College of Obstetrics and Gynecologists Annual Clinical and Scientific Meeting; May 8, 2017; San Diego, CA.
- 28.Hausmann L. Use of potentially unsafe high opioid dosage varies by demographic characteristics among veterans dually-enrolled in Veterans Affairs and Medicare. Oral presentation at: American Pain Society; May 17, 2017; Pittsburgh, PA.
- 29.Kim N, Matzon JL, Abboudi J et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98(20):e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 30.Kumar K, Gulotta LV, Dines JS et al. Unused opioid pills after outpatient shoulder surgeries given current perioperative prescribing habits. Am J Sports Med. 2017;45(3):636–641. doi: 10.1177/0363546517693665. [DOI] [PubMed] [Google Scholar]
- 31.Maughan BC, Hersh EV, Shofer FS et al. Unused opioid analgesics and drug disposal following outpatient dental surgery: a randomized controlled trial. Drug Alcohol Depend. 2016;168:328–334. doi: 10.1016/j.drugalcdep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Mylonas KS, Reinhorn M, Ott LR, Westfal ML, Masiakos PT. Patient-reported opioid analgesic requirements after elective inguinal hernia repair: a call for procedure-specific opioid-administration strategies. Surgery. 2017;162(5):1095–1100. doi: 10.1016/j.surg.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Osmundson SS, Schornack LA, Grasch JL, Zuckerwise LC, Young JL, Richardson MG. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130(1):36–41. doi: 10.1097/AOG.0000000000002095. [DOI] [PubMed] [Google Scholar]
- 34.UpToDate. Prescription of opioids for acute pain in opioid naive patients. 2017. Available at: https://www.uptodate.com. Accessed November 30, 2017.
- 35.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017;265(4):728–730. doi: 10.1097/SLA.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 36.Howard R, Fry B, Gunaseelan V et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2019;154(1):e184234. doi: 10.1001/jamasurg.2018.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Surgery Office. Annual Surgery Report. Washington, DC: Veterans Health Administration; 2018. p. 12. [Google Scholar]
- 38.Joshi GP, Rawal N, Kehlet H et al. Evidence-based management of postoperative pain in adults undergoing open inguinal hernia surgery. Br J Surg. 2012;99(2):168–185. doi: 10.1002/bjs.7660. [DOI] [PubMed] [Google Scholar]
- 39.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids prescribed after low-risk surgical procedures in the United States, 2004–2012. JAMA. 2016;315(15):1654–1657. doi: 10.1001/jama.2016.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Department of Justice, Drug Enforcement Administration. Controlled substance schedules. 2017. Available at: https://www.deadiversion.usdoj.gov/schedules. Accessed April 24, 2017.
- 41.Center for Medicare and Medicaid Services. Opioid oral morphine milligram equivalent (MME) conversion factors. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed March 30, 2017.
- 42.Vermont Department of Health. Rule governing the prescribing of opioids for pain. Available at: https://www.healthvermont.gov/sites/default/files/documents/2016/12/REG_opioids-prescribing-for-pain.pdf. Accessed July 20, 2017.
- 43.Center for Opioid Research and Education. Surgical opioid guidelines. 2018. Available at: https://www.solvethecrisis.org/best-practices. Accessed December 2, 2018.
- 44.Michigan Opioid Prescribing Engagement Network (OPEN) Prescribing recommendations. Available at: https://opioidprescribing.info. Accessed June 12, 2020.
- 45.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in opioid prescribing through evidence-based prescribing guidelines letters. JAMA Surg. 2018;153(3):285–287. doi: 10.1001/jamasurg.2017.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overton HN, Hanna MN, Bruhn WE, Hutfless S, Bicket MC, Makary MA. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227(4):411–418. doi: 10.1016/j.jamcollsurg.2018.07.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zipple M, Braddock A. Success of hospital intervention and state legislation on decreasing and standardizing postoperative opioid prescribing practices. J Am Coll Surg. 2019;229(2):158–163. doi: 10.1016/j.jamcollsurg.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Kaafarani HM, Weil E, Wakeman S, Ring D. The opioid epidemic and new legislation in Massachusetts: time for a culture change in surgery? Ann Surg. 2017;265(4):731–733. doi: 10.1097/SLA.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 49.Hartford LB, Van Koughnett JAM, Murphy PB et al. Standardization of Outpatient Procedure (STOP) Narcotics: a prospective non-inferiority study to reduce opioid use in outpatient general surgical procedures. J Am Coll Surg. 2019;228(1):81–88.e1. doi: 10.1016/j.jamcollsurg.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Reid DBC, Shah KN, Shapiro BH, Ruddell JH, Akelman E, Daniels AH. Mandatory prescription limits and opioid utilization following orthopaedic surgery. J Bone Joint Surg Am. 2019;101(10):e43. doi: 10.2106/JBJS.18.00943. [DOI] [PubMed] [Google Scholar]
- 51.Kane-Gill SL, Rubin EC, Smithburger PL, Buckley MS, Dasta JF. The cost of opioid-related adverse drug events. J Pain Palliat Care Pharmacother. 2014;28(3):282–293. doi: 10.3109/15360288.2014.938889. [DOI] [PubMed] [Google Scholar]
- 52.National Institute on Drug Abuse. Medications to treat opioid use disorder. 2018. Available at: https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/how-much-does-opioid-treatment-cost. Accessed June 6, 2019.