Abstract
Objectives. To describe county-level socioeconomic profiles associated with Kentucky’s 2017–2018 hepatitis A outbreak that predominately affected communities affected by the opioid epidemic.
Methods. We linked county-level characteristics on socioeconomic and housing variables to counties’ hepatitis A rates. Principal component analysis identified county profiles of poverty, education, disability, income inequality, grandparent responsibility, residential instability, and marital status. We used Poisson regression to estimate adjusted relative risks (RRs) and 95% confidence intervals (CIs).
Results. Counties with scores reflecting an extremely disadvantaged profile (RR = 1.21; 95% CI = 0.99, 1.48) and greater percentage of nonmarried men, residential instability, and income inequality (RR = 1.15; 95% CI = 0.94, 1.41) had higher hepatitis A rates. Counties with scores reflecting more married adults, residential stability, and lower income inequality despite disability, poverty, and low education (RR = 0.77; 95% CI = 0.59, 1.00) had lower hepatitis A rates. Counties with a higher percentage of workers in the manufacturing industry had slightly lower rates (RR = 0.97; 95% CI = 0.94, 1.00).
Conclusions. As expected, impoverished counties had higher hepatitis A rates. Evaluation across the socioeconomic patterns highlighted community-level factors (e.g., residential instability, income inequality, and social structures) that can be collected to augment hepatitis A data surveillance and used to identify higher-risk communities for targeted immunizations.
Hepatitis A is a vaccine-preventable, infectious liver disease transmitted via the fecal–oral route through direct person-to-person contact or consumption of contaminated food or water. Hepatitis A is the most common form of viral hepatitis worldwide1; however, in the United States, hepatitis A incidence decreased from 31 582 cases in 19952 to 1239 cases in 20143 after vaccine introduction in 1995. Since 2016, multiple outbreaks have resulted in greater than 15 000 incident hepatitis A cases not attributed to food or drink contamination, but that have predominately occurred in urban and rural communities that use or inject drugs and those affected by the opioid epidemic.1
The opioid epidemic and increased rates of infectious diseases are closely associated.4–6 Behaviors promoting hepatitis A virus (HAV) transmission among drug users are less clear, but likely involve poor hygiene and unsanitary drug sharing that are tied with socioeconomic challenges that increase infection vulnerability.7–9 Social and economic factors, such as personal and neighborhood poverty,10–19 income inequality,15,20,21 and lack of financial opportunities12,14,19,22,23 or social enrichment resources,24,25 are factors associated with drug-seeking behaviors,11,15–18,25 opioid use disorder,13,20,22,24 and higher prevalence of blood-borne viral infections,10,14,15,19–21,23,24 but have not been studied in relation to HAV infection. Community-level public health interventions are needed to address the contextual causes of drug use for infectious disease prevention and opioid use disorder prevention5,7,9–11 and should consider the complicated nature of socioeconomic status that is likely to be meaningful, as observed with the neighborhood deprivation index.26 In this study, we assessed the interplay of multiple county-level socioeconomic factors by using a principal component analysis (PCA) and examined socioeconomic patterns in relation to hepatitis A incidence in the context of the opioid epidemic.
Kentucky reported the highest number of hepatitis A cases in the 2017–2018 outbreak27 and had one of the highest rates of overdose deaths.28 Qualitative research in Kentucky highlighted that opioid use and risky health behaviors were thought to be driven by greater poverty, declining economic opportunity—particularly loss of coal mining jobs and out-migration—and declining social enrichment, which have disproportionately affected the eastern Kentucky region of Appalachia.24 The current study’s objective was to describe the county-level variation in socioeconomic patterns in Kentucky and to examine their associations with differential rates of the hepatitis A outbreak across Kentucky counties.
METHODS
Hepatitis A is a reportable infectious disease. Confirmed, probable, and suspected cases of hepatitis A were reported to local and state health departments in Kentucky. Local health departments and epidemiologists reviewed medical records and conducted case interviews. Patient and infectious disease outbreak information collected during this outbreak included demographic factors, clinical information, housing characteristics, behavioral factors, travel history, and contact with homeless persons, sick persons, and restaurants. Between August 2017 and December 2018, 3353 hepatitis A cases were reported in 97 of 120 Kentucky counties. In this study, we included 3349 reported hepatitis A cases with information on the county of diagnosis. The median age of hepatitis A diagnosis among the cases was 36 years, 59.2% were males, 64.9% reported illicit drug use, 52.5% had coinfections predominately from hepatitis C virus, and 9.1% reported homelessness. Nearly all hepatitis A cases (n = 3348) were serologically positive with immunoglobulin-M anti-HAV tests, and 89.8% had 2 or more symptoms consistent with infection. Among those with samples sent to the Centers for Disease Control and Prevention for HAV sequencing (n = 586), 97.8% screened positive. County-level hepatitis A rates were derived by dividing the number of reported cases in each county by the county population size and were the outcome of interest.
The American Community Survey (ACS) is an annual survey on demographic, social, economic, and housing factors conducted by the US Census Bureau from a sample of US addresses.29 We linked the county-level 5-year estimates for 2012–2016 ACS characteristics to the county-level hepatitis A rates. We excluded the 23 counties that did not report hepatitis A cases.
County-Level American Community Survey Variables
County-level demographic and social factors considered were age, race, ethnicity, familial and marital characteristics, educational attainment, grandparent responsibility for care of grandchildren, disability, residential stability, and adolescent birth rate. We examined economic factors on income and poverty, income inequality, health insurance coverage, employment, and industry types of employment as well as housing characteristics on median home value, costs, occupancy, and vehicle ownership. The median percent margin of error for the 5-year ACS variables included in the PCA was 2.8% (interquartile range = 2.0%–3.6%).
Statistical Analyses
We mapped quartiles of county-level hepatitis A rates by using ArcGIS, version 10.6 (Environmental Systems Research Institute Inc, Redlands, CA) to describe the geographic distribution of hepatitis rates. County-level socioeconomic characteristics by hepatitis A quartiles were presented to identify factors crudely related to hepatitis A rates. We examined correlation patterns between 41 socioeconomic variables with Pearson correlation coefficients. As expected, many socioeconomic variables were strongly correlated with each other with correlations greater than the absolute value of 0.70 (Table A, available as a supplement to the online version of this article at http://www.ajph.org), which prompted the use of the PCA to identify socioeconomic patterns across 97 Kentucky counties. After identifying socioeconomic status patterns from the PCA, we used Poisson regression to estimate the associations between the principal component (PC) scores with HAV infection rates.
Principal component analysis.
We conducted the PCA in SAS version 9.4 (SAS Institute Inc, Cary, NC) using PROC FACTOR to generate statistically uncorrelated PC scores using the weighted sum of the socioeconomic variables with the weights equal to the eigenvectors obtained from the PCA. We used a scree plot and a cut-off of 75% of cumulative variation explained to select the number of PCs to include in subsequent outcome analyses.30 This approach has been used in many contexts, including social and nutritional epidemiology, to develop indices related to other health outcomes.26,31 For easier interpretation of the PCs, we selected a reduced set of 12 economic and social variables that have been commonly used in the literature and based on data observations from Table 1. We examined the factor loadings and labeled each PC by the heaviest loadings greater than 0.15, which has been used as a cutpoint in previous literature.31
TABLE 1—
County-Level Socioeconomic Characteristics of 97 Kentucky Counties Overall and by Quartiles of County Hepatitis A Case Rates per 100 000 People Occurring During the 2017–2018 Outbreak
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| No. of counties | 97 | 24 | 24 | 25 | 24 |
| No. of hepatitis A cases per 100 000 (IQR) | 57.8 (27.5–149.8) | 16.5 (2.1–27.0) | 39.3 (27.5–57.5) | 82.4 (57.8–149.8) | 241.1 (153.4–474.3) |
| County median age, y (SD) | 40.3 (2.9) | 41.3 (3.6) | 39.3 (3.2) | 40.4 (2.1) | 40.3 (2.6) |
| % White, median (SD) | 96.4 (4.8) | 94.6 (5.0) | 93.6 (5.3) | 96.7 (5.2) | 97.8 (1.7) |
| % of families that are married householders, mean (SD) | 74.5 (4.4) | 76.8 (3.4) | 75.1 (4.0) | 74.1 (5.1) | 72.2 (3.7) |
| % of families with single-male heads of households, mean (SD) | 7.2 (1.7) | 6.2 (1.3) | 7.5 (1.9) | 7.4 (1.6) | 7.8 (1.8) |
| % of families with single-female heads of households, mean (SD) | 18.2 (3.5) | 17.0 (3.1) | 17.4 (3.2) | 18.5 (4.1) | 20.0 (2.8) |
| % of men who are married, mean (SD) | 53.4 (5.0) | 56.1 (3.6) | 53.3 (5.2) | 53.9 (4.7) | 50.5 (4.8) |
| % of women who are married, mean (SD) | 50.9 (4.7) | 52.4 (4.1) | 51.9 (4.9) | 49.8 (4.9) | 49.6 (4.7) |
| % educational attainment < ninth grade, mean (SD) | 9.0 (4.2) | 8.4 (3.7) | 7.1 (3.5) | 8.7 (4.1) | 11.7 (4.1) |
| % high-school graduate or higher, mean (SD) | 80.3 (6.6) | 81.5 (5.6) | 82.7 (6.1) | 80.8 (6.3) | 76.1 (6.7) |
| % of grandparents responsible for grandchildren, mean (SD) | 57.0 (11.5) | 54.2 (9.9) | 56.9 (13.2) | 53.8 (10.3) | 63.5 (10.4) |
| % with disability among noninstitutionalized civilians, median (SD) | 19.6 (5.8) | 19.0 (5.0) | 17.5 (4.9) | 20.5 (6.1) | 22.4 (5.7) |
| % with different residence than previous year, mean (SD) | 13.2 (3.7) | 11.8 (3.0) | 14.7 (4.2) | 12.5 (3.9) | 13.8 (2.9) |
| % in manufacturing industry, mean (SD) | 16.2 (6.8) | 19.5 (6.3) | 17.6 (6.2) | 16.4 (7.3) | 12.5 (6.7) |
| % in arts, entertainment, recreation, accommodation, and food services industries, mean (SD) | 7.2 (2.4) | 6.5 (2.4) | 7.7 (2.4) | 7.0 (2.6) | 7.0 (2.6) |
| % in construction industry, mean (SD) | 7.2 (2.3) | 7.7 (2.5) | 7.3 (2.5) | 7.1 (2.5) | 7.3 (2.3) |
| % in agriculture, forestry, hunting and fishing, and mining industries, median (SD) | 3.1 (3.4) | 3.4 (2.8) | 3.0 (3.0) | 3.1 (2.9) | 3.3 (4.6) |
| % of population with income below poverty line, median (SD) | 20.4 (7.6) | 21.9 (5.8) | 18.8 (7.6) | 19.1 (7.6) | 26.1 (6.9) |
| Gini index of income inequality,a mean (SD) | 0.46 (0.03) | 0.45 (0.03) | 0.46 (0.04) | 0.46 (0.04) | 0.47 (0.03) |
Note. IQR = interquartile range.
Gini Index ranges from 0 to 1.
We included percentage of population below the poverty level and the Gini index of income inequality that are commonly used income-based variables.20,21 Education variables were highly correlated with one another (> the absolute value of 0.64; Table A); we selected percentage of county with educational attainment below ninth grade and high-school graduation. On the basis of Table 1 observations, we selected a subset of 5 familial structure and marital status variables, grandparent responsibility for grandchildren, disabled population, and residential instability based on the strength of relationships observed with hepatitis A quartiles. We excluded other variables on living in nonfamily households, widows, family size, and adolescent birth rates from the PCA because they were similar across hepatitis quartiles (Table B, available as a supplement to the online version of this article at http://www.ajph.org). The loadings between these 12 socioeconomic variables and each PC score are presented in Table 2. In summary, the variables included in the PCA were the Gini index of income inequality and the percentage of the county’s population below the poverty line, with educational attainment less than ninth grade, high-school graduates, disabled, grandparents responsible for grandchildren, living in a different residence than the previous year, families living in married households, families living in households with a single-female head of household, families living in households with a single-male head of household, married adult men, and married adult women.
TABLE 2—
Principal Component (PC) Loadings Showing the Correlations Between the PC Scores and the 12 County-Level Social and Economic Variables and Percentage of the Variance Explained and Eigenvalues for Each PC: Kentucky 2017–2018 Outbreak
| PC1 | PC2 | PC3 | |
| Eigenvalues | 5.30 | 2.85 | 0.89 |
| Proportion of variability explained | 0.442 | 0.237 | 0.074 |
| Cumulative proportion of variability explained | 0.442 | 0.679 | 0.753 |
| % of all people with income below poverty level | 0.921 | 0.263 | 0.103 |
| % educational attainment in population aged ≥ 25 y < ninth grade | 0.791 | 0.480 | −0.039 |
| % civilian noninstitutionalized population with a disability | 0.729 | 0.510 | 0.025 |
| % of families that are single-female head of household | 0.721 | −0.471 | −0.076 |
| County-level Gini index of income inequality | 0.658 | 0.066 | 0.249 |
| % of grandparents responsible for grandchildren | 0.537 | 0.120 | 0.465 |
| % of families that are single-male head of household | 0.487 | −0.346 | −0.513 |
| % with different residence 1 y ago | −0.122 | −0.724 | 0.476 |
| % of women who are now married, except separated | −0.520 | 0.706 | 0.022 |
| % of men who are now married, except separated | −0.552 | 0.601 | −0.177 |
| % of families that are married householders | −0.774 | 0.517 | 0.265 |
| % high-school graduate or higher | −0.792 | −0.526 | 0.017 |
We mapped quintiles of the PC scores in ArcGIS to visualize the geospatial distribution of the socioeconomic PC scores across the state, which public health practitioners may find useful to identify their counties’ score for the 3 identified PC patterns.
Outcome analysis.
We used Poisson regression to estimate relative risks (RRs) for a 1-standard-deviation increase in each county-level PC score with a scaled deviance to account for overdispersion. County hepatitis A counts was the dependent variable, and we included an offset term for the natural log of population size. We additionally adjusted all Poisson regression models for population size and a quadratic term for population size. We included county median age and percentage of the county who were White as adjustment variables. We examined whether the adjusted associations were independent of industries alluded to as reasons underlying the rise in opioid use24 (e.g., lack of economic development or social enrichment resources). We included the percentage of the county in the industries of (1) manufacturing; (2) arts, entertainment, recreation, accommodation, and food services; (3) construction; and (4) agriculture, forestry, fishing and hunting, and mining as proxies of economic development and social enrichment resources. As a sensitivity analysis, we restricted our analyses to counties with more than 10 cases of hepatitis A. We conducted all statistical analyses in SAS version 9.4.
RESULTS
The median HAV infection rate in Kentucky was 57.8 per 100 000 (IQR = 27.5–149.8). Higher HAV infection rates tended to occur in eastern Kentucky counties (Figure 1a), which is the Appalachian region particularly affected by the opioid epidemic. Compared with low hepatitis A rate counties, counties with the highest hepatitis A rates were slightly younger, had a larger White population, were more likely to live in single-male or single-female heads of households, had more single adult men, had more grandparents responsible for grandchildren, had more individuals who were disabled, were more impoverished, and had higher income inequality and residential instability (Table 1). In addition, the populations in counties with higher hepatitis A incidence had lower educational attainment, fewer families in married households, and lower percentage in the manufacturing industry (Table 1). Counties were similar across hepatitis A quartiles in terms of family size, nonfamily households, single adult females, widows, health insurance coverage, and other top industries including arts, food services, construction, agriculture, and mining (Table B).
FIGURE 1—
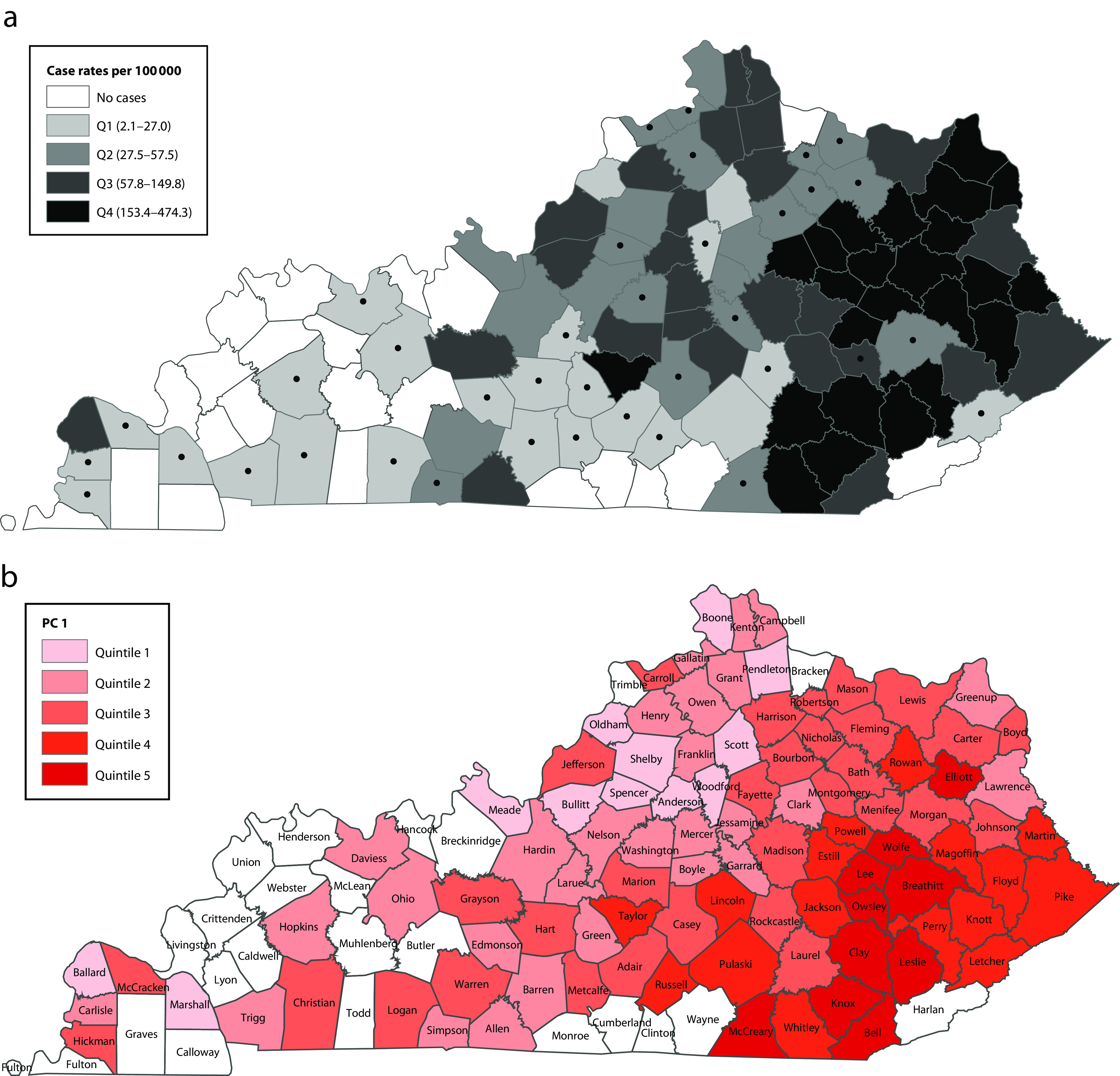
Kentucky Map Showing (a) Incidence of Outbreak-Associated Hepatitis A Cases per 100 000 by County, August 1, 2017, to December 31, 2018, and (b) Spatial Distribution of Quintiles of Principal Component 1 (PC1) Scores in 97 of the 120 Counties
Note. Q = quartile. Dots in part a indicate counties in which case rates were calculated from < 10 cases. PC2 and PC3 score maps are included in Figure B (available as a supplement to the online version of this article at http://www.ajph.org). Counties in white had no reported cases during 2017 to 2018.
Principal Component Results
Three principal components explained 75.3% of the variation in county-level socioeconomic factors (Table 2) and were the location of the elbow in the scree plot (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). High PC1 scores were correlated with high poverty (ρ = 0.921), low educational attainment (ρ = 0.791), high disability (ρ = 0.729), single-female and single-male heads of households (single-female: ρ = 0.721; single-male: ρ = 0.487), low married households (ρ = −0.774), high income inequality (ρ = 0.658), and high grandparent responsibility for grandchildren (ρ = 0.537; Table 2). Counties with high PC1 scores reflect socioeconomic factors related to extreme disadvantage. Counties in the highest quintile of PC1 scores were generally in eastern Kentucky (Figure 1b).
High PC2 scores were correlated with counties with a high proportion of married households (ρ = 0.517), married adults (married women: ρ = 0.706; married men: ρ = 0.601), high disability (ρ = 0.510), low educational attainment (high-school graduate: ρ = −0.526; education < ninth grade: ρ = 0.480), low residential instability (ρ = −0.724; i.e., high residential stability); moderately associated with poverty (ρ = 0.263); and there was a lack of an association with income inequality (ρ = 0.066; Table 2). High PC2 scores reflect a pattern of marital support and residential stability with low income inequality despite challenges of low education, high disability, and moderate poverty. Counties in the highest quintile of PC2 were distributed across the state (Figure B1, available as a supplement to the online version of this article at http://www.ajph.org).
High PC3 scores were correlated with high residential instability (ρ = 0.476), high grandparent responsibility for grandchildren (ρ = 0.465), moderate income inequality (ρ = 0.249), families living in married households (ρ = 0.265), yet lower percentages of families living in single-male head of households (ρ = −0.513) and married men (ρ = −0.177). PC3 scores were largely not correlated with poverty (ρ = 0.103), education (ρ = −0.039), disability (ρ = 0.025), married women (ρ = 0.022), or single-female heads of households (ρ = −0.076). High PC3 scores reflect a profile of residential instability, nonmarried men despite low single-male heads of households, and high income inequality. Counties in the highest quintile of PC3 were distributed across the state (Figure B2).
Outcome Poisson Regression Results
Counties scoring high on the extremely disadvantaged PC1 profile were significantly associated with higher hepatitis A incidence (RR = 1.44; 95% confidence interval [CI] = 1.19, 1.74); however, estimates attenuated after adjustment for median age of the county population, percentage of the population that was White, and percentage working in the manufacturing industry (RR = 1.21; 95% CI = 0.99, 1.48; Table 3). Counties with higher PC2 scores following the profile of high marital support and residential stability despite low education, high disability, and moderate poverty had lower HAV infection rates after adjustment for county age, race, and manufacturing (RR = 0.77; 95% CI = 0.59, 1.00). A standard deviation increase in PC3, which was characterized by counties with high residential instability, nonmarried men, and high income inequality, was associated with higher incidence after adjustment for county age, race, and manufacturing (RR = 1.15; 95% CI = 0.94, 1.41). Results were similar when restricting to counties with at least 10 reported hepatitis A cases (Table C, available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 3—
Mutually Adjusted Relative Risk (RR) Estimates (95% Confidence Intervals [CIs]) of Hepatitis A During the Kentucky 2017–2018 Outbreak for 3 Principal Components (PCs) and County-Level Adjustment Factors
| Model 1, RR (95% CI) | Model 2,a RR (95% CI) | Model 3,b RR (95% CI) | Model 4,c RR (95% CI) | |
| PC1 | 1.44 (1.19, 1.74) | 1.31 (1.09, 1.59) | 1.21 (0.99, 1.48) | 1.27 (1.04, 1.57) |
| PC2 | 0.95 (0.75, 1.21) | 0.80 (0.61, 1.04) | 0.77 (0.59, 1.00) | 0.90 (0.66, 1.23) |
| PC3 | 1.02 (0.93, 1.25) | 1.16 (0.95, 1.43) | 1.15 (0.94, 1.41) | 1.20 (0.96, 1.48) |
| % in county who were White | . . . | 1.14 (1.08, 1.20) | 1.13 (1.07, 1.19) | 1.11 (1.05, 1.18) |
| County median age | . . . | 0.99 (0.91, 1.08) | 0.98 (0.91, 1.07) | 0.98 (0.91, 1.07) |
| % of the county in the manufacturing industry | . . . | . . . | 0.97 (0.94, 1.00) | 0.96 (0.93, 1.00) |
| % of the county in the arts, entertainment, recreation, accommodation, and food services industries | . . . | . . . | . . . | 1.00 (0.90, 1.12) |
| % of the county in the agricultural, forestry, fishing and hunting, and mining industries | . . . | . . . | . . . | 0.93 (0.85, 1.00) |
| % of the county in the construction industry | . . . | . . . | . . . | 0.93 (0.84, 1.04) |
Note. All models present an estimate for a 1-standard-deviation increase in a PC score and are adjusted for population size and a square term for population size.
Model 2 included the PCs, population size, percentage of the population that was White, and county median age.
Model 3 included the PCs, population size, percentage of the population that was White, county median age, and percentage of the population in the manufacturing industry.
Model 4 included model 3 covariates and additionally included 3 other industries of interest.
Counties with a higher proportion of Whites had higher HAV infection rates (RR = 1.13; 95% CI = 1.07, 1.19; Table 3). Median age of the county population was not associated with hepatitis A rates. Counties with a higher percentage of the population in manufacturing had slightly lower rates (RR = 0.97; 95% CI = 0.94, 1.00; Table 3). The multivariable associations with HAV infection rates were null for the arts, entertainment, recreation, accommodation, and food services industries (RR = 1.00 95% CI = 0.90, 1.12) and construction (RR = 0.93; 95% CI = 0.84, 1.04), but showed an inverse association for the agriculture, forestry, fishing and hunting, and mining industries (RR = 0.93; 95% CI = 0.85, 1.00). With additional adjustment for these industries, the RR estimates for PC1, PC2, and PC3 scores were similar (Table 3, model 4; PC1: RR = 1.27 [95% CI = 1.04, 1.57]; PC2: RR = 0.90 [95% CI = 0.66, 1.23]; PC3: RR = 1.20 [95% CI = 0.96, 1.48]).
DISCUSSION
In summary, the 3 most prominent socioeconomic profiles derived from the PCA were generally geographically dispersed throughout Kentucky and were associated with differential hepatitis A case rates (PC1: RR = 1.21 [95% CI = 0.99, 1.48]; PC2: RR = 0.77 [95% CI = 0.59, 1.00]; PC3: RR = 1.15 [95% CI = 0.94, 1.41]). As expected, extremely disadvantaged counties with high poverty, high income inequality, high disability, low education, high single-family homes, and high grandparent responsibility had higher hepatitis A rates (PC1: RR = 1.21; 95% CI = 0.99, 1.48) and were predominately in the eastern Kentucky region. But even counties not correlated with poverty had similarly higher hepatitis A rates (PC3: RR = 1.15; 95% CI = 0.94, 1.41). Interestingly, counties that were characterized by poverty but had certain social and familial structures and residential stability had lower hepatitis A rates (PC2: RR = 0.77; 95% CI = 0.59, 1.00). From this study, more comes to light on potential county-level risk factors beyond the well-established risk factor of poverty (e.g., residential instability and single males who do not appear to be living in single-male heads of households), and potential county-level protective predictors are elucidated (e.g., residential stability, more married adults, lack of income inequality, and lack of single-female heads of households). These additional risk and protective factors could explain the differences in the hepatitis A epidemic severity observed across the state during the 2017–2018 outbreak.
By comparing the 3 socioeconomic profiles, we observed that more than a single marker of poverty was at play in the hepatitis A epidemic in Kentucky. Specifically, when we compared the profiles of counties with high PC3 and PC1 scores that both had similar associations with higher HAV infection rates, we observed unique factors to PC3 that may be contributing to the higher observed rates of hepatitis A, such as greater residential instability (correlation between PC3 score and residential instability: ρ = 0.476 vs correlation between PC1 score and residential instability: ρ = −0.122; Table 2) and having single males who do not appear to be living in single-male heads of households (PC3: ρ = −0.513 vs PC1: ρ = 0.487; Table 2). We also observed in counties with high PC2 scores that they experienced high poverty, high disability, and low education (PC2: ρ = 0.263; ρ = 0.510; ρ = 0.480, respectively; Table 2); yet, despite these setbacks, PC2 counties had lower rates of hepatitis A. Upon further evaluation of factors unique to PC2 counties, a few unique protective factors may explain their advantage, such as the lack of residential instability (PC2: ρ = −0.724 vs PC3: ρ = 0.476 and PC1: ρ = −0.122; Table 2), lack of income inequality (PC2: ρ = 0.066 vs PC3: ρ = 0.249 and PC1: ρ = 0.658; Table 2), having more married men and women (PC2: ρ = 0.601 for men and ρ = 0.706 for women vs PC3: ρ = −0.177 for men and ρ = 0.022 for women and PC1: ρ = −0.552 for men and ρ = −0.52 for women; Table 2), and lack of families with a single-female head of household (PC2: ρ = −0.471 vs PC3: ρ = −0.076 and PC1: ρ = 0.721; Table 2). This suggests that, despite high poverty, protective county-level factors include residential stability, lack of income inequality, being married, and lack of single-female heads of households.
Previous literature corroborates that markers of income inequality and residential instability are associated with hepatitis C and HIV,10,14,16,19–21 but less literature is available on hepatitis A.8 Higher income inequality was associated with higher HIV prevalence among persons who inject drugs20 and higher likelihood of an HIV outbreak.21 Residential instability and homelessness have been implicated in opioid use,7,10,32 risky behaviors, spread of infection, and barriers to medical care10 that are tied with the hepatitis A epidemic. While these individual markers have been widely used in the literature, there is limited research on the complex interplay among these socioeconomic markers in relation to infectious disease outbreaks, particularly in the context of the opioid epidemic or in relation to hepatitis A. One study observed that relocation to a more economically advantaged area with its inherent interplay of socioeconomic qualities was associated with disrupting an individual’s network to substance-using individuals,16 though the role of relocation on infectious disease incidence was not assessed.
The role of counties’ industrial composition on hepatitis A incidence was minimal for manufacturing (RR = 0.97; 95% CI = 0.94, 1.00). The inverse association between the percentage of the county in manufacturing and hepatitis A case rates suggests that economic development may play a role in the epidemic, though it may be relatively minor compared with the risk associated with the socioeconomic profiles.
Limitations
This was an ecologic study of county-level data subject to ecologic fallacies because the socioeconomic profiles of the individual hepatitis A cases are unknown. A limited amount of data is collected from individual hepatitis A cases that does not capture their social and economic context or their living conditions or their neighborhoods. This study suggests that improving data surveillance to gather additional information on contextual neighborhood social, economic, and housing factors may be an important avenue for understanding outbreaks associated with drug use for which little is known about the exact person-to-person transmission route. This ecologic design, while limited, aligns with ecosocial theory that macro-level contexts can shape individual behavior and population health.33 Public health practitioners can use these ecologic results and the approach to comprehensively describe the multiple socioeconomic profiles within their catchment area and identify the socioeconomic profiles most affected by the epidemic.
From this study, public health practitioners may gain insight into expanding vaccinations to high-risk communities based on neighborhood socioeconomic profiles that are not currently considered in the current Advisory Committee on Immunization Practices recommendations.34 Specifically, public health practitioners in Kentucky may consider expanding HAV vaccination programs to higher-risk communities that follow the PC1 and PC3 patterns and those with greater income inequality, more residential instability, more single-family homes, and fewer married adults.
An additional limitation of our study was that the ACS data are from a sampled population, their categorization of data may not fully capture socioeconomic attributes, and the ACS data can have higher margins of error for rural areas than urban areas. However, we included 5-year ACS estimates to improve population coverage and long-term representation of the population, and to have lower margins for error. The error in the county-level socioeconomic data in a predominately rural state is likely to be nondifferential in terms of the outcome status of HAV infection rates; hence, the exposure measurement error would bias the results toward the null making it harder to detect an association.
As with any PCA, the profiles in Kentucky may not be generalizable to other states; however, the socioeconomic county profiles may be useful to local health departments in Kentucky and to the public to appreciate the heterogeneous and complex socioeconomic profiles that are differentially affected by infectious diseases associated with the opioid epidemic. This study of contextual factors related to hepatitis A takes a comprehensive approach to examine multiple social, economic, and housing factors’ associations with hepatitis A and to gain a greater appreciation of the complexities of socioeconomic patterns on health in Kentucky, instead of focusing on single predictors that ignore complex correlations among socioeconomic factors. Furthermore, the current study addressed an understudied infectious disease associated with the opioid epidemic for which the behaviors and environments leading to hepatitis A outbreaks and transmission were less clear.
Public Health Implications
In conclusion, socioeconomic county profiles were modestly associated with hepatitis A incidence rates in Kentucky. This approach went beyond observing poverty as a risk factor and shed light on additional county-level risk factors (e.g., residential instability and single males who do not appear to be living in single-male heads of households) and protective factors (e.g., residential stability, lack of income inequality, more married adults, and lack of single-female heads of households). This may be useful to public health practitioners looking to expand immunization programs to higher-risk communities not currently included in the recommendations, such as communities with more income inequality, residential instability, and single-family homes, and fewer married adults. Even with the expansion of HAV vaccination programs, this study supports the notion that there are several community-level socioeconomic profiles associated with severity of hepatitis A outbreaks that should be explored with other opioid epidemic–related outcomes. These findings may also support public health practitioners in augmenting hepatitis A data surveillance by capturing personal- and community-level social, economic, and housing characteristics that are not routinely collected, if resources allow.
ACKNOWLEDGMENTS
The authors received no financial support for the research, authorship, or publication of this article.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose nor funding that would compromise the integrity of the analysis or interpretation.
HUMAN PARTICIPANT PROTECTION
This study was approved by the Kentucky Cabinet for Health and Family Services’ institutional review board.
Footnotes
REFERENCES
- 1.Foster MA, Hofmeister MG, Kupronis BA et al. Increase in hepatitis A virus infections—United States, 2013–2018. MMWR Morb Mortal Wkly Rep. 2019;68(18):413–415. doi: 10.15585/mmwr.mm6818a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1995. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00044418.htm. Accessed June 11, 2019.
- 3.Adams DA, Thomas KR, Jajosky RA et al. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;63(54):1–152. doi: 10.15585/mmwr.mm6354a1. [DOI] [PubMed] [Google Scholar]
- 4.Zibbell JE, Asher AK, Patel RC et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. doi: 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwetz TA, Calder T, Rosenthal E, Kattakuzhy S, Fauci AS. Opioids and infectious diseases: a converging public health crisis. J Infect Dis. 2019;220(3):346–349. doi: 10.1093/infdis/jiz133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdiserri R, Khalsa J, Dan C et al. Confronting the emerging epidemic of HCV infection among young injection drug users. Am J Public Health. 2014;104(5):816–821. doi: 10.2105/AJPH.2013.301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushel M. Hepatitis A outbreak in California—addressing the root cause. N Engl J Med. 2018;378(3):211–213. doi: 10.1056/NEJMp1714134. [DOI] [PubMed] [Google Scholar]
- 8.Villano SA, Nelson KE, Vlahov D, Purcell RH, Saah AJ, Thomas DL. Hepatitis A among homosexual men and injection drug users: more evidence for vaccination. Clin Infect Dis. 1997;25(3):726–728. doi: 10.1086/513757. [DOI] [PubMed] [Google Scholar]
- 9.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182–186. doi: 10.2105/AJPH.2017.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galea S, Vlahov D. Social determinants and the health of drug users: socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135–S145. [PMC free article] [PubMed] [Google Scholar]
- 11.Galea S, Ahern J, Vlahov D. Contextual determinants of drug use risk behavior: a theoretic framework. J Urban Health. 2003;80(4 suppl 3):iii50–iii58. doi: 10.1093/jurban/jtg082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoras CA, Karanika S, Velmahos E et al. Correlation of opioid mortality with prescriptions and social determinants: a cross-sectional study of Medicare enrollees. Drugs. 2018;78(1):111–121. doi: 10.1007/s40265-017-0846-6. [DOI] [PubMed] [Google Scholar]
- 13.Fink DS, Hu R, Cerdá M et al. Patterns of major depression and nonmedical use of prescription opioids in the United States. Drug Alcohol Depend. 2015;153:258–264. doi: 10.1016/j.drugalcdep.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Handel MM, Rose CE, Hallisey EJ et al. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr. 2016;73(3):323–331. doi: 10.1097/QAI.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph AE, Crawford ND, Latkin C, Fowler JH, Fuller CM. Individual and neighborhood correlates of membership in drug using networks with a higher prevalence of HIV in New York City (2006–2009) Ann Epidemiol. 2013;23(5):267–274. doi: 10.1016/j.annepidem.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linton SL, Cooper HLF, Luo R et al. People and places: relocating to neighborhoods with better economic and social conditions is associated with less risky drug/alcohol network characteristics among African American adults in Atlanta, GA. Drug Alcohol Depend. 2016;160:30–41. doi: 10.1016/j.drugalcdep.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandi A, Glass TA, Cole SR et al. Neighborhood poverty and injection cessation in a sample of injection drug users. Am J Epidemiol. 2010;171(4):391–398. doi: 10.1093/aje/kwp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper HLF, West B, Linton S et al. Contextual predictors of injection drug use among Black adolescents and adults in US metropolitan areas, 1993–2007. Am J Public Health. 2015;106(3):517–526. doi: 10.2105/AJPH.2015.302911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zierler S, Krieger N, Tang Y et al. Economic deprivation and AIDS incidence in Massachusetts. Am J Public Health. 2000;90(7):1064–1073. doi: 10.2105/ajph.90.7.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman SR, Tempalski B, Brady JE et al. Income inequality, drug-related arrests, and the health of people who inject drugs: reflections on seventeen years of research. Int J Drug Policy. 2016;32:11–16. doi: 10.1016/j.drugpo.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolopoulos GK, Fotiou A, Kanavou E et al. National income inequality and declining GDP growth rates are associated with increases in HIV diagnoses among people who inject drugs in Europe: a panel data analysis. PLoS One. 2015;10(4):e0122367. doi: 10.1371/journal.pone.0122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pear VA, Ponicki WR, Gaidus A et al. Urban–rural variation in the socioeconomic determinants of opioid overdose. Drug Alcohol Depend. 2019;195:66–73. doi: 10.1016/j.drugalcdep.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian WJ, Hopenhayn C, Christian A, McIntosh D, Koch A. Viral hepatitis and injection drug use in Appalachian Kentucky: a survey of rural health department clients. Public Health Rep. 2010;125(1):121–128. doi: 10.1177/003335491012500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloud DH, Ibragimov U, Prood N, Young AM, Cooper HLF. Rural risk environments for hepatitis C among young adults in Appalachian Kentucky. Int J Drug Policy. 2019;2:47–54. doi: 10.1016/j.drugpo.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudolph AE, Young AM, Havens JR. Examining the social context of injection drug use: social proximity to persons who inject drugs versus geographic proximity to persons who inject drugs. Am J Epidemiol. 2017;186(8):970–978. doi: 10.1093/aje/kwx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messer LC, Laraia BA, Kaufman JS et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Widespread outbreaks of hepatitis A across the United States. 2019. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed June 3, 2019.
- 28.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Census Bureau. American Community Survey—history. Available at: https://www.census.gov/history/www/programs/demographic/american_community_survey.html. Accessed June 20, 2019.
- 30.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning With Applications in R. New York, NY: Springer; 2013. [Google Scholar]
- 31.Hu FB, Rimm E, Smith-Warner SA et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 32.Wooten DA. Forgotten but not gone: learning from the hepatitis A outbreak and public health response in San Diego. Top Antivir Med. 2019;26(4):117–121. [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 34.Doshani M, Weng M, Moore KL, Romero JR, Nelson NP. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68(6):153–156. doi: 10.15585/mmwr.mm6806a6. [DOI] [PMC free article] [PubMed] [Google Scholar]


