Abstract
Background
Little attention has been paid to whether snoring frequency is associated with body composition in menopausal women, particularly in China. This study objected to investigate the association between self-reported snoring and body composition in (peri-post) menopausal Chinese women as well as metabolic indicators.
Methods
This cross-sectional study enrolled 715 participants aged 40–67 years from the Menopause Clinic in the Shanghai Sixth People’s Hospital. Participants were categorized into four subgroups stratified by self-reported snoring frequency: never, rarely (< 1 night per week), occasionally (1–2 nights per week), regularly (≥3 nights per week), while body composition was measured using bioelectrical impedance analysis (BIA). Besides, blood sample were collected to test the glycolipid indicators.
Results
In our sample of investigation, regular snoring (≥3 nights per week) was found to be an independent risk factor for higher fat mass (total, upper limbs, trunk), with the highest risk of 2.4 times for fat mass of trunk after adjusting for metabolic confounders(p = 0.003). Meanwhile, regular snoring was independently associated with higher fat mass (total and each segment) only in menopausal transition (p = 0.023).
Conclusions
We suggested that self-reported regular snoring may be taken as a simple alternative to predict higher fat mass (≥17.11 kg, upper quartile) in menopausal women. Similarly, body composition should be attached to the great importance to those who in menopausal transition in order to help to prevent obstructive sleep apnea (OSA).
Keywords: Body composition, Snoring, Menopausal transition
Background
Snoring, the manifestation of increased upper airway resistance, is commonly regarded as a reliable proxy marker of obstructive sleep apnea (OSA) [1, 2]. Moreover, regular snoring has been suggested to be correlated with obesity [3], hypertension [4] and diabetes mellitus [5]. OSA is supposed to be more prevalent in men than women, however the gap was narrowed when women approach menopause [6, 7]. Women in menopause transition are more likely to report perspective poor sleep, snoring [8], which largely affected quality life of menopausal women. In addition, previous studies have reported that menopause was an important risk factor for snoring mainly due to the declining ovarian hormones [9, 10]. Thus, it is important to combat snoring in (peri-post) menopausal women.
Meanwhile, menopause is a vital window for variations in the body composition and rising in the body weight caused by hormonal alterations [11]. However, body mass index, BMI, is not a valid measure of true obesity status in menopausal women [12]. Changes in menopause-related body composition may be covered and underestimated by stable BMI since the counteractive effect of loss of lean mass and gain of fat mass when aging. Therefore, body composition by bioelectrical impedance analysis (BIA) may be a more representative and precise instrument rather than BMI among menopausal Chinese women [13].
So far, current studies on the association of snoring and obesity have focused primarily on men and children [3, 14], while underrepresented women. In addition, any association between snoring and body composition in menopausal women has received little attention. Since it’s possible that glycolipid metabolism may confound the association, and whether snoring is associated with body composition in menopausal women independently of glycolipid metabolism confounders remains unknown. Given the evidence of the cross interplay among snoring, obesity and menopause, we aim to explore the association with snoring and body composition in menopausal women.
Methods
Study participants
This cross-sectional study enrolled participants who visited the Menopause Clinic in the Shanghai Sixth People’s Hospital. Han-Chinese woman aged 40–67 years passing through the menopause were recruited. Exclusion criteria were (1) with rhinitis; (2) having severe internal illnesses and/or diseases such as myocardial infarction, stroke, and cancer; (3) current smoking (at least once per week for the previous 6 months); (4) excessive alcohol drinking (at least one pack per month for the previous 6 months); (5) suffering from thyroid disease; (6) having tubercle and cachexy; (7) missing data. Ultimately,715 participants were recruited in this study.
General questionnaire
Baseline sociodemographic information was collected from a questionnaire through face-to-face interview, which has been previously employed [8] (seen in supplementary file 1); Variables included age, marital status, employment status, education level, income per month, menopausal age, menopausal status, history of chronic disease (i.e., hypertension, diabetes mellitus, rhinitis, other diseases), besides, lifestyle (i.e., smoke, alcohol consumption) were recorded. Guiding by the Stages of Reproductive Aging Workshop (STRAW + 10) [15],participants were divided into three different menopausal subgroups, namely menopausal transition group (consecutive irregularities for over 7 days of menstrual cycle), early postmenopausal group (absence of menstrual periods for 12 months − 5 years) and late postmenopausal group (absence of menstrual periods for ≥5 years). Hypertension was defined by any prior diagnosis from the questionnaire or by the criteria recommended by the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7, [16]). While diabetes mellitus was identified by FPG ≥ 7 mmol/L or received any treatment for diabetes according to the WHO criteria [17].
Snoring frequency assessment
Participants were asked by the question to assess the sleep snoring frequency, which was applied previously [18, 19]. “Over the past 4 weeks, did you snore? And if did, how many times per week?” and the options for responses were “never”, “rarely”, “occasionally”, and “regularly”, corresponding to “never”, “<1 night per week”, “1–2 nights per week”, and “≥ 3 nights per week”, respectively (seen in supplementary file 1).
Anthropometric and lab tests
We measured and recorded participants’ weight, height. Body mass index (BMI) was computed by dividing weight in kilograms by the square of their height in meters. We took the blood pressure for all participants on the right arm three consecutive times after 5-min sitting (systolic blood pressure (SBP), diastolic blood pressure (DBP)). Blood samples were collected for the detection of serum concentration of triglyceride (TG), cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and fasting blood glucose (FBG) after an overnight fast.
Body composition
We measured the body composition by BIA (TBF-418B analyzer; TANITA) of lean mass (LM), fat mass (FM), and fat-free mass (FFM), and each segment included upper /lower limbs, and trunk. We also recorded basal metabolic rate (BMR) concurrently [20]. The well-trained staff guided the participants to take off heavy clothes, socks and shoes, and hold the hand electrodes, standing barefoot in contact with footpad electrodes [21]. Fat mass (total and each segment) and lean mass (total and each segment) were stated in the dichotomized form, with a cutoff of the highest quartile as the higher one (comparing the highest to the lower two tertiles). We defined ≥17.11 kg, ≥1.41 kg and ≥ 9.11 kg as higher total fat mass, higher fat mass of upper limbs and higher fat mass of trunk respectively.
Statistical analyses
All statistical analyses were taken by SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Data were tested for normal distribution by the Kruskal WallisH-test. Levene’s test of homogeneity of variance was also performed. Variables were presented as mean ± standard deviation (SD) when they showed normal distributions, whereas medians (inter quartile range) or values (%). One-way ANOVA (normal distributions), the Kruskal Wallis H-test (skewed continuous variables) and χ2 test (categorical variables) were carried out to compare the differences among the four groups. Snoring was analyzed as a categorical variable with never as the reference group. Relationship between body composition and snoring frequency was computed by multiple logistic regression analysis. Covariates included TG, TC, HDL, LDL, FBG, SBP, DBP, age, marital status, employment status, education level, income per month, menopausal age, menopausal status, hypertension, diabetes mellitus. Two-sided p < 0.05 was considered significant.
Results
Characteristics of the study participants based on snoring frequency
A total of 715 participants were finally entered into the study. The basic characteristics among the four groups divided by the snoring frequency (never, rarely, occasionally, regularly) were presented in Table 1. Participants were on average 51.50 ± 4.71 years of age with a mean weight of 57.45 ± 7.97 kg, and the average BMI was 22.23 ± 2.76 kg/m2. The mean lean mass, fat mass and fat free mass were 37.64 ± 3.10 kg, 17.52 ± 5.47 kg, and 39.95 ± 3.39 kg, respectively. Compared with non-snorers, rare and occasional snorers, regular snorers tended to be older, showed higher triglyceride, lower HDL-C, and had less income (p < 0.05). Moreover, there was an ascending trend in the incidence of hypertension in different snoring frequency subgroups,with 19.28% in non-snorers increasing to 40.51% in regular snorers (p < 0.05). However, we did not observe the difference among three menopausal status respect to the snoring frequency.
Table 1.
Body composition and characteristics of the women distributed by snoring frequency
| Variables | Snoring Frequency | P value | ||||
|---|---|---|---|---|---|---|
| Never | Rarely | Occasionally | Regularly | Total | ||
| n = 508 | n = 76 | n = 56 | n = 76 | n = 715 | ||
| Age (years) | 51.37 ± 4.85 | 51.3 ± 4.48 | 52.79 ± 4.02 | 51.58 ± 4.41 | 51.50 ± 4.71 | 0.047 |
| Weight (Kg) | 56.68 ± 7.69 | 57.48 ± 8.12 | 58.86 ± 7.52 | 61.36 ± 8.73 | 57.45 ± 7.97 | 0.307 |
| Height (cm) | 160.60 ± 4.88 | 161.24 ± 4.46 | 160.46 ± 4.74 | 160.85 ± 4.64 | 160.68 ± 4.8 | 0.580 |
| BMI (Kg/m2) | 21.95 ± 2.61 | 22.06 ± 2.60 | 22.88 ± 2.92 | 23.71 ± 3.16 | 22.23 ± 2.76 | 0.034 |
| BMR | 1154.8 ± 109.79 | 1166.97 ± 121.7 | 1175.58 ± 99.35 | 1210.67 ± 120.69 | 1163.93 ± 112.73 | 0.555 |
| TG (mmol/l) | 1.07 (0.79–1.49) | 1.05 (0.82–1.57) | 1.18 (0.80–1.69) | 1.37 (0.94–2.05) | 1.11 (0.80–1.60) | 0.003 |
| TC (mmol/l) | 5.21 ± 0.95 | 5.23 ± 1.05 | 5.38 ± 0.93 | 5.21 ± 1.07 | 5.23 ± 0.97 | 0.440 |
| HDL-C (mmol/l) | 1.61 ± 0.39 | 1.56 ± 0.37 | 1.52 ± 0.34 | 1.14 ± 0.14 | 1.55 ± 0.39 | <0.001 |
| LDL-C (mmol/l) | 3.05 ± 0.76 | 3.01 ± 0.89 | 3.13 ± 0.71 | 3.18 ± 0.78 | 3.07 ± 0.77 | 0.192 |
| FPG (mmol/L) | 5.16 (4.82–5.52) | 5.05 (4.80–5.33) | 5.24 (4.75–5.55) | 5.37 (4.90–5.86) | 5.16 (4.81–5.53) | 0.129 |
| SBP (mmHg) | 119.77 ± 15.26 | 122.21 ± 13.98 | 121.91 ± 17.85 | 126.19 ± 19.02 | 120.91 ± 15.91 | 0.088 |
| DBP (mmHg) | 73.29 ± 9.84 | 75.72 ± 8.85 | 75.09 ± 10.93 | 78.19 ± 9.8 | 74.23 ± 9.94 | 0.392 |
| Chronic disease, n (%) | ||||||
| Hypertension | 97 (19.28%) | 15 (19.74%) | 18 (31.58%) | 32 (40.51%) | 162 (22.66%) | <0.001 |
| Diabetes | 16 (3.18%) | 2 (2.63%) | 2 (3.51%) | 7 (8.86%) | 27 (3.78%) | 0.094 |
| Marital status, n (%) | 0.904 | |||||
| Married | 490 (97.42%) | 75 (98.68%) | 56 (98.25%) | 77 (97.47%) | 698 (97.62%) | |
| Single/Separated/Divorced/Widowed | 13 (2.58%) | 1 (1.32%) | 1 (1.75%) | 2 (1.53%) | 17 (2.38%) | |
| Menopausal status, n (%) | 0.393 | |||||
| Perimenopause | 217 (43.14%) | 32 (42.11%) | 20 (35.09%) | 31 (39.24%) | 300 (41.96%) | |
| Early postmenopause | 189 (37.57%) | 34 (44.74%) | 24 (42.11%) | 27 (34.18%) | 274 (38.32%) | |
| Late postmenopause | 97 (19.28%) | 10 (13.16%) | 13 (22.81%) | 21 (26.58%) | 141 (19.72%) | |
| Employment, n (%) | 306 (60.83%) | 42 (55.26%%) | 29 (50.88%) | 45 (56.96%) | 422 (59.02%) | 0.448 |
| Education, n (%) | 0.053 | |||||
| Junior or below | 87 (17.30%) | 15 (19.74%) | 12 (21.05%) | 19 (24.05%) | 133 (18.60%) | |
| Senior high | 160 (31.81%) | 28 (36.84%) | 18 (31.58%) | 36 (45.57%) | 242 (33.85%) | |
| College or above | 256 (50.89%) | 33 (43.42%) | 27 (47.37%) | 24 (30.38%) | 340 (47.55%) | |
| Income (RMB/month), n (%) | 0.011 | |||||
| < 1000 | 35 (6.96%) | 7 (9.21%) | 5 (8.77%) | 12 (15.19%) | 59 (8.25%) | |
| 1000–3000 | 113 (22.47%) | 23 (30.26%) | 18 (31.58%) | 31 (39.24%) | 185 (25.87%) | |
| 3000–5000 | 156 (31.01%) | 18 (23.68%) | 16 (28.07%) | 22 (27.85%) | 212 (29.65%) | |
| 5000–10,000 | 131 (26.04%) | 17 (22.37%) | 11 (19.30%) | 9 (11.39%) | 168 (23.50%) | |
| > 10,000 | 68 (13.52%) | 11 (14.47%) | 7 (12.28%) | 5 (6.33%) | 91 (12.73%) | |
| Lean mass (kg) | 37.45 ± 3.07 | 37.53 ± 3.13 | 37.95 ± 2.92 | 38.71 ± 3.25 | 37.64 ± 3.10 | 0.007 |
| Upper | 3.46 ± 0.42 | 3.45 ± 0.44 | 3.56 ± 0.38 | 3.71 ± 0.46 | 3.50 ± 0.43 | <0.001 |
| Trunk | 21.08 ± 1.95 | 21.23 ± 1.94 | 21.22 ± 1.59 | 21.68 ± 2.42 | 21.17 ± 1.99 | 0.087 |
| Lower | 12.91 ± 1.36 | 12.86 ± 1.43 | 13.17 ± 1.57 | 13.32 ± 1.65 | 12.97 ± 1.42 | 0.063 |
| Fat mass (kg) | 16.94 ± 5.23 | 17.66 ± 5.18 | 18.58 ± 5.47 | 20.24 ± 6.34 | 17.52 ± 5.47 | 0.027 |
| Upper | 1.38 ± 0.53 | 1.44 ± 0.55 | 1.56 ± 0.61 | 1.76 ± 0.69 | 1.45 ± 0.57 | 0.002 |
| Trunk | 9.04 ± 3.33 | 9.56 ± 3.20 | 10.03 ± 3.48 | 10.94 ± 3.97 | 9.39 ± 3.46 | 0.044 |
| Lower | 6.55 ± 1.50 | 6.68 ± 1.53 | 7.02 ± 1.54 | 7.55 ± 1.90 | 6.71 ± 1.59 | 0.027 |
| Fat-free (kg) | 39.74 ± 3.34 | 39.83 ± 3.44 | 40.29 ± 3.14 | 41.13 ± 3.56 | 39.95 ± 3.39 | 0.695 |
| Upper limbs | 3.70 ± 0.45 | 3.70 ± 0.49 | 3.80 ± 0.42 | 3.97 ± 0.48 | 3.74 ± 0.46 | 0.789 |
| Trunk | 22.36 ± 2.14 | 22.53 ± 2.13 | 22.52 ± 1.70 | 23.05 ± 2.65 | 22.47 ± 2.17 | 0.476 |
| Lower limbs | 13.70 ± 1.47 | 13.63 ± 1.54 | 13.99 ± 1.68 | 14.15 ± 1.80 | 13.76 ± 1.54 | 0.645 |
Body composition of the study participants distributed by snoring frequency
As presented in Table 1, compared with non-snorers, rare and occasional snorers, regular snorers had higher fat mass (upper limbs, trunk, lower limbs). In addition, we found that there was an increasing trend in the fat mass of upper limbs, trunk and lower limbs and also in lean mass of upper limbs with the increase of the sleep snoring frequency (p < 0.05).
Odds ratio of snoring frequency for body composition by multiple logistic regression analysis
We next investigated the odds ratio of snoring frequency in predicting for body composition (p < 0.05 in univariate analysis) after adjusting for potential confounders. As depicted in Fig. 1, compared with non, rare and occasional snoring, regular snoring was the risk predictor for higher total fat mass (≥17.11 kg) (OR = 1.970, 95%CI (1.099,3.530), P = 0.023), higher fat mass of upper limbs (≥1.41 kg) (OR = 1.845, 95%CI (1.004,3.391), P = 0.049), higher fat mass of trunk (≥9.11 kg) (OR = 2.400, 95%CI (1.336,4.313), P = 0.003), while other segments showed no significance after adjustments. In addition, regular snoring increased the highest odds ratio (OR) of 2.4 for fat mass of trunk among the other statistically significant body compositions.
Fig. 1.
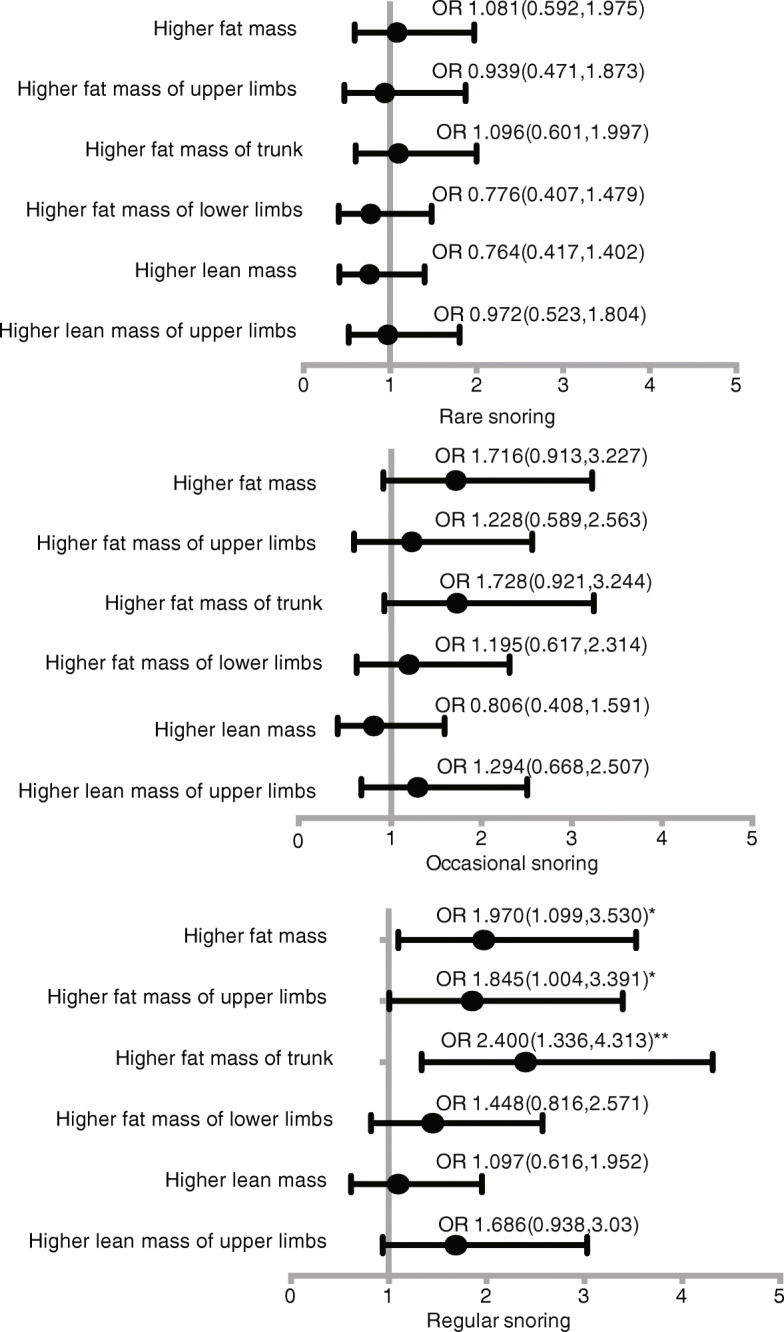
Odds ratios (95%CI) of snoring frequency for body composition in women analyzed by multivariate logistic regression. Covariates: age, BMI, TG, TC, HDL, LDL, FBG, SBP, DBP, hypertension, diabetes mellitus, menopause, income, education, employment status. *means p<0.005
Independent determinants for regular snoring stratified by menopausal status
We also investigated the independent roles of body composition for predicting regular snoring in multivariate logistic regression analysis in Table 2, however, we did not observe any significance of body composition in predicting for regular snoring after adjusting confounders. Interestingly, when the participants were stratified by different menopausal status (menopausal transition, early postmenopause, late postmenopause), we observed that fat mass segments were independently associated with regular snoring in menopausal transition but not postmenopause. Total fat mass (OR = 1.134, 95%CI (1.018,1.263), P = 0.023), fat mass of upper limbs (OR = 3.162, 95%CI (1. 183,8.452), P = 0.022), fat mass of trunk (OR = 1.206, 95% CI (1.016,1.431), P = 0.033), fat mass of lower limbs (OR = 1.548, 95%CI (1.069,2.242), P = 0.021) were independent indicators for regular snoring after adjusting for confounders in menopausal transition.
Table 2.
Odds ratio of body composition for regular snoring stratified by menopausal status by logistic regression
| Regular snoring | |||
|---|---|---|---|
| Perimenopause n = 300 | Early postmenopause n = 274 | Late postmenopause n = 141 | |
| Odds ratio (95% CI), P-value | Odds ratio (95% CI), P-value | Odds ratio (95% CI), P-value | |
| Fat mass (kg) | 1.134 (1.018,1.263),0.023 | 0.970 (0.875,1.074),0.556 | 1.012 (0.876,1.170),0.867 |
| Fat mass of upper limbs (kg) | 3.162 (1.183,8.452),0.022 | 1.007 (0.389,2.606),0.989 | 0.838 (0.196,3.587),0.812 |
| Fat mass of trunk (kg) | 1.206 (1.016,1.431),0.033 | 0.941 (0.801,1.105),0.456 | 1.048 (0.834,1.316),0.689 |
| Fat mass of lower limbs (kg) | 1.548 (1.069,2.242),0.021 | 0.928 (0.641,1.345),0.695 | 0.955 (0.608,1.502),0.843 |
Discussion
To our knowledge, this is the first study to document associations of snoring and body composition as well as metabolic indicators in women with regard to menopausal status. The main finding was that regular snoring (≥3 nights per week) was an independent risk factor for higher fat mass (total, trunk, upper limbs) in menopausal women after adjusting for well-established metabolic variables. Of special concern was that regular snoring had a 2.4 times significantly higher odds of higher fat mass of trunk, which was the highest among other significant body composition. This finding was in concordant with the previous study that OSA was more inclined to a central-obesity phenotype than a whole-obesity pattern [22].
Several mechanisms can interpret this association. Upper airway resistance and collapsibility caused by regular snoring could result in intermittent hypoxia and sympathetic activation, thus leading to the aggravation of obesity, especially for abdominal fat [23]. In addition, protective role of progesterone and estrogen in respiratory control vanished after menopause, which was associated with continuum from increased airway resistance (manifested as snoring) [24–26]. Taken together, menopause make women lose protective effects against snoring and further augment snore-obesity association, especially snore-central-obesity association.
Interests in obesity and OSA as regards to “which is the chicken or the egg?” has existed since the dawn of history [23, 27]. Thus, to identify the mutual effect of snoring and obesity, we also assessed the role of body composition in predicting the snoring. We found that fat mass was an independent risk factor for regular snoring only in menopausal transition not postmenopause in a multi-variable model. Taken together, we suggested that the rise in obesity may serve as a key contributor to the burgeoning prevalence of snoring in women, while menopausal transition not postmenopause period may mark this relationship.
The reason can be explained by the fact that menopausal transition is more concerned with fluctuation of sex hormone than postmenopause, which predisposed to modulate sleep regulation and breathing, thus leading to the snoring.
Besides, other independent factors for regular snoring such as higher TG, lower LDL were compatible with one study [28]. Although self-reported snoring was closely related with hypertension and diabetes. Unexpectedly, we did not find that hypertension was related with regular snoring after multiple adjustments. These divergent findings may be attributed to difference in sample size, ethnicity, culture, and the definition of hypertension and diabetes, etc. Another possible explanation is that many previous studies did not consider menopause status in to account, which may aggravate the snore-obesity association, thus overshadow the snore-hypertension/diabetes association.
However, our study should be interpreted in light of the following limitations. First, one limitation of the present study is that the cross-sectional design does not permit conclusion of causality, further prospective studies are needed to verify the association between snoring frequency and body composition. Second, self-reported snoring frequency but not polysomnography (the gold standard for diagnosing OSA), which could bring about the statistical error. However, precious study has suggest that self-report is a reliable measure [29].
Conclusions
Regular snoring (≥ 3 times per week) may be an independent strong predictor for fat mass of trunk in menopausal women, while fat mass in turn serves as a strong predictor for regular snoring only in menopausal transition. Taken together, early detection and interventions of participant showing regular snoring and higher fat mass in menopause could have important preventive implications.
Supplementary information
Acknowledgements
The authors would like to acknowledge all women who consented to take part of this study. We are also thankful for the support and cooperation from staff members of obstetrics and gynecology in Shanghai Jiao Tong University Affiliated Sixth People’s Hospital.
Abbreviations
- BIA
Bioelectrical impedance analysis
- OSA
Obstructive sleep apnea
- TG
Triglyceride
- TC
Cholesterol
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- FBG
Fasting blood glucose
- BMI
Body mass index
- FM
Fat mass
- LM
Lean mass
- FM
Fat mass
- FFM
Fat-free mass
- BMR
Basal metabolic rate
Authors’ contributions
MT conceived the study, and YT designed the study. [YZ]1 drafted and critically revised the manuscript. FL designed the questionnaire and analyzed the data. CL, [YZ]2, JH, [YZ]3, LG and SJ administered the questionnaire survey and managed the data. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Science and Technology Commission of Shanghai Municipality (154119050202). The role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript should be declared.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All participants had provided verbal informed consent after full explanation, because the study posed no threat to the health of patients. This study was submitted to and approved by the ethics committees of Institutional Review Board in Shanghai Sixth People’s Hospital affiliated for Shanghai Jiaotong University (No:2016-R07).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Zhou and Fei Liu contributed equally to this work.
Contributor Information
Yincheng Teng, Email: ycteng@sjtu.edu.cn.
Minfang Tao, Email: taomf@sjtu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12905-020-01025-2.
References
- 1.Lee YH, Kweon SS, Choi JS, et al. A Gender-Specific Association between Self-Reported Snoring and Hemoglobin A1c Levels in a General Population without Type 2 Diabetes Mellitus. Yonsei Med J. 2017;58(6):1152–1159. doi: 10.3349/ymj.2017.58.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song J, Wang C, Ma A, et al. Self-reported snoring is associated with chronic kidney disease independent of metabolic syndrome in middle-aged and elderly Chinese. J Diabetes Investig. 2019;10(1):124–130. doi: 10.1111/jdi.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggs SN, Tamanyan K, Walter LM, et al. Overweight and obesity add to behavioral problems in children with sleep-disordered breathing. Sleep Med. 2017;39:62–69. doi: 10.1016/j.sleep.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Shivashankar R, Kondal D, Ali MK, et al. Associations of Sleep Duration and Disturbances With Hypertension in Metropolitan Cities of Delhi, Chennai, and Karachi in South Asia: Cross-Sectional Analysis of the CARRS Study. Sleep. 2017;40(9). 10.1093/sleep/zsx119 PubMed PMID: 28934524; PubMed Central PMCID: PMCPMC5806550. eng. [DOI] [PMC free article] [PubMed]
- 5.Wu HB, Wang H, Hu RY, et al. The association between sleep duration, snoring and prevalent type 2 diabetes mellitus with regard to gender and menopausal status: the CKB study in Zhejiang rural area, China. Acta Diabetol. 2017;54(1):81–90. doi: 10.1007/s00592-016-0918-1. [DOI] [PubMed] [Google Scholar]
- 6.Phillips BA, Collop NA, Drake C, et al. Sleep disorders and medical conditions in women. Proceedings of the Women & Sleep Workshop, National Sleep Foundation, Washington, DC, March 5–6, 2007. J Womens Health (Larchmt) 2008;17(7):1191–1199. doi: 10.1089/jwh.2007.0561. [DOI] [PubMed] [Google Scholar]
- 7.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health (Larchmt) 2009;18(8):1211–1219. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Yang R, Li C, et al. Sleep disorder, an independent risk associated with arterial stiffness in menopause. Sci Rep. 2017;7(1):1904. doi: 10.1038/s41598-017-01489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young T, Finn L, Austin D, et al. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 10.Li QY, Huang SG, Li M, et al. BMI is an independent risk factor for snoring in Chinese women aged over 30 years. Sleep Breath. 2009;13(3):289–293. doi: 10.1007/s11325-008-0236-0. [DOI] [PubMed] [Google Scholar]
- 11.Stenholm S, Harris TB, Rantanen T, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banack HR, Wactawski-Wende J, Hovey KM, et al. Is BMI a valid measure of obesity in postmenopausal women? Menopause. 2018;25(3):307–313. doi: 10.1097/gme.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson R, Brinkworth GD, Buckley JD, et al. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007;26(6):771–777. doi: 10.1016/j.clnu.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet (London, England) 2005;365(9464):1046–1053. doi: 10.1016/s0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 15.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97(4):843–851. doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenfant C, Chobanian AV, Jones DW, et al. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178–1179. doi: 10.1161/01.hyp.0000075790.33892.ae. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Yang Y, Song X, et al. An evaluation of the International Diabetes Federation definition of metabolic syndrome in Chinese patients older than 30 years and diagnosed with type 2 diabetes mellitus. Metabolism. 2006;55(8):1088–1096. doi: 10.1016/j.metabol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Endeshaw Y, Rice TB, Schwartz AV, et al. Snoring, daytime sleepiness, and incident cardiovascular disease in the health, aging, and body composition study. Sleep. 2013;36(11):1737–1745. doi: 10.5665/sleep.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HB, Yan WH, Dou JT, et al. Association between Self-reported Snoring and Prediabetes among Adults Aged 40 Years and Older without Diabetes. Chin Med J. 2017;130(7):791–797. doi: 10.4103/0366-6999.202741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Zheng Y, Li C, et al. Association of body composition with menopausal symptoms in (peri-)menopausal women. Climacteric. 2018;21(2):179–183. doi: 10.1080/13697137.2018.1428295. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka NI, Hanawa S, Murakami H, et al. Accuracy of segmental bioelectrical impedance analysis for predicting body composition in pre- and postmenopausal women. J Clin Densitometry. 2015;18(2):252–259. doi: 10.1016/j.jocd.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Gasa M, Lopez-Padros C, Monasterio C, et al. Anthropometrical phenotypes are important when explaining obstructive sleep apnea in female bariatric cohorts. J Sleep Res. 2019:e12830. 10.1111/jsr.12830 PubMed PMID: 30740836; eng. [DOI] [PubMed]
- 23.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31(Suppl 2):S303–S309. doi: 10.2337/dc08-s272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behan M, Kinkead R. Neuronal control of breathing: sex and stress hormones. Comprehensive Physiology. 2011;1(4):2101–2139. doi: 10.1002/cphy.c100027. [DOI] [PubMed] [Google Scholar]
- 25.Behan M, Zabka AG, Thomas CF, et al. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136(2–3):249–263. doi: 10.1016/S1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- 26.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 27.Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. 2018;84:76–84. doi: 10.1016/j.metabol.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhang N, Chen Y, Chen S, et al. Self-Reported Snoring Is Associated with Dyslipidemia, High Total Cholesterol, and High Low-Density Lipoprotein Cholesterol in Obesity: A Cross-Sectional Study from a Rural Area of China. Int J Environ Rese Public Health. 2017;14(1). 10.3390/ijerph14010086 PubMed PMID: 28106727; PubMed Central PMCID: PMCPMC5295337. eng. [DOI] [PMC free article] [PubMed]
- 29.Telakivi T, Partinen M, Koskenvuo M, et al. Periodic breathing and hypoxia in snorers and controls: validation of snoring history and association with blood pressure and obesity. Acta neurologica Scandinavica. 1987;76(1):69–75. doi: 10.1111/j.1600-0404.1987.tb03547.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


