Abstract
Objective
To estimate the prognostic value of positive surgical margins (PSM) location and perineural invasion (PNI) for biochemical recurrence (BCR) in patients undergoing radical prostatectomy (RP).
Methods
All men with prostate cancer (PCa) who received RP in the second hospital of Tianjin Medical University from 2014 to 2018 were retrospectively identified. All patients met the following criteria: no neoadjuvant or adjuvant treatment, absence of lymph node invasion, or distant metastasis confirmed by surgery or imaging. Comparisons were made between cases with only apex positive (AM), isolated nonapical positive (OM), multiple positive (MM), and negative surgical margins (NSM). Patients were also subdivided according to the Gleason score and pathological tumor stage for analysis.
Results
A total of 416 patients available for analysis, of which 132 (31.7%) were PSM, 43 were AM, 37 were OM, and 52 were MM at a median follow-up of 27 months. The PNI was in 30.5% of patients. BCR occurred in 22.6% of patients during follow-up. Both AM and MM were noticed to be independent predictors of BCR with a hazard ratio of 4.192 (95% CI 2.185–8.042; p < 0.001) and 2.758 (95% CI 1.559–4.880; p < 0.001), respectively, when compared to NSM. Though the correlation was significant in univariate analysis, PNI was not an independent risk factor for BCR (p = 0.369). Subgroup analyses suggested that MM was not particularly predictive for BCR in the Gleason score < 8. The hole Cox regression model for the C-index was 0.843
Conclusions
PSM location was a significant independent predictor of BCR in PCa, especially in patients with AM or MM, while PNI is a non-independent risk factor. Compared with other locations, AM has a higher BCR risk.
Keywords: Positive surgical margins, Location, Perineural invasion, Biochemical recurrence, Prostate cancer
Background
Positive surgical margin (PSM) after radical prostatectomy (RP) for prostate cancer (PCa) has been consistently considered an effective predictor of postoperative biochemical recurrence (BCR) [1–4]. Owing to patient selection, the experience of surgeons, surgical technique, and pathological specimen analyses, the incidence of PSM varies obviously between different researches and ranges from 6.5 to 38.4% [5–8]. The risk of PSM was reported to be related to high serum prostate-specific antigen (PSA), low prostate volume, high Gleason score (GS), and interfascial nerve [9]. The number, length, and GS of PSM have been followed in previous discussions. However, the impact of PSM location on BCR was reported several and remains controversial [10–12].
Perineural invasion (PNI) was defined as the trajectory of tumor cells along or around nerve fibers, which has been a recognized mechanism of tumor spread [9]. The existence of PNI is related to the adverse outcome for several malignancies, while the clinical significance of PNI was still controversial. Previous studies have shown that PNI is a predictor of adverse pathological and clinical features, as well as a strong predictor of BCR in PCa [13]. However, as a predictor of BCR, the independent value of PNI has not yet been established.
The aim of our study is to discuss the correlation of PSM location and PNI on the prediction of BCR for PCa (pathological stage T2–T3 patients), as well as those impacts on different subgroups.
Patients and methods
Patient selection
From 2014 to 2018, 416 patients of PCa who underwent RP in the second hospital of Tianjin Medical University were included. All patients met the following criteria: no neoadjuvant or adjuvant treatment, absence of lymph node invasion, or distant metastasis confirmed by surgery or imaging. The surgical techniques for RP differed among patients: open RP or laparoscopic RP. Both interventions were conducted by two experienced surgical teams. BCR was defined as a serum PSA level ≥ 0.2 ng/mL twice 3 months after RP. Early salvage therapy was conducted in patients with BCR. This study was approved by the local ethics committee.
Pathology analysis
The Stanford protocol and Gleason system were used to processing all RP specimens. All pathologic specimens were step-sectioned for complete evaluation tumor grade, volume, and margins. The definition of PSM was the presence of definite tumor cells on the edge of the RP specimen [8]. In this study, we divided PSM locations into the apex, peripheral (included anterior, posterior, lateral), and base firstly. Negative surgical margins (NSM), a solitary positive apical margin (AM), a solitary nonapical positive margin (OM), a solitary positive margin (AOM), and multiple positive surgical margins (MM) were subdivided by margin status. Whenever two or more of these locations were positive, the PSM was deemed as MM.
Statistical analysis
Frequencies and proportions were used to describe statistics of categorical variables. Interquartile ranges (IQR) and medians were presented for continuous variables. The Kruskal-Wallis H test and Chi-square test or Fish exact test were performed in continuous variables and categorical variables for statistical analysis. Kaplan-Meier survival analysis was conducted to evaluate the probability of BCR-free survival. A comparison of survival distributions was performed with the log-rank test or Breslow test. A backward stepwise multivariable cox regression analysis (entry-level at p ≤ 0.1) was modeled to evaluate BCR. Spearman’s rank was used for the correlations analysis, using Harrell consistency Index (c-index) to evaluate the discriminant ability of PSM and PNI state models. All analyses were performed with IBM SPSSv.23.0 and Stata15. All p values were bilateral, with p values < 0.05 considered statistically significant.
Result
Table 1 summarizes patients’ characteristics of the 416 cases. Among the patients included in the study, 132 (31.7%) patients exhibit PSM. Of these, 43 (10.3%) were reported to have AM, 37 (8.9%) were OM, and 52 (12.5%) were MM. The median age and BMI were 68 and 24.9 kg/m2, respectively. The median prostate volume of patients was 24.6 ml and the median preoperative PSA was 15.8 ng/ml. In total, 152 (37%) patients were conducted by open RP, and the remainder used laparoscopic RP. The rate of BCR was 36.2% in PNI patients versus 16.6% in without PNI patients. When compared to a PSM with NSM cases, PSM cases revealed a higher preoperative PSA, GS, pathological tumor stage (pT), and PNI. The median follow-up time was 27 months (IQR 20–47). During follow-up, BCR occurred in 22.6% cases.
Table 1.
Clinical and histological characteristics of patients according to the location of positive surgical margin (PSM)
| Characteristics | Total | Surgical margin | p value | |||
|---|---|---|---|---|---|---|
| NSM | AM | OM | MM | |||
| Patients, no. (%) | 416 (100) | 284 (100) | 43 (100) | 37 (100) | 52 (100) | - |
| Age, years, median (IQR) | 68 (62–72) | 67 (62–72) | 68 (63–71) | 68 (64–71) | 68 (61–75) | 0.922 |
| BMI, kg/m2, median (IQR) | 24.9 (23.5–27.0) | 25.0 (23.7–27.1) | 24.7 (23.8–27.1) | 25.1 (22.9–27.0) | 24.0 (22.9–27.0) | 0.078 |
| Volume, ml, median (IQR) | 24.6 (18.7–34.6) | 24.6 (18.7–36.1) | 19.5 (16.6–28.1) | 25.7 (18.1–36.3) | 26.4 (16.4–35.9) | 0.167 |
| PSA, ng/ml, median (IQR) | 15.8 (8.8–29.8) | 13.1 (8.3–25.7) | 15.8 (7.3–25.6) | 26.0 (13.8–57.0) | 22.6 (14.0–55.9) | < 0.001 |
| Surgical approach, no. (%) | ||||||
| ORP | 152 (37) | 125 (44) | 8 (19) | 12 (32) | 7 (13) | < 0.001 |
| LRP | 264 (63) | 159 (56) | 35 (81) | 25 (68) | 45 (87) | |
| Gleason score, no. (%) | ||||||
| < 8 | 246 (59) | 193 (68) | 25 (58) | 14 (38) | 14 (27) | < 0.001 |
| ≥ 8 | 170 (41) | 91 (32) | 18 (42) | 23 (62) | 38 (73) | |
| pTNM, no. (%) | ||||||
| T2 | 270 (65) | 187 (66) | 34 (79) | 15 (41) | 34 (65) | 0.004 |
| T3 | 146 (35) | 97 (34) | 9 (21) | 22 (59) | 18 (35) | |
| Perineural invasion, no. (%) | ||||||
| No | 289 (69) | 213 (75) | 30 (70) | 22 (59) | 24 (46) | < 0.001 |
| Yes | 127 (31) | 71 (25) | 13 (30) | 15 (41) | 28 (54) | |
| BCR | ||||||
| No | 322 (77) | 238 (84) | 30 (70) | 24 (65) | 30 (58) | < 0.001 |
| Yes | 94 (23) | 46 (16) | 13 (30) | 13 (35) | 22 (42) | |
| Follow-up time, median (IQR) | 27 (20–47) | 32 (23–44) | 23 (19–29) | 25 (17–31) | 19 (16–26) | < 0.001 |
NSM negative surgical margins, AM a solitary positive apical margin, OM a solitary nonapical positive margin, MM multiple positive surgical margins, PSA prostate-specific antigen, BCR biochemical recurrence
Regarding all PSM locations, the most common was the apex, exhibited in 82/191 cases. For the peripheral and base, 53/191 and 56/191 cases exhibited those locations respectively (data not shown). In cases with PSM, 36.3% develop BCR compared with just 16.2% in NSM. AM, OM, and MM cases had higher rates of BCR (30.2%, 35.1%, and 42.3%, respectively) than those with NSM. Among them, MM experienced BCR earlier than the AM and OM.
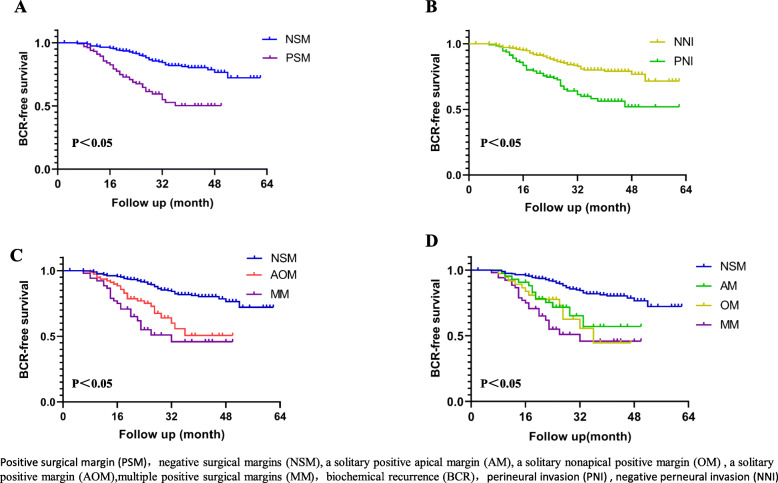
BCR-free survival at 3 years was 81.9% for patients with NSM versus 50.3% for those with PSM (p < 0.001), 51.5%, 57.1%, 44.6%, and 45.9% for AOM, AM, OM, and MM, respectively (p < 0.001). As for the patients with PNI, BCR-free survival at 3 years was 58.2% compared to 80% without PNI (p < 0.05, Fig. 1). We discovered that patients with PSM and PNI were more likely to develop BCR.
Fig. 1.
Kaplan-Meier curves showing biochemical recurrence (BR)-free survival following. a PSM and NSM. b PNI and NNI. c NSM, AOM, and MM. d NSM, AM, OM, and MM
The results of univariable and multivariable cox regression analyses for predicting PSM are shown in Table 2. In the univariable analysis, PSA level, GS, pT, surgical margin, and the PNI were all significant risk factors for a BCR (p < 0.001). AM, OM, and MM were statistically significant in univariable analysis, while in a multivariable analysis, only AM and MM remained independently significant predictors for BCR. The BCR risk between AM and NSM increased by 4.192 times (95% CI 2.185–8.042; p < 0.001), and that between MM and NSM increased by 2.758 times (95% CI 1.559–4.880; p < 0.001). There was also a connection between pT and BCR: pT3 were associated with a higher risk of recurrence compared to pT2 (HR = 2.526, 95% CI 1.614–3.955; p < 0.001), as well as GS < 8 with a lower risk of recurrence compared to GS ≥ 8 (HR = 4.629, 95% CI 2.718–7.883; p < 0.001). The preoperative PSA level was also significantly associated with BCR (HR = 1.012, 95% CI 1.009–1.016; p < 0.001). It was worth to be noted that BMI or PNI was not an independent predictor for BCR, though PNI was statistically significant in univariable analysis.
Table 2.
Univariate and multivariate model and hazard ratio calculations for variables associated with biochemical recurrence
| Variables | Univariable cox regression | Multivariable cox regression (backward stepwise) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age (years) | 0.993 (0965–1.023) | 0.646 | ||
| BMI (kg/m2) | 0.939 (0.875–1.008) | 0.081 | 0.975 (0.906–1.050) | 0.508 |
| Volume(ml) | 1.005 (0.995–1.015) | 0.349 | ||
| TPSA (ng/ml) | 1.016 (1.013–1.018) | < 0.001 | 1.012 (1.009–1.016) | < 0.001 |
| Surgical approach | ||||
| ORP | 1.000 (Ref.) | 0.979 | ||
| LRP | 1.006 (0.665–1.521) | |||
| Gleason score | ||||
| < 8 | 1.000 (Ref.) | < 0.001 | 1.000 (Ref.) | < 0.001 |
| ≥ 8 | 7.796 (4.702–12.925) | 4.629 (2.718–7.883) | ||
| pT stage | ||||
| T2 | 1.000 (Ref.) | < 0.001 | 1.000 (Ref.) | < 0.001 |
| T3 | 3.239 (2.136–4.911) | 2.526 (1.614–3.955) | ||
| Surgical margin | < 0.001 | < 0.001 | ||
| NSM | 1.000 (Ref.) | 1.000 (Ref.) | ||
| AM | 2.687 (1.443–5.005) | 0.002 | 4.192 (2.185–8.042) | < 0.001 |
| OM | 3.147 (1.689–5.862) | <0.001 | 1.501 (0.794–2.838) | 0.211 |
| MM | 4.530 (2.702–7.595) | <0.001 | 2.758 (1.559–4.880) | < 0.001 |
| Perineural invasion | ||||
| No | 1.000 (Ref.) | < 0.001 | 0.369 | |
| Yes | 2.503 (1.669–3.753) | 1.219 (0.791–1.877) | ||
In spearman’s rank-order correlation analysis for PNI, there was a significant correlation of PNI with GS and pT (r1 = 0.277, r2 = 0.267). Subgroup analysis for BCR, PSM, and AM remained independent predictors in both GS and pT stage group. Neither OM nor PNI was predictive for BCR after patients adjusted for the variable in a multivariable cox regression analysis. OM was even not a predictor for BCR in the pT2 group (Tables 3 and 4).
Table 3.
Cox regression analysis evaluating the value of surgical margins and perineural invasion as a predictor of time to biochemical recurrence in a subgroup analysis regarding Gleason Score
| Variable | Gleason Score < 8 (n = 246) | Gleason Score ≥ 8 (n = 170) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable1 | Univariable | Multivariable1 | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Surgical margins | 0.041 | 0.033 | 0.002 | 0.001 | ||||
| NSM | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | ||||
| AM | 4.4 (1.5–12.7) | 0.006 | 5.1 (1.7–14.7) | 0.003 | 2.2 (1.0–4.9) | 0.049 | 3.8 (1.6–8.7) | 0.002 |
| OM | - | 0.984 | 2.2 (1.2–4.4) | 0.012 | 1.6 (0.8–3.1) | 0.164 | ||
| MM | 3.0 (0.7–13.7) | 0.15 | 2.0 (0.4–9.2) | 0.394 | 2.9 (1.6–5.1) | <0.001 | 3.0 (1.6–5.6) | 0.001 |
| Perineural invasion | ||||||||
| No | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | ||||
| Yes | 1.4 (0.5–3.8) | 0.561 | 1.8 (1.1–2.8) | 0.016 | 1.2 (0.7–1.9) | 0.493 | ||
1Adjusted for BMI, TPSA, and pathological tumor stage
Table 4.
Cox regression analysis evaluating the value of surgical margins and perineural invasion as a predictor of time to biochemical recurrence in a subgroup analysis regarding pathological tumor stage
| Variable | pT2 (n = 270) | pT3 (n = 146) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable1 | Univariable | Multivariable1 | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Surgical margins | < 0.001 | 0.002 | < 0.001 | 0.011 | ||||
| NSM | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | ||||
| AM | 4.8 (2.1–11.2) | < 0.001 | 4.9 (2.1–11.8) | < 0.001 | 2.3 (0.8–6.5) | 0.122 | 3.7 (1.2–11.1) | 0.019 |
| OM | 2.1 (0.5–9.1) | 0.343 | 2.6 (0.6–11.5) | 0.216 | 2.6 (1.3–5.3) | 0.007 | 1.3 (0.6–2.6) | 0.51 |
| MM | 6.5 (3.0–14.5) | < 0.001 | 3.1 (1.3–7.5) | 0.014 | 4.5 (2.2–9.2) | < 0.001 | 2.8 (1.4–5.9) | 0.005 |
| Perineural invasion | ||||||||
| No | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | |||||
| Yes | 2.4 (1.2–4.6) | 0.01 | 0.9 (0.4–2.0) | 0.808 | 1.7 (1.0–2.9) | 0.044 | 1.5 (0.9–2.6) | 0.157 |
1Adjusted for BMI, TPSA, and Gleason Score
The prediction accuracy of the COX regression model for NSM, AM, OM, and MM stratification was 0.843. When surgical margin location status or PNI was removed from the hole model, the C-index was 0.827 or 0.841, respectively.
Discussion
In the present large, single-center study of RP cases, we have shown that patients with AM and MM showing as an independent predictor of BCR when compared to patients with NSM on multivariate analysis. Patients with AM were more important for the prediction of BCR relative to patients with MM and OM. Interestingly, patients with PNI and OM exhibited significantly worse BCR prognosis in univariable analysis; however, this significance was lost on multivariate analysis. The same conclusion was conducted by subgroup analysis. BR-free survival at 3 years was 81.9% for patients with NSM versus 50.3% for those with PSM. The BR-free survival rates seemed to be lower than in previous studies. Preisser et al. who identified 8770 patients suggested that the 72-month BCR-free survival rates of PSM and NSM group after RP were 77.7% and 89.0%, respectively [14]. A potential explanation may be the patient’s selection. In our study, patients with high stage and GS occupied a considerable number. All patients were not accepted adjuvant treatment before BCR. As for the patients with PNI, the BR-free survival rates were 80% versus 58.2%. Similar conclusions could be found in previous studies [15].
Though PSM revealed a significant hazard ratio by adjusted cox regression analysis, the location result from RP is not equivalent to BCR. Our study suggested that BCR was independently associated with AM and MM but not with OM, in addition to BMI, PSA level, GS, pT stage, and PNI. The AM appeared to have greater effects across all margin locations and a significant contributor to BCR across all statistical models, particularly when compared to OM: 4.192 versus 1.501. However, the significance of PSM location on BCR was still controversial in the current literature, especially in AM. In a large series of 4001 patients, Dev et al. suggested that the apex is an important factor affecting BCR, and it seems to be the strongest among all the risk factors [16]. Choo et al. and Porpiglia et al. also found that positive apical is a significant risk factor of BCR compared with nonapical margins [17, 18]. Nevertheless, Gautier et al. showed that independently from the pT stage, GS, and lymph nodes invasion, focal apical PSM had no significant effect on BCR, while extensive apical PSM significantly increased the risk of BCR [19].
Further research on PSM patients revealed that apex was the most common PSM site for RP. This may be owing to its location that closes to the dorsal venous plexus and neurovascular bundles plexus with a little capsule. Furthermore, the surgeons also attempt to conserve maximum urethral length, in order to protect the sexual and urine function better [10]. The possible reason for AM being more likely to BCR may also be caused by its location with abundant blood supplement. In addition to the special location, some patients with AM suggested that the tumor might invade part of the urethra with the epithelium of transitional cells, which is metastasis and recurrence. Our study also discovered that AM patients’ prostate volume was less than others. Recent research found men with smaller prostates were at greater risk of progression after RP. For a given age, the small prostate may be associated with lower androgen levels and the total concentration of growth factors in the prostate. Owing to PSA-driven biopsies, men with larger prostates can detect their tumors earlier [20]. Those may explain why we should focus on patients with AM.
We also demonstrated the greater impact of PSM on BCR after subgroup analysis, especially in AM. The correlation between OM and PNI was still found in univariable analysis. Interestingly, after adjusting for BMI, PSA level, and GS, we could not find any significant association between MM and BCR in GS < 8 group. In addition to the lack of statistical efficiency, one possible explanation may be that MM was only a significant predictor for BCR in high-risk PCa.
Preoperative multi-parametric MRI may play an important role in the staging and surgical planning of PCa, and it may also have an impact on PSM. For the patients with TNM stage < T3, the RP can be a curative treatment. However, for patients with extracapsular extension, seminal vesicle invasion, and distant metastasis, the preoperative MRI evaluation of tumor stage mainly determine the surgical planning and affect the prognosis of patients [21]. A study involved 353 patients showed that the initial surgical plan was changed in 26% of patients after the surgery reviewed MRI images. Of these patients, the majority was intermediate or high-risk groups and 90% of patients chose a more conservative operation [22]. Jaderling et al. investigated 557 patients demonstrated that MRI examination would increase the rate of non-nerve sparing operation and reduce PSM, while Rud et al.’s research observed a possible benefit of MRI in patients only with T1 [23, 24].
Different surgical planning and technique may also have an impact on the surgical margin during RP. According to the mode of operation, RP can be divided into bilateral nerve sparing, unilateral, or non-nerve sparing. Previous studies have found that compared with unilateral or non-nerve sparing, bilateral nerve sparing patients had better sexual and urinary function especially in terms of men with good baseline sexual function [25]. Preston et al. evaluated 6120 patients who underwent open, laparoscopic, or robotic RP with the stage from T2 to T3 and demonstrated that there was a significant difference between robotic and open RP, while the bilateral nerve sparing was only associated with increased risk of PSM in the stage of T2. They assumed that nerve sparing should be performed in patients with a good sexual function and patients who could accept the risk of PSM after the operation [26]. A similar study conducted by Atsushi et al. also showed that the PSM rates were 27.6% (open), 18.4% (laparoscopic), and 13.4% (robotic), respectively, and surgical approach (open vs robot) were independent risk factors for PSM [6]. In our study, all patients were evaluated for sexual function before the operation. Nerve sparing therapy would be conducted in patients with earlier stage and better physical condition. For patients with and without nerve preservation, the surgical dissection carried above and below Denonvillier’s fascia, respectively. Open and laparoscopic RP patients also found a significant difference in PSM (p < 0.001).
Our results showed that 30.5% of patients had PNI, while the incidence in other research ranged from 21.1 to 50.2% [13, 27–30]. The variation between these studies may be associated with different patient groups or pathological criteria. Though the patients with PNI had reported that short BCR-free survival time and advanced pathological outcomes were observed in some studies, the relationship between PNI and BCR after RP is still controversial [13, 15, 29]. Kang et al. found that PNI is an important predictor in PCa patients treated with RP; however, its prognostic value has not been detected in localized PCa [28]. Jung et al. reported that PNI was independently related to the aggressive pathological features of PCa, while it did not predict BCR [31]. Loeb et al. also claimed that PNI was not an independent risk factor for BCR after RP, while it was still an independent factor of advanced pathology features in multivariate analysis [32]. The results above were in line with our findings. Although our study discovered a significant correlation between PNI and BCR in univariable analysis, after adjusting for BMI, PSA level, GS, and pT stage, the relationship was no longer statistically significant. A similar conclusion was observed in the subgroup analysis. Patients with PNI in GS < 8 even could not find a correlation with BCR in univariable analysis.
In our study, the higher risk of BCR also may occur if a PSM is present with a higher PSA level, pT3, and GS ≥ 8. Preisser et al. and Max Kates et al. illustrated that a higher GS at the PSM is independently associated with early BCR. In these cases, adjuvant therapy should be considered after RP [14, 33].
The study also has some limitations. First, there is no consistent record of PSM length in the available pathological reports and further study could not be analyzed. Secondly, we have a relatively short follow-up period. Further follow-up of these patients should be continued for disease progression or overall survival. Finally, large-scale multicenter studies were still needed to illustrate the exact prognostic value of PSM location and PNI in PCa patients.
Conclusions
We recommend careful evaluation of patients with PSM location following RP, especially if they are AM or MM with higher PSA level, pT3, and GS ≥ 8. In these cases, adjuvant therapy should be considered after radical surgery.
Acknowledgements
The authors thank professor Aixiang Wang, professor Yuanchun Qu, and professor Ya Wang for acquisition of data. We also thank the whole project team who worked on this study.
Abbreviations
- PSM
Positive surgical margin
- RP
Radical prostatectomy
- PCa
Prostate cancer
- BCR
Biochemical recurrence
- GS
Gleason score
- PSA
Prostate-specific antigen
- PNI
Perineural invasion
- AM
Solitary apical positive margin
- OM
Solitary nonapical positive margin
- MM
Multiple positive surgical margins
- AOM
Solitary positive margin
- IQR
Medians and interquartile
- pT
Pathological tumor stage
Authors’ contributions
Study concept and design: Ranlu Liu and Xiaoming Wang. Acquisition of data: Zhenpeng Lian, Zhaowei He, and Shenfei Ma. Analysis and interpretation of data: Zhenpeng Lian and Hongtuan Zhang. Drafting of the manuscript: Zhenpeng Lian, Hongtuan Zhang, and Ranlu Liu. Critical revision of the manuscript for important intellectual content: Zhenpeng Lian and Hongtuan Zhang. Statistical analysis: Zhenpeng Lian. Obtaining funding: Ranlu Liu, Xiaoming Wang, and Hongtuan Zhang. The authors read and approved the final manuscript.
Funding
This work was supported by the Fund program: the Key Project of Tianjin Municipal Science and Technology Commission (No. 19ZXDBSY00050) and National Natural Science Foundation of China (NSFC 81972412)
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The retrospective study was approved by the institutional ethical committee of the second hospital of Tianjin Medical University.
Consent for publication
Not applicable
Competing interests
The authors report no conflicts of interest in this work
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenpeng Lian and Hongtuan Zhang contributed equally to this work.
Contributor Information
Xiaoming Wang, Email: doctorwxm2013@163.com.
Ranlu Liu, Email: liuranlu1976@126.com.
References
- 1.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, Cangiano T, Schroder FH, Scardino PT, Kattan MW. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–1250. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 2.Mauermann J, Fradet V, Lacombe L, Dujardin T, Tiguert R, Tetu B, Fradet Y. The impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013;64:19–25. doi: 10.1016/j.eururo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Lin SX, Wirth GJ, Lu M, Lu J, Subtelny AO, Wang Z, Dahl DM, Olumi AF, Wu CL. Impact of multifocality and multilocation of positive surgical margin after radical orostatectomy on oredicting oncological outcome. Clin Genitourin Cancer. 2019;17:e44–e52. doi: 10.1016/j.clgc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, Yuan J. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018;16:124. doi: 10.1186/s12957-018-1433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravi CA, Tin A, Vertosick E, Mazzone E, Martini A, Dell'Oglio P, Stabile A, Gandaglia G, Fossati N, Suardi N, et al. The impact of experience on the risk of surgical margins and biochemical recurrence after robot-assisted radical prostatectomy: a learning curve study. J Urol. 2019;202:108–113. doi: 10.1097/JU.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koizumi A, Narita S, Nara T, Takayama K, Kanda S, Numakura K, Tsuruta H, Maeno A, Huang M, Saito M, et al. Incidence and location of positive surgical margin among open, laparoscopic and robot-assisted radical prostatectomy in prostate cancer patients: a single institutional analysis. Jpn J Clin Oncol. 2018;48:765–770. doi: 10.1093/jjco/hyy092. [DOI] [PubMed] [Google Scholar]
- 7.Novara G, Ficarra V, Mocellin S, Ahlering TE, Carroll PR, Graefen M, Guazzoni G, Menon M, Patel VR, Shariat SF, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Yossepowitch O, Bjartell A, Eastham JA, Graefen M, Guillonneau BD, Karakiewicz PI, Montironi R, Montorsi F. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol. 2009;55:87–99. doi: 10.1016/j.eururo.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Harnden P, Shelley MD, Clements H, Coles B, Tyndale-Biscoe RS, Naylor B, Mason MD. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer. 2007;109:13–24. doi: 10.1002/cncr.22388. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot PA, Mansour AM. Reporting positive surgical margins after radical prostatectomy: time for standardization. BJU Int. 2013;111:E290–E299. doi: 10.1111/j.1464-410X.2012.11640.x. [DOI] [PubMed] [Google Scholar]
- 11.Keller EX, Bachofner J, Britschgi AJ, Saba K, Mortezavi A, Kaufmann B, Fankhauser CD, Wild P, Sulser T, Hermanns T, et al. Prognostic value of unifocal and multifocal positive surgical margins in a large series of robot-assisted radical prostatectomy for prostate cancer. World J Urol. 2019;37:1837–1844. doi: 10.1007/s00345-018-2578-y. [DOI] [PubMed] [Google Scholar]
- 12.Sooriakumaran P, Dev HS, Skarecky D, Ahlering T. The importance of surgical margins in prostate cancer. J Surg Oncol. 2016;113:310–315. doi: 10.1002/jso.24109. [DOI] [PubMed] [Google Scholar]
- 13.Vukovic M, Kavaric P, Magdelinic A, Nikomanis P, Tomovic S, Pelicic D. Perineural invasion on biopsy specimen as predictor of tumor progression in aging male treated with radical prostatectomy. Could we use it for pre-surgical screening? Aging Male. 2019:1–6. [DOI] [PubMed]
- 14.Preisser F, Coxilha G, Heinze A, Oh S, Chun FK, Sauter G, Pompe RS, Huland H, Graefen M, Tilki D. Impact of positive surgical margin length and Gleason grade at the margin on biochemical recurrence in patients with organ-confined prostate cancer. Prostate. 2019;79:1832–1836. doi: 10.1002/pros.23908. [DOI] [PubMed] [Google Scholar]
- 15.Jeon HG, Bae J, Yi JS, Hwang IS, Lee SE, Lee E. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int J Urol. 2009;16:682–686. doi: 10.1111/j.1442-2042.2009.02331.x. [DOI] [PubMed] [Google Scholar]
- 16.Dev HS, Wiklund P, Patel V, Parashar D, Palmer K, Nyberg T, Skarecky D, Neal DE, Ahlering T, Sooriakumaran P. Surgical margin length and location affect recurrence rates after robotic prostatectomy. Urol Oncol. 2015;33:109. doi: 10.1016/j.urolonc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Choo MS, Cho SY, Ko K, Jeong CW, Lee SB, Ku JH, Hong SK, Byun SS, Kwak C, Kim HH, et al. Impact of positive surgical margins and their locations after radical prostatectomy: comparison of biochemical recurrence according to risk stratification and surgical modality. World J Urol. 2014;32:1401–1409. doi: 10.1007/s00345-013-1230-0. [DOI] [PubMed] [Google Scholar]
- 18.Porpiglia F, Fiori C, Manfredi M, Grande S, Poggio M, Bollito E, Papotti M, Scarpa RM. Surgical margin status of specimen and oncological outcomes after laparoscopic radical prostatectomy: experience after 400 procedures. World J Urol. 2012;30:245–250. doi: 10.1007/s00345-011-0711-2. [DOI] [PubMed] [Google Scholar]
- 19.Marcq G, Michelet A, Hannink G, Rizk J, Sauvain J, Villers A, Saffarini M, Rochat CH. Risk of biochemical recurrence based on extent and location of positive surgical margins after robot-assisted laparoscopic radical prostatectomy. BMC Cancer. 2018;18:1291. doi: 10.1186/s12885-018-5229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, Presti JC, Jr, Kane CJ. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 21.de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70:233–245. doi: 10.1016/j.eururo.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 22.Park BH, Jeon HG, Jeong BC, Seo SI, Lee HM, Choi HY, Jeon SS. Influence of magnetic resonance imaging in the decision to preserve or resect neurovascular bundles at robotic assisted laparoscopic radical prostatectomy. J Urol. 2014;192:82–88. doi: 10.1016/j.juro.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Jaderling F, Akre O, Aly M, Bjorklund J, Olsson M, Adding C, Oberg M, Blomqvist L, Nyberg T, Wiklund P, Carlsson S. Preoperative staging using magnetic resonance imaging and risk of positive surgical margins after prostate-cancer surgery. Prostate Cancer Prostatic Dis. 2019;22:391–398. doi: 10.1038/s41391-018-0116-z. [DOI] [PubMed] [Google Scholar]
- 24.Rud E, Baco E, Klotz D, Rennesund K, Svindland A, Berge V, Lundeby E, Wessel N, Hoff JR, Berg RE, et al. Does preoperative magnetic resonance imaging reduce the rate of positive surgical margins at radical prostatectomy in a randomised clinical trial? Eur Urol. 2015;68:487–496. doi: 10.1016/j.eururo.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Avulova S, Zhao Z, Lee D, Huang LC, Koyama T, Hoffman KE, Conwill RM, Wu XC, Chen V, Cooperberg MR, et al. The effect of nerve sparing status on sexual and urinary function: 3-year results from the CEASAR study. J Urol. 2018;199:1202–1209. doi: 10.1016/j.juro.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Preston MA, Breau RH, Lantz AG, Morash C, Gerridzen RG, Doucette S, Mallick R, Eastham JA, Cagiannos I. The association between nerve sparing and a positive surgical margin during radical prostatectomy. Urol Oncol. 2015;33:18 e11–18 e16. doi: 10.1016/j.urolonc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Gorin MA, Chalfin HJ, Epstein JI, Feng Z, Partin AW, Trock BJ. Predicting the risk of non-organ-confined prostate cancer when perineural invasion is found on biopsy. Urology. 2014;83:1117–1121. doi: 10.1016/j.urology.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang M, Oh JJ, Lee S, Hong SK, Lee SE, Byun SS. Perineural invasion and lymphovascular invasion are associated with increased risk of biochemical recurrence in patients undergoing radical prostatectomy. Ann Surg Oncol. 2016;23:2699–2706. doi: 10.1245/s10434-016-5153-z. [DOI] [PubMed] [Google Scholar]
- 29.Peng LC, Narang AK, Gergis C, Radwan NA, Han P, Marciscano AE, Robertson SP, He P, Trieu J, Ram AN, et al. Effects of perineural invasion on biochemical recurrence and prostate cancer-specific survival in patients treated with definitive external beam radiotherapy. Urol Oncol. 2018;36:309 e307–309 e314. doi: 10.1016/j.urolonc.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Chen J, Zhang M, Tang X, Sun G, Zhu S, Liu J, Zhang H, Zhang X, Yin X, et al. The clinical significance of perineural invasion in patients with de novo metastatic prostate cancer. Andrology. 2019;7:184–192. doi: 10.1111/andr.12578. [DOI] [PubMed] [Google Scholar]
- 31.Jung JH, Lee JW, Arkoncel FR, Cho NH, Yusoff NA, Kim KJ, Song JM, Kim SJ, Rha KH. Significance of perineural invasion, lymphovascular invasion, and high-grade prostatic intraepithelial neoplasia in robot-assisted laparoscopic radical prostatectomy. Ann Surg Oncol. 2011;18:3828–3832. doi: 10.1245/s10434-011-1790-4. [DOI] [PubMed] [Google Scholar]
- 32.Loeb S, Epstein JI, Humphreys EB, Walsh PC. Does perineural invasion on prostate biopsy predict adverse prostatectomy outcomes? BJU Int. 2010;105:1510–1513. doi: 10.1111/j.1464-410X.2009.08845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kates M, Sopko NA, Han M, Partin AW, Epstein JI: Importance of Reporting the Gleason Score at the Positive Surgical Margin Site: Analysis of 4,082 Consecutive Radical Prostatectomy Cases. J Urol. 2016;195:337–42. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.