Abbreviation
- EPAS1
Endothelial PAS domain‐containing protein 1
- GO
Gene Ontology
- HIF‐2α
Hypoxia‐inducible factor‐2α
- HRF
HIF‐1α related factor
- ISH
In Situ Hybridization
- JAM‐A
junctional adhesion molecule‐A
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- NTA
Nano particle tracking analysis
- Q‐PCR
Quantitative‐polymerase chain reaction
- TDEs
Tumor‐derived exosomes
- TEM
Transmission electron microscopy
- TME
tissue microarray
- VE‐cad
VE‐cadherin
Dear editor,
Ovarian cancer is one of the most prevalent gynecological malignant tumors. Its five‐year overall survival rate is less than 30%, mostly due to advanced metastasis and chemotherapy resistance [1]. However, the mechanisms involved in ovarian cancer metastasis are still poorly understood. Hypoxia is a tumor microenvironment (TME) factor that facilitates tumor progression [2]. Changes in TME and cross‐talk signaling pathway is crucial for ovarian cancer metastasis and indicate that these tumor biological characteristics are ruled by lots of conserved mechanisms [3, 4]. Exosomes are crucial signalosomes between various cell types crosstalk and can carry complex mRNAs and microRNAs (miRNAs) functions between cells [5]. Tumor‐derived exosomes (TDEs) have been shown to not only contribute to TME remodeling but also as a contributor to premetastatic niche formation [6]. There are evidence showing that cancer‐secreted miRNAs may mediate cell‐cell communication in many diseases, including ovarian cancer. For instance, Liu X et al. [7] reported that miR‐199a‐5p, as a tumor suppressor, could inhibit ovarian cancer cell proliferation and invasion by inhibiting the expression of NF‐κB1. Song K et al. [8] reported that emodin had inhibiting effects on ovarian cancer cells colony formation by activating the FOXD3/miR‐199a‐5p axis. These, therefore, suggest that miR‐199a‐5p may be a promising target in ovarian cancer treatment. However, till present, the ovarian cancer cell‐secreted exosomal miR‐199a‐5p interaction with Wnt/β‐catenin in ovarian cancer has not been explored, especially in relation to the TME. In this study, we aimed to explore the role of ovarian cancer cell‐derived exosomal miR‐199a‐5p in ovarian cancer metastasis as a new potential target for ovarian cancer treatment.
We analyzed exosome‐miRNA enriched in ovarian cancer cells under normoxia and hypoxia conditions. The materials and methods for this study are provided in the “supplementary file”. Transmission electron microscopy (TEM) and nano particle tracking analysis (NTA) confirmed the observed round vesicles as exosomes with diameters of 30∼150 nm (Figure 1A, B). Besides, NTA found that A2780 cells cultured under hypoxia condition produced more exosomes than those cultured in normoxia condition (P = 0.03, Figure 1C). The exosome surface‐proteins CD81 and CD63 on the exosomes were positive in Western blotting but negative in cell lysis (Figure 1D). High‐Throughput Sequencing (Illumina PE150) detected a total of 359 differentially expressed miRNAs, of which 153 were up‐regulated (red) and 206 were down‐regulated (green) (Supplementary Fig. S1A). A heatmap was constructed to show the top 50 miRNAs which enriched the up‐regulated top 25 and down‐regulated top 25 miRNA in order of |Fold Change| from large to small via miRNA sequence. (|Fold Change| ≥ 2, corrected P values ≤ 0.050) (Figure 1E). The first 10 significantly downregulated miRNAs were: miR‐10a‐5p, miR‐214‐5p, miR‐199a‐5p, miR‐486‐5p, miR‐200b‐3p, miR‐200a‐3p, miR‐199a‐3p, miR‐199b‐3p, miR‐451a and miR‐203a. Of them, miR‐199a‐5p demonstrated the most significant difference in ovarian cancer cell hypoxia condition compared with normoxia condition (P = 0.003). We further predicted its target gene by using the miRwalk software. The Gene Ontology (GO) analysis mainly enriched the foundation of miR‐199a‐5p in cell‐cell adhesion and transcription factor activity. Subsequently, the Kyoto Encyclopedia of Genes and Genomes (KEGG) showed the enrichment of the TGF‐β signaling pathway, Wnt‐signaling pathway, and more. GO and KEGG were conducted using the DAVID tools (Supplementary Fig. S1 B, C). We detected the level of miR‐199a‐5p in four ovarian cancer cell lines (UWB, HEY, A2780, and Anglne) and the exosomes derived from them using Quantitative‐polymerase chain reaction (Q‐PCR). We found that the expression of miR‐199a‐5p, both in cells and exosomes under hypoxia condition, was dramatically lower than those in normoxia condition (Supplementary Fig. S2A, B). The expression of miR‐199a‐5p expression in ovarian cancer tissue and normal fallopian tube were detected by Q‐PCR and in situ hybridization (ISH), respectively. Q‐PCR results showed that the expression of miR‐199a‐5p in ovarian cancer tissue was significantly lower than in normal fallopian tube (Supplementary Fig. S2C). Consistently, ISH detection of tissue microarrays (TMA) showed the same trend about miR‐199a‐5p expression in ovarian cancer and fallopian tubes (Supplementary Fig. S2D, E). These results demonstrated that the expression of miR‐199a‐5p in ovarian cancer tissue was downregulated. After culturing in hypoxia, the miR‐199a‐5p level in both ovarian cancer cells and exosomes were found to decrease further.
FIGURE 1.
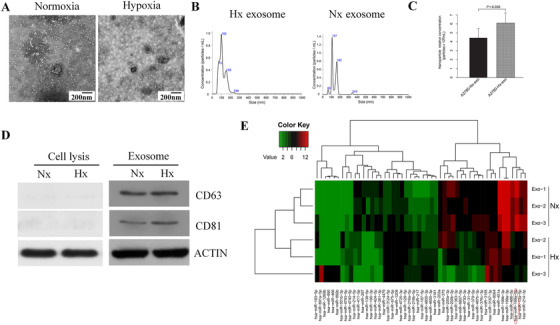
Characterization and analysis of exosomal miRNA derived from ovarian cancer cells under normoxia and hypoxic conditions. (A) Identification of the typical cup‐shape morphology of exosomes by TEM. (B) Particle size and concentration of exosome analyzed by NTA. (C) Cancer cells, in hypoxic conditions, can produce more exosomes than normoxia (P = 0.030). (D) Surface molecular markers of exosome excreted with Western Blot (WB). (E) Hierarchical clustering enriched the top 50 different expression levels of miRNAs between A‐2780‐derived normoxia and hypoxic exosomes, in order of |Fold Change| from big to small. The top 25 upregulated miRNA and top 25 downregulated miRNA were enriched to ensure clearer figure. The significantly up‐regulated genes were represented in red, the downregulated genes were represented in green, and the non‐significant genes were represented in black.
Abbreviations: TEM: Transmission electron microscopy; NTA: Nano particle tracking analysis; WB: Western Blot; DAPI: 4′,6‐diamidino‐2‐phenylindole; FITC: fluorescein isothiocyanate; Hx, hypoxic; Nx, normoxia
The correlation between miR‐199a‐5p expression and the clinicopathological characteristics of the 57 ovarian cancer on TMA was analyzed by immunohistochemistry. It was identified that the miR‐199a‐5p expression in ovarian cancer was negatively correlated with tumor infiltration (P < 0.001), tumor size (≤ 5 cm vs. > 5 cm, P < 0.001), lymphatic metastasis (absent vs. present, P < 0.001) and TNM stage (T1‐T2 vs. T3‐T4, P < 0.001) (Table 1). However, no statistical significance in miR‐199a‐5p expression was found associated with age (≤ 50 years vs. > 50 years, P = 0.574). These results indicated that the downregulation of miR‐199a‐5p might occur in ovarian cancer progression, and could serve as a promising therapeutic marker for ovarian cancer.
TABLE 1.
Correlation between the exosomal miR‐199a‐5p expression to the clinicopathological characteristics of ovarian cancer patients (N = 57)
| Relative expression of miR‐199a‐5p | ||||
|---|---|---|---|---|
| Clinicopathological characteristics | Cases n | High [cases, (%)] | Low [cases, (%)] | P |
| Age (years) | 0.574 | |||
| ≤ 50 | 22 | 12 (21.1%) | 10 (17.5%) | |
| > 50 | 35 | 19 (33.3%) | 16 (28.1%) | |
| Tumor size(cm) | < 0.001 | |||
| ≤ 5 | 20 | 15 (26.3%) | 5 (8.8%) | |
| > 5 | 37 | 5 (8.8%) | 32 (56.1%) | |
| Infiltration | < 0.001 | |||
| Absent | 14 | 11 (19.3%) | 3 (5.3%) | |
| Present | 43 | 10 (17.5%) | 33 (57.9%) | |
| Lymphatic | < 0.001 | |||
| metastasis | ||||
| Absent | 52 | 40 (70.2%) | 12 (21.1%) | |
| Present | 5 | 1 (1.7%) | 4 (7.0%) | |
| T stage | < 0.001 | |||
| T1+T2 | 21 | 19 (33.3%) | 2 (3.5%) | |
| T3+T4 | 36 | 10 (17.6%) | 26 (45.6%) | |
Wound‐healing assays showed that A2780 cells transfected with miR‐199a‐5p plasmid had lower migratory ability than the control cells in hypoxia and normoxia condition (Figure 2A‐C). Meanwhile, the cell migration and Matrigel Transwell assay showed that transfected A2780 cells with miR‐199a‐5p plasmid also had lower invasion (Supplementary Fig. S3A‐D) and migration ability in contrast to the miR‐NC group, while transfection with miR‐199a‐5p inhibitor could promote A2780 migration and infiltration, under normoxia and hypoxia condition. These results demonstrated that the downregulation of miR‐199a‐5p might promote the invasion and migratory ability of ovarian cancer.
FIGURE 2.
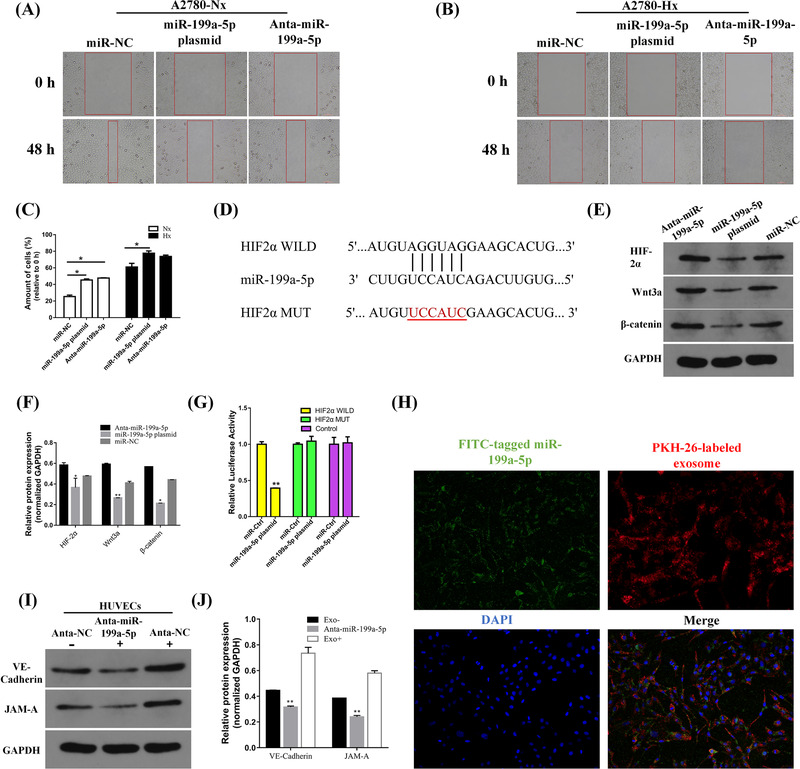
Exosomal miR‐199a‐5p regulating the migration process in ovarian cancer cell line (A2780), miR‐199a‐5p inhibiting the Wnt/β‐catenin signaling pathway, and ovarian cancer cell‐derived exosomal miR‐199a‐5p enhancing the tight junction of HUVECs. (A‐B) Wound‐healing assay in normoxia and hypoxic condition A2780 cells transfected with the miR‐199a‐5p plasmid, inhibitor, or NC. (C) Cartogram of the proportion of 48h migrating cells compared with 0h in each group. (*P < 0.050 and **P < 0.010). (D) Seed sequence alignment of miR‐199a‐5p‐blinding site with portions of HIF‐2α mRNA 3′UTR. (E) miR‐199a‐5p inhibits the Wnt/β‐catenin signaling pathway. HIF‐2α, Wnt3a, and β‐catenin protein expression in A2780 cells transfected with Anta‐miR‐199a‐5p, miR‐199a‐5p plasmid, or miR‐NC were analyzed by immunoblot. GAPDH was used as a reference. (F) The intensity for each band shows the relative protein expression normalized for GAPDH. Anta‐miR‐199a‐5p: miR‐199a‐5p inhibitor, miR‐199a‐5p plasmid, miR‐NC: negative control. (G) Luciferase assay demonstrating that has‐miR‐199a‐5p plasmid significantly suppressed the luciferase activity of HIF‐2α wild‐type group, compared to the HIF‐2α mutant‐type group. (H) Co‐culture of exosomes from ovarian cancer A2780 cells transfected with miR‐199a‐5p‐stable expressing cells. Representative pictures of HUVECs uptake of exosomal miR‐199a‐5p. (FITC: FITC‐marked miR‐199a‐p (green), PKH26: PKH26‐labeled exosomes (red), DAPI, cell nuclei (blue)). (I, J) Anti‐NC or Anti‐miR‐199a‐5p transfected HUVECs were cultured with or without A2780‐derived exosomes, then analyzed using Western Blot. GAPDH was used as an internal control. The intensity for each band shows relative protein expression normalized for GAPDH.
Abbreviations: HIF‐2α: Hypoxia‐inducible factor‐2α
The potential target gene of miR‐199a‐5p was predicted using the online software miRwalk which contains twelve online databases. We selected target genes that were associated with hypoxia from the intersection in at least three databases. The potential target gene of miR‐199a‐5p might be the hypoxia‐inducible factor (HIF‐2α) (Figure 2D). We found that the overexpression of miR‐199a‐5p could significantly inhibit the expression of HIF‐2α mRNA, while the miR‐199a‐5p inhibitor had the opposite effects (Figure 2E, F). To further verify this hypothesis, we performed Dual‐luciferase report assay, which showed that has‐miR‐199a‐5p plasmid could significantly suppress the luciferase activity of HIF‐2α wild‐type group compared with HIF‐2α mutant‐type group (P < 0.010) (Figure 2G); hinting that miR‐199a‐5p could directly combine with the 3′UTR of HIF‐2α. Additionally, Western blot and immunohistochemical staining of HIF‐2α levels in ovarian cancer tissues and normal fallopian tissues were tested and the results demonstrated that the relationship between miR‐199a‐5p and HIF‐2α may have a negative correlation (Supplementary Fig. S4A, B, Supplementary Fig. S5A‐D). These results suggested that miR‐199a‐5p could directly regulate HIF‐2α. The Wnt/β‐catenin signaling pathway has been reported to be involved in ovarian cancer progression [9], and in this study, we detected the Wnt3a and β‐catenin expression in A2780 cell and tissues. Western blot analysis showed that Wnt3a and β‐catenin expression were downregulated after miR‐199a‐5p was upregulated in A2780 cells (Figure 2E, F). Meanwhile, the upregulation of Wnt3a and β‐catenin in ovarian cancer tissues and downregulation in normal fallopian tissues indicated that miR‐199a‐5p may be negatively correlated with Wnt3a and β‐catenin expression (Figure 2E, F).
To further study whether ovarian cancer cell‐derived exosomal miR‐199a‐5p could be engulfed by HUVECs, hypoxia ovarian cancer A2780 cell‐secreted exosome was labeled with PKH26 (red fluorescent color) and the miR‐199a‐5p was tagged with fluorescein isothiocyanate (FITC) (green fluorescent color). Then, we co‐cultured endothelial cells with A2780 cell‐derived exosomal miR‐199a‐5p under hypoxic conditions. The internalized exosomal miR‐199a‐5p was shown as yellow fluorescence dots around HUVECs nuclei (Figure 2H). The expression of VE‐cadherin (VE‐Cad) and junctional adhesion molecule‐A (JAM‐A) were detected with Western Blot. The results showed that anti‐NC was cultured with A2780‐derived exosome, resulting in an increased expression of VE‐Cad and JAM‐A in HUVECs. The transfection of anti‐miR‐199a‐5p into HUVECs remarkably reduced the expression of A2780‐secreted exosome to reduce the expression of VE‐cad and JAM‐A in HUVECs (Figure 2I, J). Moreover, ovarian cancer tissues had low VE‐cadherin and JAM‐A staining compared with the normal fallopian tubes according to histologic scoring, meaning that the expression of VE‐cad and JAM‐A in ovarian cancer tissues were downregulated (Supplementary Fig. S5E‐L). These results uncovered the crucial role of ovarian cancer cell‐secreted miR‐199a‐5p on a vascular level and indicated that ovarian cancer cell‐derived exosomal miR‐199a‐5p might enhance the tight junction of HUVECs.
Hypoxia is a hallmark of the TME, and also an underling factor promoting tumor angiogenesis and metastatic progression [2]. Recently, hypoxia has been reported to promote exosomes release from cancer cells [6]. Our findings demonstrated that hypoxia OvCa cell excreted exosomal miR‐199a‐5p played a negative regulatory role in cancer metastasis. In other words, the down‐regulation of exosomal miR‐199a‐5p promoted cancer metastasis. This effect was achieved through HIF‐2α regulating the Wnt/β‐catenin pathway. Exchange of cellular ingredients between cells through paracrine mechanisms is a vital way of intercellular communication and can be mediated by exosomes. Emerging evidence shows that an increment in vascular permeability was linked with an escalation in cancer metastasis [10]. Findings from this study revealed that exosomes mediated the interaction between cancer cells and TME, which increased the endothelial monolayers junction integrity to inhibit cancer metastasis. miR‐199a‐5p secreted by hypoxic ovarian cancer cells acted as a promising tumor suppressor factor. Exosomal miR‐199a‐5p is phagocytized by endothelial cells and increases tight junction in endothelial cells. The crosstalk between ovarian cancer cells and endothelial cells in TME confirms that miR‐199a‐5p may be a potential biomarker, which can be used as a novel therapeutic target for ovarian cancer treatment.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of those hospitals. All human ovarian cancer tissues were obtained with informed consent.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA
Data can be requested from the corresponding author upon reasonable request.
COMPETING INTERESTS
The authors declare no conflict of interest.
FUNDING
This study was supported by the National Natural Science Foundation of China (81301789), the Shandong Province Natural Science Foundation (ZR2011HQ013), the Key Research and Development Projects in Shandong Province, China (2016GSF201150), and the Scientific and Technological Development Projects of Shandong Province Medical and Health Science (No. 2015WS0244).
AUTHOR CONTRIBUTIONS
XYL and HZ designed the study. XYL contributed to perform the experiments and data analysis. XL, PZ, SQH, RXT, and QL contributed to collect samples. All those works were supervised by JC. All authors read and approved the final manuscript.
Supporting information
supporting information
supporting information
supporting information
supporting information
supporting information
supporting information
ACKNOWLEDGMENT
The authors would like to thank all the support received by the National Natural Science Foundation of China, Shandong Science and technology department.
REFERENCES
- 1. Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309(7):C444‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863(3):382‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park GB, Kim D. MicroRNA‐503‐5p Inhibits the CD97‐Mediated JAK2/STAT3 Pathway in Metastatic or Paclitaxel‐Resistant Ovarian Cancer Cells. Neoplasia. 2019;21(2):206‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Natarajan S, Foreman KM, Soriano MI, Rossen NS, Shehade H, Fregoso DR, et al. Collagen Remodeling in the Hypoxic Tumor‐Mesothelial Niche Promotes Ovarian Cancer Metastasis. Cancer Res. 2019;79(9):2271‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. elife. 2016;5:e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu Y, Hung J, Chang W, Lin Y, Pan Y, Tsai P, et al. Hypoxic lung cancer‐secreted exosomal miR‐23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO‐1. Oncogene. 2017;36(34):4929‐42. [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Yao B, Wu Z. miRNA‐199a‐5p suppresses proliferation and invasion by directly targeting NF‐κB1 in human ovarian cancer cells. Oncology letters. 2018;16(4):4543‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song K, Lv T, Chen Y, Diao Y, Yao Q, Wang Y. Emodin inhibits TGF‐β2 by activating the FOXD3/miR‐199a axis in ovarian cancer cells in vitro. Oncology Reports. 2018;39(5):2063‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β‐catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. Journal of Ovarian Research. 2019;12(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tichet M, Prod'Homme V, Fenouille N, Ambrosetti D, Mallavialle A, Cerezo M, et al. Tumour‐derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nature Communications. 2015;6(1):1‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supporting information
supporting information
supporting information
supporting information
supporting information
supporting information
Data Availability Statement
Data can be requested from the corresponding author upon reasonable request.


