Abstract
BACKGROUND
Despite an increased risk of subsequent human papillomavirus (HPV)–related malignancies, HPV vaccine initiation rates among cancer survivors remain critically low. The purpose of this study was to determine the relationship between HPV vaccine intent and subsequent vaccine initiation among cancer survivors by linking data from a cross-sectional survey with state-based immunization registry records.
METHODS
Cancer survivors who were 9 to 26 years old were surveyed 1 to 5 years after their treatment to assess their HPV vaccine initiation status, HPV vaccine intent, sociodemographic factors, and vaccine-related health beliefs. HPV vaccine doses/dates were abstracted from the Georgia Registry for Immunization Transactions for 3.5 years after survey participation. Logistic regression models identified factors associated with vaccine intent and subsequent vaccine initiation.
RESULTS
Among survivors who were HPV vaccine–naive at survey participation (n = 103), factors associated with vaccine intent included the following: 1) provider recommendation for the HPV vaccine (odds ratio [OR], 5.0; 95% confidence interval [CI], 1.4–18.4; P = .014), 2) positive general attitude toward vaccines (OR, 4.8; 95% CI, 2.0–11.2; P < .001), and 3) perceived severity of HPV disease (OR, 3.5; 95% CI, 1.2–9.9; P = .02). Of the vaccine-naive patients, 28 initiated the HPV vaccine at a median of 1.1 years after the survey. Initiation was more likely among survivors who had reported vaccine intent (OR, 3.9; 95% CI, 1.2–12.5; P = .02) and was less likely among older survivors (OR per year, 0.7; 95% CI, 0.6–0.9; P < .001).
CONCLUSIONS
These findings suggest that provider recommendation for the HPV vaccine plays a role in establishing intent, which then translates into subsequent initiation.
Keywords: adolescent, cancer survivors, papillomavirus vaccines, young adult
INTRODUCTION
Human papillomavirus (HPV) is an attributable cause of more than 90% of cervical cancers and more than 60% of vaginal, vulvar, penile, anal, rectal, and oropharyngeal cancers in the United States.1 Populations that experience prolonged immunosuppression are more likely to develop a persistent HPV infection, which is associated with an increased risk for HPV-related cancers.2–5 In comparison with age-matched peers in the general population, the excess risk for HPV-related malignancies among survivors of childhood cancer is 40% for female survivors and 150% for male survivors.6 Survivors of allogenic stem cell transplantation, a curative treatment for a variety of malignancies and hematologic disorders, are at significantly higher risk for HPV-related cancers in comparison with the general population.5
The nonavalent HPV vaccine provides protection against the 7 oncogenic HPV types associated with the large majority of HPV-related cancers.7 For most cancer survivors, vaccinations, including the HPV vaccine, are recommended to be begun 6 months to 1 year after cancer treatment completion.8,9 Unfortunately, uptake of the vaccine among cancer survivors has remained critically low and below that of the general population; 23.8% of cancer survivors aged 13 to 26 years report 1 or more doses of the vaccine, whereas 40.5% of age-matched peers in the general population do.10 This leaves the majority of young cancer survivors potentially vulnerable to HPV acquisition and subsequent malignancies.
A lack of a provider recommendation for the HPV vaccine is the strongest predictor of vaccine noninitiation among the general population11,12 and among cancer survivors.10 Intent to receive the HPV vaccine has been associated with and is predictive of subsequent vaccine initiation in the general population13; however, this relationship has not been explored in cancer survivors. Cancer survivors have frequent visits with oncology providers during and after treatment.14 Although provider recommendation has been identified as a critical factor related to HPV vaccine initiation,11,12 most survivors report not receiving a provider recommendation,10 and this suggests that survivorship-focused care may not include a discussion of the HPV vaccine. Many oncology providers may not routinely offer the HPV vaccine within their clinical setting and, therefore, may view discussion of the HPV vaccine as beyond their oncology/survivorship-focused role. Understanding the relationship between intent and subsequent initiation—and determining the influence of provider recommendation on these concepts—may inform future interventions targeted toward increasing the provision of HPV vaccine recommendations by oncology providers.
Although studies of HPV vaccine initiation among cancer survivors to date have been cross-sectional in design and based on self-report,10,15,16 state-based immunization registries offer an opportunity to link cross-sectional data with vaccine records to assess HPV vaccine initiation among cancer survivors over time. The primary purpose of this study was to determine the relationship between HPV vaccine intent and subsequent initiation among cancer survivors. We also identified factors associated with HPV vaccine intent among vaccine-naive cancer survivors.
MATERIALS AND METHODS
Data Source and Sample: Cancer Survivor Cohort Data
Participant recruitment and survey collection occurred between 2012 and 2014 at Emory University/Children’s Healthcare of Atlanta (CHOA) as part of the HPV Vaccine in Cancer Survivors Study (HPVCSS; NCT01492582). The study was approved by the Emory University institutional review board. Eligibility criteria for the study included cancer survivors aged 9 to 26 years who were scheduled for a follow-up visit at Emory University/CHOA and were English- or Spanish-speaking, in remission, and 1 to 5 years past the completion of treatment. Eligible survivors (or their parents if they were aged 9–17 years) were approached regarding study participation in person during a routine clinic visit. Participants provided informed consent/assent in the patient’s or parent’s preferred language according to institutional review board requirements and completed a 1-time survey designed to elicit the prevalence of HPV vaccine uptake and relevant sociodemographic and behavioral variables.10
Variables of Interest
HPVCSS survey data
At study entry, young adult participants (18–26 years old) and parents of minor participants (9–17 years old) completed a survey indicating the number of HPV vaccine doses received; among those reporting zero or no previous vaccine doses, participants indicated future intent to receive the HPV vaccine. Self-reported HPV vaccine noninitiation was defined as a survey response indicating prior receipt of 0 doses of HPV vaccine. Survivor sociodemographic characteristics self-reported by survey participants included race, ethnicity, education, and household income.
The HPVCSS was guided by an integrative framework informed by both the Theory of Planned Behavior and the Health Belief Model. The Theory of Planned Behavior is focused on motivational factors that affect an individual’s health behavior, and it posits that intention is the strongest determinant of performing a behavior.17,18 The Health Belief Model is focused on individual-level factors that influence health behavior, including the perception of threat (eg, consequence of HPV-related disease) and the value of actions that reduce the threat (eg, HPV vaccination).19 Survey data collected from HPVCSS participants assessed concepts from both theories with standardized items adapted from previous research.20–24 Items were rated on Likert-type scales, with an average score calculated for each construct, and higher scores were more reflective of the construct being measured (eg, increased perceived vulnerability to HPV-related diseases and more positive attitudes toward vaccines). A 9-item scale measuring HPV-related knowledge, adapted from Brabin et al,21 was summed, with higher scores indicating greater HPV-related knowledge. Social and environmental influences on HPV vaccine–related decision making were assessed with an 11- to 13-item scale tailored to participant sex,22,23 with higher scores indicating greater social/environmental influences on vaccine decision making. Participant-reported receipt of a health care provider (“provider”) recommendation for the HPV vaccine (yes/no) and perceived health insurance coverage for the HPV vaccine (yes/no) were also assessed.
Medical record data
Data abstracted from the medical record included the survivor’s date of birth, sex, cancer diagnosis, date of cancer diagnosis, date of treatment completion, and treatment modalities (yes/no for chemotherapy, surgery, radiation, and/or hematopoietic cell transplantation [HCT]). The participant’s cancer diagnosis, cancer stage, and cancer therapy were used to categorize the treatment intensity as low, moderate, high, or very high according to the Intensity of Treatment Rating Scale, version 3.0 (ITR-3).25
State-based immunization registry data
Since 1996, the state of Georgia has mandated that vaccine providers record all immunizations administered to state residents in the Georgia Registry for Immunization Transactions (GRITS), and this makes the registry a unique tool for vaccine tracking. Immunizations can be recorded in GRITS either through manual entry by the provider/practice or through an interface with the provider’s electronic medical record; immunizations are required to be recorded in GRITS within 30 days of administration.26 GRITS captures 86.7% of Georgia’s adolescent vaccine recipients and 66.7% of adult vaccine recipients.27
In January 2018, participating survivors were linked to GRITS records through the institutional electronic medical record, and all dates/doses of the HPV vaccine received before survey completion and during the 3.5-year follow-up period were abstracted. A 3.5-year follow-up period was chosen because all survivors were at least 3.5 years past survey completion at the time of GRITS data linkage.
Outcome variables
HPV vaccine intent was assessed with 4 survey items22: “How likely is it that you (your son/daughter) will i) start the HPV vaccine within the next month; ii) within the next 6 months; iii) within the next 12 months; and iv) get vaccinated in the future?” Response options were based on a 7-point Likert scale and ranged from definitely will not (0) to definitely will (6). To examine an overall measure of intent, it was necessary to construct a compound variable to analyze this concept. A binary variable was derived from these data, and the following responses were categorized as HPV vaccine intent: likely to (4), very likely to (5), or definitely will (6) start the vaccine within the next 6 months or very likely to (5) or definitely will (6) start the vaccine within the next 12 months or in the future. All other responses were defined as HPV vaccine nonintent.
Subsequent initiation was defined as a documented first dose of the HPV vaccine in GRITS during the follow-up period.
Statistical Analysis
Descriptive statistics
These included means, medians, standard deviations, and ranges for continuous variables and frequencies and percentages for categorical data.
Logistic regression models
Univariable models
For survivors who had not initiated the HPV vaccine at the time of survey completion, univariable logistic regression models were initially developed to identify sociodemographic factors, clinical characteristics, and health beliefs related to the 2 outcome variables of interest: 1) subsequent vaccine initiation and 2) vaccine intent. The following variables were examined in the univariable logistic regression models for both outcome variables: age at survey; sex; race and ethnicity; household income; educational level; diagnosis; age at diagnosis; time from diagnosis; time from therapy completion; history of HCT; intensity of treatment (ITR-3 rating); receipt of provider recommendation for the HPV vaccine; perceived insurance coverage for the HPV vaccine; HPV disease–related knowledge; perceived vulnerability to and severity of HPV-related disease; general attitude toward vaccines; perceived barriers to HPV vaccine initiation; perceived self-efficacy to initiate HPV vaccine; and social influences on HPV vaccine decision making such as the perspectives of family, friends, and media. In addition, vaccine intent was examined as a potential predictor of subsequent vaccine initiation with univariable logistic regression.
Multivariable models
Variables with P values < .1 in univariable analyses were included in the multivariable logistic regression models constructed for each outcome variable.
Parsimonious models
Using only variables with P values < .1 in the multivariable models (except for survivor sex and age at survey, which were included in the models a priori, regardless of P values), we further used stepwise backward variable elimination to develop and select a final parsimonious model for each outcome variable. In these final parsimonious models, 2-sided tests with P < .05 were considered statistically significant.
SAS (version 9.4; SAS Institute, Cary, North Carolina) was used to perform all statistical analyses.
RESULTS
Patient Characteristics
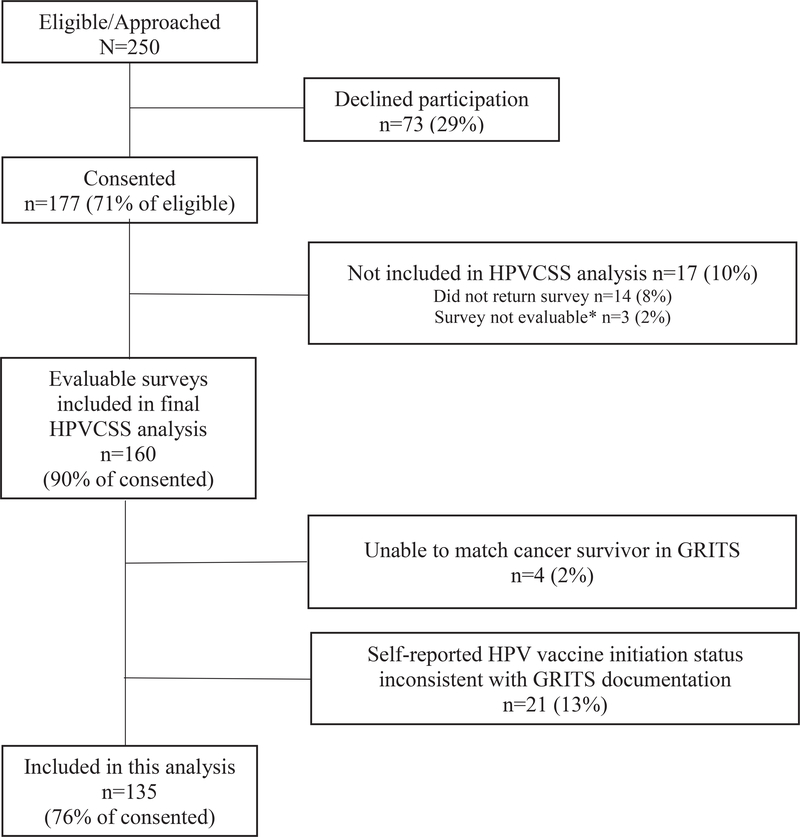
Of the 250 eligible cancer survivors approached for HPVCSS study participation at Emory University/CHOA, 177 (71%) consented, and 160 HPV-vaccinated and vaccine-naive participants were eligible for this analysis (90% of those who consented). Study participants were older than nonparticipants (mean age, 15.8 vs 14.4 years; P = .008). There were no differences in sex, race, diagnosis, or time off treatment between participants and nonparticipants. A total of 25 participants were excluded from this analysis either because their self-reported HPV vaccine initiation status was inconsistent with GRITS documentation (n = 21) or because the participants could not be linked with a GRITS record (n = 4; Fig. 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram of cohort derivation. GRITS indicates Georgia Registry for Immunization Transactions; HPV, human papillomavirus; HPVCSS, HPV Vaccine in Cancer Survivors Study.
At the time of survey completion, the 135 survivors included in this analysis had a median age of 15.4 years (range, 9.04–25.77 years) and were 3.05 years (range, 1.04–4.99 years) from the completion of cancer therapy. The majority of survivors were male (51%) and non-Hispanic white (64%) and had a diagnosis of leukemia or lymphoma (58%). Most survivors’ cancer treatment was rated as high-intensity or very high-intensity (40% and 23%, respectively) according to ITR-325; 12% of the survivors underwent HCT (Table 1).
TABLE 1.
Cancer Survivor Demographic, Clinical, and Treatment Characteristics (n = 135)
| Characteristic | Value |
|---|---|
| Patient age at survey, y | |
| Median (range) | 15.40 (9.04–25.77) |
| Mean (standard deviation) | 15.34 (4.12) |
| Age at diagnosis, y | |
| Median (range) | 10.30 (2.41–21.43) |
| Mean (standard deviation) | 10.60 (4.75) |
| Time from diagnosis (at survey participation), y | |
| Median (range) | 4.60 (1.53–10.79) |
| Mean (standard deviation) | 4.74 (1.91) |
| Time from therapy completion (at survey participation), y | |
| Median (range) | 3.05 (1.04–4.99) |
| Mean (standard deviation) | 3.01 (1.23) |
| Sex, No. (%) | |
| Female | 66 (49) |
| Male | 69 (51) |
| Race/ethnicity, No. (%) | |
| Non-Hispanic white | 87 (64) |
| Other | 48 (36) |
| Household income, No. (%)a | |
| ≤$50,000 | 40 (34) |
| >$50,000 | 77 (66) |
| Educational level, No. (%)a,b | |
| Low/appropriate | 67 (52) |
| Exceeds | 62 (48) |
| Diagnosis, No. (%) | |
| Leukemia/lymphoma | 78 (58) |
| Solid tumor | 57 (42) |
| HCT, No. (%) | |
| No | 119 (88) |
| Yes | 16 (12) |
| ITR-38 | |
| Low/moderate | 50 (37) |
| High | 54 (40) |
| Very high | 31 (23) |
| HPV vaccine doses at time of survey | |
| 0 | 103 (76) |
| ≥1 | 32 (24) |
Abbreviations: GED, General Education Development; HCT, hematopoietic cell transplantation; HPV, human papillomavirus; ITR-3, Intensity of Treatment Rating Scale, version 3.0.
Statistics were calculated for this table by the exclusion of patients with missing values for the characteristics.
The education level was based on the age of the respondent, and for adults (parent respondents and patients 20 years old or older), it was categorized as low (less than a high school graduate), appropriate (high school graduate/GED or some college/technical degree), or exceeds (college degree or higher). The education level for adolescents (patients 18–19 years old) was categorized as low (less than high school), appropriate (some high school or high school graduate/GED), or exceeds (some college or more).
Prevalence of HPV Vaccine Initiation
Among the 135 survivors included in this analysis, 32 (24%) had initiated the HPV vaccine before survey completion; an additional 28 (21%) initiated the vaccine during the follow-up period; and 75 (55%) remained unvaccinated against HPV. Among the 28 HPV vaccine–naive survivors who initiated the vaccine during the follow-up period, 13 (46%) initiated within 1 year, and 15 (54%) initiated 1 to 3.5 years after survey completion, with a median time to initiation of 1.1 years. Among the 103 participants who were vaccine-naive at the time of survey completion, 24 (24%) reported receipt of a health care provider recommendation for the vaccine (Table 2).
TABLE 2.
HPV Vaccine Intent, Report of Health Care Provider Recommendation, and Subsequent Vaccine Initiation Among HPV Vaccine–Naive Cancer Survivors (n = 103)
| Characteristic | No. (%) |
|---|---|
| Intent to receive HPV vaccine | |
| No | 69 (67) |
| Yes | 34 (33) |
| Health care provider recommendation for HPV vaccinea | |
| No | 75 (76) |
| Yes | 24 (24) |
| Subsequent (postsurvey) initiation of HPV vaccine | |
| No | 75 (73) |
| Yes | 28 (27) |
Abbreviation: HPV, human papillomavirus.
Statistics were calculated for this table by the exclusion of patients with missing values for the characteristics.
Intent and Subsequent Initiation of the HPV Vaccine
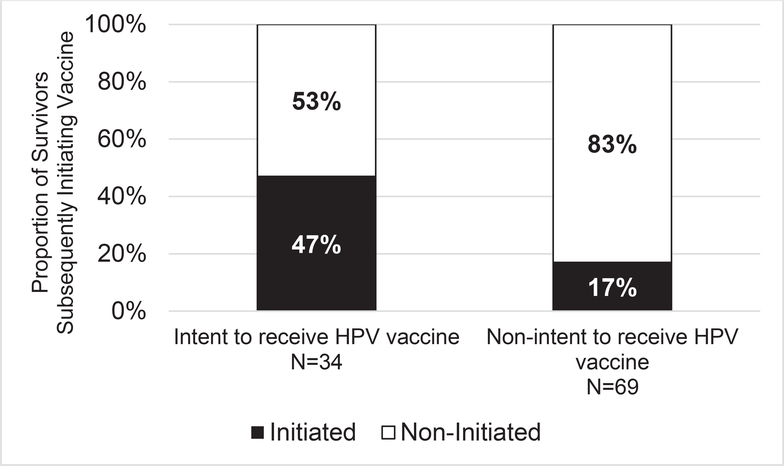
Among the 103 survivors who were HPV vaccine–naive at the time of survey completion, 34 (33%) reported intent to receive the vaccine, and 28 (27%) subsequently initiated the vaccine during the follow-up period. Forty-seven percent (16 of 34) of those reporting intent to receive the vaccine subsequently initiated, whereas only 17% (12 of 69) of those not reporting intent subsequently initiated the vaccine (Fig. 2). Intent to receive the HPV vaccine was predictive of subsequent initiation (odds ratio [OR], 3.9; 95% confidence interval [CI], 1.2–12.5; P = .02), and subsequent initiation was less likely among older survivors (OR per year, 0.7; 95% CI, 0.6–0.9; P < .001; Table 3). Although on univariable analysis, provider recommendation for the HPV vaccine met criteria for inclusion in the multivariable model, this variable was subsequently eliminated through backward elimination during development of the parsimonious multivariable model.
Figure 2.
Subsequent HPV vaccine initiation rates by vaccine intent among survivors who were HPV vaccine–naive at the time of survey participation (n = 103). HPV indicates human papillomavirus.
TABLE 3.
Parsimonious Multivariable Model of Factors Predicting Subsequent Initiation Among HPV Vaccine–Noninitiated Cancer Survivors (n = 103)
| Variable | Comparison | Multivariable Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| Attitudes toward vaccines (continuous)a | 2.03 | 0.92–4.48 | .079 | |
| Sex | Female vs male | 2.85 | 0.94–8.70 | .065 |
| Patient age at survey participation (continuous) | 0.74 | 0.62–0.88 | <.001 | |
| Intent to receive HPV vaccine | Yes vs no | 3.87 | 1.20–12.51 | .024 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; OR, odds ratio.
Measured on a 5-point Likert scale (from most negative attitude [1] to most positive attitude [5]).
Factors Related to Intent to Receive the HPV Vaccine
In a multivariable parsimonious model, the odds of HPV vaccine intent were increased among participants who reported receipt of a provider recommendation for the vaccine (OR, 5.0; 95% CI, 1.4–18.1; P = .01), a more favorable attitude toward vaccines in general (OR, 4.8; 95% CI, 2.0–11.2; P < .001), and increased perceived severity of HPV-related illness (OR, 3.5; 95% CI, 1.2–9.9; P = .02; Table 4).
TABLE 4.
Parsimonious Multivariable Model of Factors Associated With Vaccine Intent Among HPV Vaccine–Noninitiated Cancer Survivors (n = 103)
| Variable | Comparison | Multivariable Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| Patient age at survey participation (continuous) | 0.94 | 0.83–1.06 | .299 | |
| Sex | Female vs male | 0.98 | 0.34–2.79 | .969 |
| Perceived severity of HPV disease (continuous)a | 3.50 | 1.24–9.86 | .018 | |
| General attitude toward vaccines (continuous)b | 4.75 | 2.02–11.15 | <.001 | |
| Health care provider recommendation for HPV vaccine | Yes vs no | 5.02 | 1.39–18.14 | .014 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; OR, odds ratio.
Measured on a 5-point Likert scale (from lowest perceived severity [1] to highest perceived severity [5]).
Measured on a 5-point Likert scale (from most negative attitude [1] to most positive attitude [5]).
By linking cross-sectional survey and immunization registry data, this study identifies factors associated with human papillomavirus vaccine intent and subsequent initiation among young cancer survivors. Vaccine intent is strongly predictive of subsequent human papillomavirus vaccine initiation, and provider recommendation is strongly associated with human papillomavirus vaccine intent in young cancer survivors.
DISCUSSION
By linking data from a cross-sectional survey of young cancer survivors with data from a state-based vaccine registry, we found that HPV vaccine intent was strongly predictive of subsequent vaccine initiation. Almost half of HPV vaccine–naive cancer survivors reporting vaccine intent subsequently initiated the HPV vaccine during the follow-up period, whereas less than 1 in 5 survivors reporting no vaccine intent initiated the vaccine within the same time period. Importantly, survivors who reported vaccine intent at the survey were 4 times more likely to subsequently initiate the vaccine during the follow-up period in comparison with those not reporting intent. These findings mirror those seen in the general population, in which HPV vaccine intent is predictive of later initiation.13,28 Younger age was also predictive of subsequent vaccine initiation in our study, and this is consistent with the current focus on routine HPV vaccination for preadolescents in the general population.7 Although this analysis identifies important factors related to HPV vaccine intent and subsequent initiation, it is also important to note that the majority of cancer survivors in this cohort remained unvaccinated and potentially vulnerable to HPV infection. Thus, in the population of young cancer survivors, identifying factors associated with vaccine intent may be critical for developing targeted interventions to improve vaccine uptake in this vulnerable group.
Among vaccine-naive cancer survivors, we found that reporting prior receipt of a provider recommendation for the vaccine, having a more positive attitude toward vaccines in general, and reporting increased perceived severity of HPV-related diseases were associated with HPV vaccine intent. Provider recommendation has been consistently identified as an important predictor of HPV vaccine initiation across populations29–31 and among cancer survivors.10 Previously, we reported the relationship between provider recommendation and prior receipt of the HPV vaccine10; in this study, our findings suggest that provider recommendation may play an important role in intent to receive the vaccine. In the general population, provider recommendation has been shown to be related to secondary acceptance of the HPV vaccine among parents who initially decline the HPV vaccine for their adolescent child.32 Yet, in a study of subspecialty providers, approximately half of providers reported that they do not routinely recommend the HPV vaccine to their patients with chronic illness.33 In this study of cancer survivors, only 24% of vaccine-naive survivors reported receiving a provider recommendation for the HPV vaccine, and this suggests the need for subspecialty providers, including oncologists, to promote and facilitate HPV vaccine uptake in adolescent and young adult cancer survivors.
We also found that more favorable attitudes toward vaccines were associated with intent to receive the HPV vaccine. In the general population, positive attitudes toward vaccines overall have been associated with increased HPV vaccine uptake.34,35 In a study of 2025 parents of adolescent daughters, Ogilvie et al36 found that the odds of initiating the HPV vaccine were 8.5 times higher (95% CI, 6.1–11.9) among parents with positive (vs negative) attitudes toward vaccines. In our study, increased perceived severity of HPV-related diseases was also associated with intent to receive the HPV vaccine. This finding is similar to that of Gargano et al,31 who showed that perceived severity of vaccine-targeted diseases is significantly associated with intent to receive any adolescent vaccine, including the HPV vaccine. Perceived severity of HPV-related disease and HPV-associated cancers is particularly relevant to cancer survivors, who experience a higher prevalence and earlier onset of HPV-related cancers in comparison with general population peers.6 Our data highlight a potential opportunity to increase HPV vaccine intent and subsequent vaccine initiation in this population by framing the HPV vaccine as a cancer prevention strategy.37
We acknowledge some limitations of this study. Participants were recruited at a single site, and we used a single state-based immunization registry. Because of the cross-sectional nature of the survey, we were unable to determine the temporal relationship between self-reported variables associated with vaccine intent. For example, it is possible that participant intent to receive the HPV vaccine was present before the receipt of a provider recommendation. In addition, we relied on GRITS as the gold standard for verifying receipt of the HPV vaccine and excluded participants whose self-reported vaccine initiation status was inconsistent with GRITS documentation; however, it is possible that GRITS vaccine records for some survivors could be incomplete or inaccurate. Finally, although we found that survivor report of receiving a provider recommendation for the HPV vaccine was significantly associated with vaccine intent, we did not assess the type of provider that recommended the vaccine (eg, oncologist or primary care provider). We also did not assess participants’ reasons for not intending to receive the vaccine. Despite these limitations, this study was the first to evaluate the relationship between HPV vaccine intent and subsequent initiation among cancer survivors with state-based immunization registry records.
In conclusion, in this study of young cancer survivors, we have found that provider recommendation for the HPV vaccine is strongly associated with intent to receive the vaccine and that vaccine intent predicts for subsequent vaccine initiation. These findings suggest that provider recommendation plays a role in establishing intent, which then translates into subsequent initiation. Future research is needed to develop effective interventions to increase HPV vaccination among cancer survivors; our findings support the incorporation of strategies that focus on the promotion of health care provider recommendations for the vaccine among adolescent and young adult cancer survivors to increase HPV vaccine uptake in this vulnerable population.
Acknowledgments
FUNDING SUPPORT
This work was supported by the National Cancer Institute (R01CA166559; principal investigators Wendy Landier and James L. Klosky); it was also supported in part by the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp (MISP 40083; principal investigator Wendy Landier) and by the American Lebanese Syrian Associated Charities’ support of the Consortium for Pediatric Intervention Research. Brooke Cherven received support from the Robert Wood Johnson Future of Nursing Scholars Program (RWJF 72509) and the American Cancer Society Doctoral Scholarship in Cancer Nursing (17-078-01-SCN).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Jocelyn M. York reports stock ownership in Intelligent Medical Objects by an immediate family member. Melissa M. Hudson serves in an advisory role for the following organizations: the Coleman Supportive Oncology Initiative for Children With Cancer, the Oncology Research Information Exchange Network, the Pfizer advisory board for Genotropin, and the Princess Maxima Center. Wendy Landier reports nonfinancial support given to her institution by Merck Sharp & Dohme for a related clinical trial. The other authors made no disclosures.
This trial is registered at ClinicalTrials.gov (NCT01492582).
REFERENCES
- 1.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661–666. [DOI] [PubMed] [Google Scholar]
- 2.Sasadeusz J, Kelly H, Szer J, Schwarer AP, Mitchell H, Grigg A. Abnormal cervical cytology in bone marrow transplant recipients. Bone Marrow Transplant. 2001;28:393–397. [DOI] [PubMed] [Google Scholar]
- 3.Socie G, Curtis RE, Deeg HJ, et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. [DOI] [PubMed] [Google Scholar]
- 4.Katz RL, Veanattukalathil S, Weiss KM. Human papillomavirus infection and neoplasia of the cervix and anogenital region in women with Hodgkin’s disease. Acta Cytol. 1987;31:845–854. [PubMed] [Google Scholar]
- 5.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. [DOI] [PubMed] [Google Scholar]
- 6.Ojha RP, Tota JE, Offutt-Powell TN, et al. Human papillomavirus–associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8:e70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–304. [PMC free article] [PubMed] [Google Scholar]
- 8.Cesaro S, Giacchino M, Fioredda F, et al. Guidelines on vaccinations in paediatric haematology and oncology patients. Biomed Res Int. 2014;2014:707691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. [DOI] [PubMed] [Google Scholar]
- 10.Klosky J, Hudson MM, Chen Y, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35:3582–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine. 2016;34:1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–839. [DOI] [PubMed] [Google Scholar]
- 13.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride ML, Lorenzi MF, Page J, et al. Patterns of physician follow-up among young cancer survivors: report of the Childhood, Adolescent, and Young Adult Cancer Survivors (CAYACS) research program. Can Fam Physician. 2011;57:e482–e490. [PMC free article] [PubMed] [Google Scholar]
- 15.Klosky JL, Russell KM, Simmons JL, et al. Medical and sociodemographic factors associated with human papillomavirus (HPV) vaccination adherence among female survivors of childhood cancer. Pediatr Blood Cancer. 2015;62:1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman L, Okcu MF, Dreyer ZE, Suzawa H, Bryant R, Middleman AB. Human papillomavirus vaccination in female pediatric cancer survivors. J Pediatr Adolesc Gynecol. 2012;25:305–307. [DOI] [PubMed] [Google Scholar]
- 17.Ajzen I The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 18.Glanz K, Rimer B. Theory at a Glance: A Guide for Health Promotion Practice. 2nd ed. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 19.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–183. [DOI] [PubMed] [Google Scholar]
- 20.Cox DS, Cox AD, Sturm L, Zimet G. Behavioral interventions to increase HPV vaccination acceptability among mothers of young girls. Health Psychol. 2010;29:29–39. [DOI] [PubMed] [Google Scholar]
- 21.Brabin L, Roberts SA, Farzaneh F, Kitchener HC. Future acceptance of adolescent human papillomavirus vaccination: a survey of parental attitudes. Vaccine. 2006;24:3087–3094. [DOI] [PubMed] [Google Scholar]
- 22.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007;40:108–115. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117:1486–1493. [DOI] [PubMed] [Google Scholar]
- 24.Klosky J, Russell KM, Canavera KE, et al. Risk factors for non-initiation of the human papillomavirus vaccine among adolescent survivors of childhood cancer. Cancer Prev Res (Phila). 2013;6:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the Intensity of Treatment Rating Scale: classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer. 2012;59:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgia Department of Public Health. Georgia Registry of Immunization Transactions and Services (GRITS) Software User Agreement. https://www.gritstest.state.ga.us/docs/GRITS_User_Agreement_2015.pdf.
- 27.Centers for Disease Control and Prevention. Immunization Information System Annual Report. https://www.cdc.gov/vaccines/programs/iis/annual-report-iisar/2016-data.html.
- 28.Rodriguez SA, Savas LS, Baumler E, et al. Parental predictors of HPV vaccine initiation among low-income Hispanic females aged 11–17 years. Vaccine. 2018;36:5084–5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890–895. [DOI] [PubMed] [Google Scholar]
- 31.Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: who later accepts and why? Acad Pediatr. 2018;18:S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofstetter AM, Lappetito L, Stockwell MS, Rosenthal SL. Human papillomavirus vaccination of adolescents with chronic medical conditions: a national survey of pediatric subspecialists. J Pediatr Adolesc Gynecol. 2017;30:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Visser R, Waites L, Parikh C, Lawrie A. The importance of social norms for uptake of catch-up human papillomavirus vaccination in young women. Sex Health. 2011;8:330–337. [DOI] [PubMed] [Google Scholar]
- 35.Ogunbajo A, Hansen CE, North AL, Okoloko E, Niccolai LM. “I think they’re all basically the same”: parents’ perceptions of human papilloma virus (HPV) vaccine compared with other adolescent vaccines. Child Care Health Dev. 2016;42:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvie G, Anderson M, Marra F, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: parental factors associated with HPV vaccine receipt. PLoS Med. 2010;7:e1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. A Report to the President of the United States From the President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]