To the Editor:
In a recent article in the Journal, Gattinoni and colleagues (1) reported that patients with coronavirus disease (COVID-19) fulfilling the Berlin criteria of acute respiratory distress syndrome (ARDS) presented an atypical form of the syndrome characterized by the “dissociation between their relatively well-preserved lung mechanics and the severity of hypoxemia” that is in sharp contrast with what is expected in severe ARDS. We believe that these findings are actually similar to what we have seen in prematurely born infants with severe respiratory distress syndrome (RDS) caused by surfactant deficiency.
We reviewed data from pulmonary function testing we had performed at the Children’s Hospital of Pittsburgh in neonates during the first week of life as part of an institutional review board–approved study of the natural course of respiratory failure in the neonatal period (2). Twelve prematurely born neonates who were mechanically ventilated because of respiratory distress syndrome (RDS group) were compared with 13 term infants with ARDS due to meconium aspiration syndrome (MAS group) requiring extracorporeal membrane oxygenation. Ten term newborns without lung disease, who had been briefly intubated for procedures under anesthesia, served as controls. The testing was done under sedation or general anesthesia with or without muscle relaxants.
The lung function was evaluated with the deflation flow–volume curve technique that has been described in detail elsewhere (3). In brief, volume history was established by inflating the lungs to TLC with an anesthesia bag system, using a standard inflating pressure of +40 cm H2O. The lungs were then rapidly deflated by opening the endotracheal tube to negative pressure reservoir via a three-way slide valve generating a standard pressure of −40 cm H2O for up to 3 seconds. Pressures of +30 cm H2O and −30 cm H2O were used for all neonates weighing <1,000 g. The lungs were immediately reinflated to TLC after the deflation. The produced airflow and integrated volume signals were plotted as a flow–volume curve (Figure 1). The procedure was repeated until three superimposed curves were obtained. The following indices were calculated: FVC, maximum expiratory flow rate at 25% of the FVC (measured from the residual volume) (MEF25), and the ratio MEF25/FVC. Respiratory system compliance (Crs) was calculated from partial flow–volume curves produced by a modification of the technique described by LeSouef and colleagues (4) Specifically, the lungs were inflated to TLC and then passively deflated from a standard pressure of 10 cm H2O. All values were adjusted for body weight and are presented as mean ± SD. Comparisons between the groups were made with one-way ANOVA and the Student-Newman-Keuls test. A P value less than 0.05 was considered statistically significant.
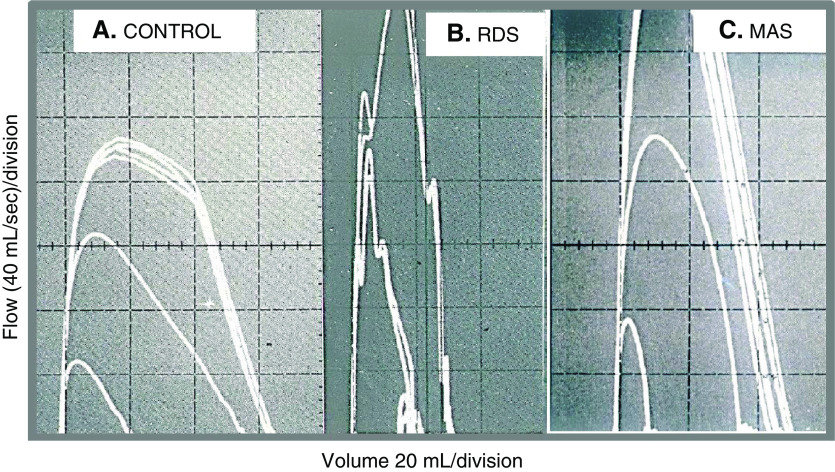
Figure 1.
Deflation flow–volume curves (DFVCs) in intubated infants. (A) Term newborn without lung disease. The outer curves are superimposed DFVCs obtained with inflating pressure of +40 cm H2O and deflating pressure of −40 cm H2O; the middle curve is a passive flow–volume curve after the lungs were inflated with a pressure of +40 cm H2O; the small inner curve is a passive flow–volume curve from a standard pressure of +10 cm H2O and is used to calculate respiratory system compliance and resistance. (B and C) DFVCs from newborns with RDS and MAS. Note the tall and narrow configuration of the curves that illustrate the very high airway conductance seen in both conditions. MAS = meconium aspiration syndrome; RDS = respiratory distress syndrome.
The demographic information and the results of the pulmonary function testing for all patients are presented in Table 1. The FVC/kg and the MEF25/kg as well as the Crs/kg were significantly decreased in the ARDS (MAS) group. In contrast, the lung volume and the Crs were near normal in RDS. The ratio MEF25/FVC was significantly elevated in both the RDS and MAS groups, suggesting abnormally high upstream conductance (5). There were no adverse effects during the testing in any patient studied with the deflation flow–volume curve technique.
Table 1.
Demographic Data and Indices of Lung Mechanics and Function
| MAS (n = 13) | RDS (n = 12) | Control (n = 10) | |
|---|---|---|---|
| Postconceptional age, wk | 39.5 ± 1.9 | 29.0 ± 2.7* | 39.9 ± 0.8 |
| Postnatal age, d | 3.9 ± 2.0 | 4.0 ± 1.7* | 2.7 ± 2.0 |
| Weight, g | 3,280 ± 397 | 1,256 ± 511* | 3,174 ± 390 |
| FVC/kg, ml/kg | 19.7 ± 10.6† | 39.1 ± 12.3 | 41.1 ± 7.3 |
| MEF25, ml/s/kg | 37.9 ± 15.3 | 67.1 ± 40.4* | 43.3 ± 16.0 |
| MEF25/FVC | 2.2 ± 0.8† | 1.9 ± 1.4 | 1.1 ± 0.4 |
| Crs, ml/cm H2O/kg | 0.6 ± 0.5† | 1.6 ± 0.4 | 1.7 ± 0.6 |
Definition of abbreviations: Crs = respiratory system compliance; MAS = meconium aspiration syndrome; MEF25 = maximum expiratory flow rate at 25% of the FVC (measured from the residual volume); RDS = respiratory distress syndrome.
P < 0.05 compared with MAS and with control.
P < 0.001 compared with RDS and with control.
Our findings suggest that despite similarities in clinical and often radiographic manifestation, the lung mechanics are very different between RDS and ARDS. Specifically, in RDS, the lung volume and the Crs (adjusted for body weight) are near normal, but they are severely decreased in MAS. Both conditions show very high airway conductance (reflected by the elevated MEF25/FVC) probably due to lack of surfactant. In RDS, the surfactant is normally absent because its production only starts at around 28 weeks of gestation. Because the lung volume and respiratory system compliance are near normal (for gestational age), prematurely born infants can be successfully managed with supplemental oxygen and noninvasive continuous positive airway pressure even without exogenous surfactant (6). In contrast, in MAS, the surfactant is present but inactivated owing to meconium-induced inflammation, and its production is impaired because of alveolar damage (specifically of the surfactant-producing type II pneumocytes) (7).
Observations of patients presenting in the emergency room with severe hypoxemia but preserved lung mechanics have been reported even in the lay press (8). It has been suggested that there are different phenotypes of COVID-19 that will probably require different treatments (9). We believe that the presumed phenotypes may be in fact different stages of the same continuum, that starts with a surfactant-deficient RDS-type picture that causes severe hypoxemia because of extensive alveolar collapse. In that stage, adult patients respond to oxygen and noninvasive positive airway pressure in a similar way to the premature infants. Mechanical ventilation in that stage may be detrimental (especially when instituted by untrained personnel in the emergency room). Because the virus may affect other organs beyond the lungs, the patients may progress to full-blown ARDS that can become refractory both to oxygen and to invasive mechanical ventilation.
Whether early administration of exogenous surfactant could alter the course and severity of COVID-19 is not known. Trials of exogenous surfactant in typical ARDS have not been successful in the past (10), often because the intervention took place when the lungs had already suffered irreparable damage. Because children (especially newborns) are not just “small adults,” it would be prudent to verify our findings in adult patients. Then a randomized controlled trial should start with the surfactant given as early in the course of the disease as possible, and not as a rescue. Several practical aspects such as dose, frequency, and mode of administration need to be determined. It is a complicated path but one worth investigating.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202004-1471LE on June 24, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome [letter] Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koumbourlis AC, Motoyama EK, Mutich RL. Changes in lung function in premature infants with respiratory insufficiency during the first four weeks of life. Am Rev Respir Dis. 1990;141:A157. [Google Scholar]
- 3.Motoyama EK, Fort MD, Klesh KW, Mutich RL, Guthrie RD. Early onset of airway reactivity in premature infants with bronchopulmonary dysplasia. Am Rev Respir Dis. 1987;136:50–57. doi: 10.1164/ajrccm/136.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Lesouef PN, England SJ, Bryan AC. Passive respiratory mechanics in newborns and children. Am Rev Respir Dis. 1984;129:552–556. [PubMed] [Google Scholar]
- 5.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 6.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019;115:432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopincova J, Calkovska A. Meconium-induced inflammation and surfactant inactivation: specifics of molecular mechanisms. Pediatr Res. 2016;79:514–521. doi: 10.1038/pr.2015.265. [DOI] [PubMed] [Google Scholar]
- 8.Levitan R. The New York Times; 2020. How we can get ahead of Covid-19. April 26;Sect SR:3. [Google Scholar]
- 9.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dushianthan A, Cusack R, Goss V, Postle AD, Grocott MP. Clinical review: exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome--where do we go from here? Crit Care. 2012;16:238. doi: 10.1186/cc11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.