Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) exacerbations are prone to nonrecovery, but there are no data about the effectiveness of retreatment for these prolonged events. We examined whether further therapy with ciprofloxacin for incompletely resolved COPD exacerbations prolonged the time until the next event.
Objectives: To assess whether incompletely recovered COPD exacerbations benefit from additional treatment with ciprofloxacin, at Day 14.
Methods: In a multicenter, randomized double-blind placebo-controlled trial, we studied retreatment with oral ciprofloxacin 500 mg or matched placebo twice daily for 7 days in patients with Global Initiative for Chronic Obstructive Lung Disease stage II–IV COPD and persistent symptoms and/or serum C-reactive protein ≥8 mg/L initiated 14 (±3) days after an index COPD exacerbation. The primary outcome was the time to the next exacerbation within a 90-day period.
Measurements and Main Results: Among 826 patients screened at four centers, 144 eligible participants with incomplete recovery were randomized to receive ciprofloxacin (n = 72) or placebo (n = 72). Within 90 days of randomization, 57% of the patients in the ciprofloxacin group and 53% in the placebo group experienced one or more exacerbations. The median time to the next exacerbation was 32.5 days (interquartile range 13–50) in the placebo arm and 34 days (interquartile range 17–62) in the ciprofloxacin arm, which was not significantly different (adjusted hazard ratio, 1.07; 95% confidence interval, 0.68–1.68; P = 0.76). No significant differences were seen in quality-of-life scores or lung function between the treatment groups.
Conclusions: In patients with persistent symptoms and/or raised C-reactive protein 14 days after a COPD exacerbation, an additional course of ciprofloxacin resulted in no additional benefit compared with placebo. This suggests that nonrecovered exacerbations are not driven by ongoing bacterial infection and may potentially be targeted with antiinflammatory therapy.
Clinical trial registered with www.clinicaltrials.gov (NCT02300220).
Keywords: chronic obstructive pulmonary disease, exacerbations, retreatment, ciprofloxacin, incomplete recovery
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) exacerbations are prone to nonrecovery, but there are no data about the effectiveness of retreatment for these prolonged events. We examined whether further therapy with 7 days of ciprofloxacin in patients with COPD exacerbations and ongoing symptoms or inflammation would delay a subsequent reexacerbation.
What This Study Adds to the Field
This multicenter, double-blind randomized placebo-controlled trial is the first to evaluate the effect of ciprofloxacin retreatment. The intervention was administered at Day 14 in patients with an acute exacerbation of COPD that remained incompletely recovered despite initial treatment with antibiotics and/or corticosteroids. These patients, who had persistent symptoms and/or raised C-reactive protein, derived no additional benefit from the supplementary course of ciprofloxacin compared with placebo. This suggests that nonrecovered exacerbations are not driven by ongoing bacterial infection.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPDs) are important events that have significant adverse consequences for patients (1). Frequent exacerbations are associated with accelerated lung function decline (2, 3), impaired quality of life (4), and increased mortality (5). They also have significant implications for healthcare costs (6).
Exacerbations associated with sputum purulence or increased sputum volume are treated with antibiotics, leading to faster resolution of the exacerbation and a longer time to the next AECOPD (7, 8). Despite antibiotic treatment, however, recovery is often delayed. More than 25% of patients experience another event within 8 weeks after an AECOPD (9), 25% do not recover to baseline by 5 weeks (10), and more than 33% do not recover by 3 months (11). These recurrent events are associated with substantially increased mortality (11), and this has led to financial incentives for healthcare services aimed at avoiding hospital readmissions (12, 13).
We previously reported that serum CRP (C-reactive protein) measured 14 days after an exacerbation was higher (mean = 8.8 mg/dl) in patients who experienced another exacerbation within the next 50 days (recurrent exacerbation) than in those who did not (mean = 3.4 mg/dl) (14). A recent trial demonstrated the utility of point-of-care measurement of CRP at the onset of an AECOPD to target antibiotic treatment successfully, without negative effects on health status outcomes (15). At present, there are no studies assessing the efficacy of further antibiotic treatment for nonrecovery of an AECOPD. We therefore evaluated a novel treatment approach using point-of-care measurement of CRP and/or respiratory symptom review to guide antibiotic retreatment 14 days after an index exacerbation. Our aim was to determine whether retreatment at Day 14 would prolong the time until the next exacerbation and hasten recovery of the index event.
This work was presented as an abstract at the 29th Annual Congress of the European Respiratory Society, 2019 (16).
Methods
Study Design
This study was a randomized, parallel-group, double-blind placebo-controlled trial performed in four academic health centers in the United Kingdom. Patients were assessed for incomplete recovery at 14 ± 3 days after the first day of antibiotic treatment for an AECOPD. Eligible patients were then randomized in a 1:1 ratio to receive oral ciprofloxacin 500 mg or matched placebo twice daily for 7 days. Patients were instructed to continue their current COPD inhaled or oral treatment, but if further oral corticosteroids or antibiotics other than the investigational medicinal product were clinically indicated at randomization, these individuals were excluded. Permuted block randomization with stratification for site and current smoking status was used via an online portal (Sealed Envelope Ltd).
The trial received approval from the National Research Ethics Committee, West Midlands – Edgbaston (13/WM/0364). All patients gave written informed consent. The trial was registered on EudraCT 2012-002198-72 and ClinicalTrials.gov (Identifier: NCT02300220).
Patients
Patients whose exacerbations were treated with antibiotics and/or systemic corticosteroids in accordance with Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidance (17) were screened for participation in this trial. The main inclusion criteria were persistent symptoms and/or a serum CRP of ≥8 mg/L on Day 14 after the initial treatment. This CRP cutoff was selected based on previous work from our group, in which we examined Day 14 CRP levels in 73 patients with AECOPD (14). Persistent symptoms were defined as any one or more of the following: increased breathlessness, increased sputum volume, and increased sputum color compared with baseline. CRP was measured at the point of care using a Quikread Go CRP analyzer (Orion Diagnostica). Eligible patients had an established diagnosis of GOLD stage II–IV COPD (based on clinical history and spirometry), were ≥45 years of age, and were current smokers or had a smoking history of ≥10 pack-years. A full list of the inclusion and exclusion criteria is provided in the online supplement.
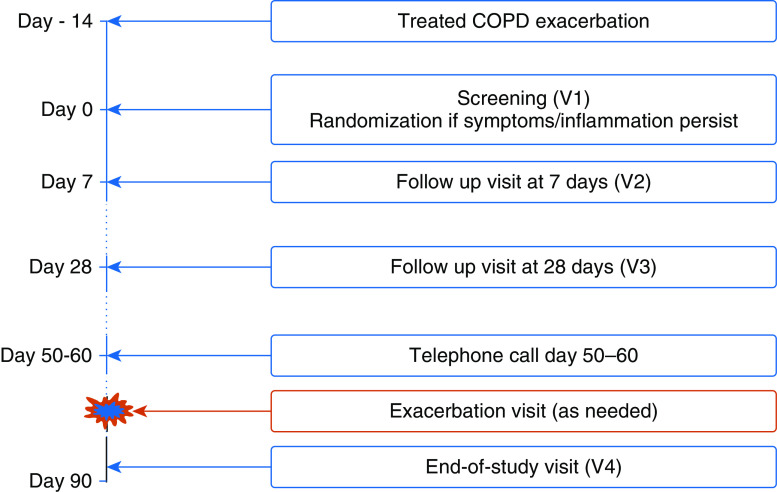
Patients were followed for 90 days after randomization and underwent assessment visits on Days 7 (visit 2 [V2]), 28 (V3), and 90 (V4) after randomization. The participants were asked to record worsening or new daily respiratory symptoms on diary cards and to self-report and attend for exacerbation visits if necessary (Figure 1). New exacerbations during follow-up were assessed by the study physician based on the patient’s symptoms and treatment record on the diary card or when seen in the clinic. An exacerbation was defined as an increase in respiratory symptoms for two consecutive days, with at least one major symptom (dyspnoea, sputum purulence, or sputum volume) plus either another major symptom or a minor symptom (wheeze, cold, sore throat, or cough). The first day of these increased symptoms was defined as the day of onset of the exacerbation (10). The full trial protocol is available in the online supplement.
Figure 1.
The trial study design. COPD = chronic obstructive pulmonary disease; V = visit.
At clinic visits, post-bronchodilation spirometry was measured with a Flow Screen II spirometer (eResearchTechnology GmbH) in accordance with American Thoracic Society/European Respiratory Society guidance (18). Blood samples were collected for measurement of CRP in the hospital laboratory for a longitudinal analysis of CRP during the study. Vital signs were recorded and respiratory symptoms were assessed using the COPD assessment test (CAT) questionnaire. At randomization and at Day 90, the St. George’s Respiratory Questionnaire (SGRQ) was completed by the patient. Spontaneous sputum samples were obtained when possible and sent for routine bacterial culture; sputum induction was not performed.
Main Efficacy Outcomes
The primary outcome was the time from randomization to the next exacerbation within a 90-day period. Prespecified secondary outcomes included the duration of the initial exacerbation and changes in CRP and health status (assessed by SGRQ and CAT).
Sample Size
The required sample size was calculated as 72 patients per group, 144 in total, to detect a difference in the primary endpoint at a two-sided significance level of 0.05 with 80% power. This was a novel study design, and in the absence of available prior data, the power calculation was based on a survival analysis assuming proportional hazards with reexacerbation within 90 days of 70% in the placebo arm and 45% in the treatment arm (hazard ratio [HR], 0.46). This sample size allowed for a dropout rate of 5% and recruitment to target within the study centers.
Statistical Analysis
The primary endpoint was assessed using a Cox’s proportional hazard model with prespecified adjustment for patients’ self-reported history of exacerbations over the previous year and with stratification for study center. Survival data were censored at 90 days or at patient withdrawal. Patient demographics are reported as mean (SD), median (interquartile range [IQR]), or percentage. Differences in patient characteristics between sites were compared by ANOVA, Kruskal-Wallis, or chi-squared test as appropriate. Differences in the number of exacerbations during the 90 days of observation were assessed using a negative binomial regression with adjustment for time under observation. CAT scores were analyzed by random effect linear models to accommodate repeated measurements on the same patient. All analyses were performed using STATA 12.1 (StataCorp LLC) and according to the principal of intention-to-treat. All statistical tests were two-sided and a P value < 0.05 was considered significant. Apart from the primary outcome, all statistical analyses should be considered as exploratory.
The full study protocol and prespecified statistical analysis plan are included in the online supplement.
Results
Patients
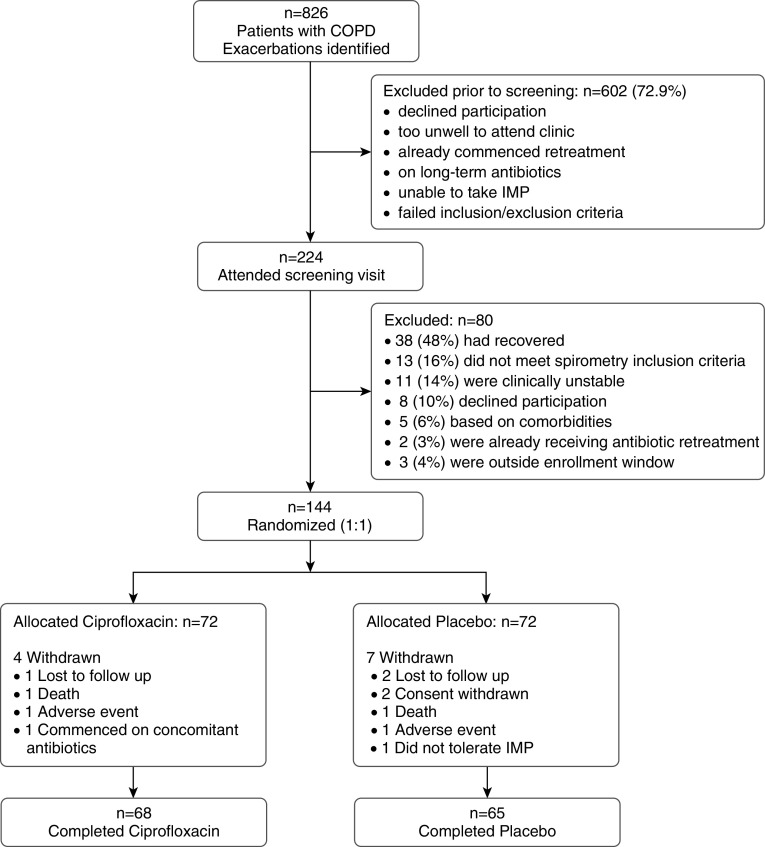
Between July 2014 and November 2017, 826 patients with an AECOPD attending an emergency department or respiratory outpatient clinic were identified at the four hospitals. A total of 224 of these patients were screened at 14 days and 144 were randomized to either ciprofloxacin (n = 72) or placebo (n = 72) (Figure 2). The most common reason for screening failure was recovery in symptoms of the index exacerbation (n = 38 [17%]; see Figure 2). Demographic and clinical characteristics of the randomized subjects were well balanced across treatment arms (Table 1). There were no significant differences in adherence between groups; the median number of treatment doses taken by patients was 14 (IQR, 14–14) in the ciprofloxacin arm and 14 (IQR, 14–14) in the placebo arm (chi-squared test, P = 0.945).
Figure 2.
Consolidated Standards of Reporting Trials diagram of the targeted retreatment of exacerbations in chronic obstructive pulmonary disease (COPD): a double-blind, randomized, placebo-controlled, multicenter, phase III trial. IMP = investigational medicinal product.
Table 1.
Characteristics of Patients at Randomization, by Treatment Group
| Ciprofloxacin (n = 72) | Placebo (n = 72) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, yr | 69.1 (8.8) | 69.1 (7.4) | 1.000 |
| Weight, kg | 77.4 (16.5) | 77.1 (21.0) | 0.909 |
| Height, m | 1.68 (0.11) | 1.70 (0.09) | 0.295 |
| Body mass index | 27.5 (5.8) | 26.5 (6.0) | 0.312 |
| Sex, M, n (%) | 44 (61.1) | 47 (65.2) | 0.604 |
| Current smoker, n | 24 | 26 | 0.726 |
| Pack-years | 48.7 (3.5) | 43.3 (23.9) | 0.231 |
| Annual exacerbation rate (IQR) | 3 (1–4) | 2 (1–4) | 0.879 |
| Anthonisen criteria | |||
| Type I | 11 | 11 | 0.763 |
| Type II | 15 | 19 | 0.467 |
| Type III | 31 | 32 | 0.859 |
| Examination findings | |||
| FEV1, L | 1.29 (0.52) | 1.38 (0.51) | 0.269 |
| FEV1% predicted | 48.7 (16.5) | 50.0 (14.9) | 0.608 |
| FVC, L | 2.79 (0.89) | 2.91 (0.76) | 0.380 |
| FEV1/FVC | 0.47 (0.14) | 0.48 (0.13) | 0.734 |
| Oxygen saturation, % | 94.8 (2.24) | 95.0 (1.97) | 0.555 |
| Respiration rate | 17.4 (2.7) | 17.8 (2.8) | 0.322 |
| Point-of-care sampling | |||
| CRP | 15.2 (12.2) | 19.9 (16.3) | 0.158 |
| Disease burden questionnaires | |||
| SGRQ total | 53.4 (18.1) | 52.4 (17.2) | 0.732 |
| CAT | 21.1 (8.3) | 19.8 (7.7) | 0.324 |
Definition of abbreviations: CAT = chronic obstructive pulmonary disease assessment test; CRP = C-reactive protein; IQR = interquartile range; SGRQ = St. George’s Respiratory Questionnaire.
Data are shown as mean (SD) unless otherwise noted.
Clinical Presentation
Among the 144 participants, 119 reported continuing symptoms (22 with type I, 34 with type II, and 63 with type III Anthonisen criteria exacerbations based on symptom questionnaires recorded at randomization) (Table 1). There was a good balance between treatment arms. In addition to the 22 participants with type I exacerbations, there were 19 patients with persistent sputum purulence. Sixty-four of the randomized participants had a positive CRP. Overall, the mean CRP for included participants was 15.3 mg/L (SD = 9.23); 73 patients had ongoing sputum purulence and/or Anthonisen type I and/or a raised CRP.
Primary Endpoint
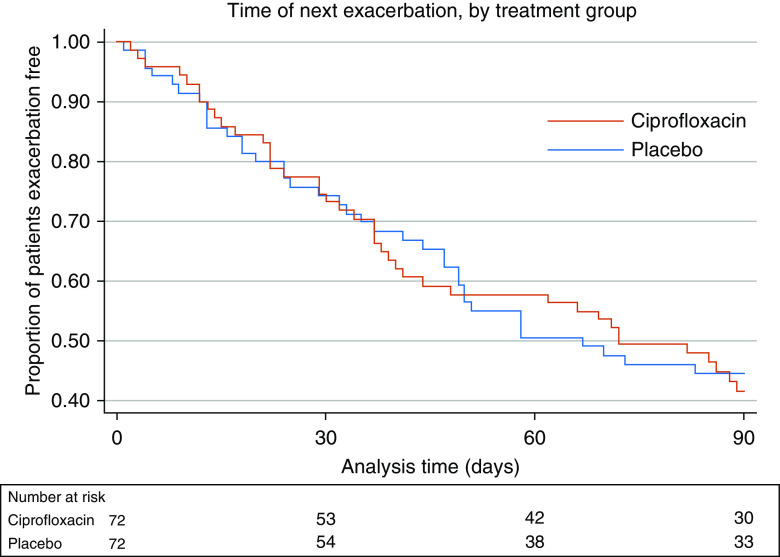
At 90 days after commencing retreatment, 41 patients (56.9%) in the ciprofloxacin group and 38 (52.8%) in the placebo group had experienced one or more exacerbations. The median time to the first exacerbation was 34 days (IQR, 17–62) in the ciprofloxacin group and 32 days (IQR, 13–50) in the placebo group. There was no significant difference between treatment arms in a prespecified Cox proportional hazard model analysis that adjusted for the number of exacerbations in the previous year and stratified by center (HR, 1.071; 95% confidence interval [CI], 0.684–1.676; P = 0.764; Figure 3). Patients with a history of more exacerbations in the previous year were at greater risk of another event (HR, 1.18; 95% CI, 1.060–1.319; P = 0.003).
Figure 3.
Kaplan-Meier survival estimate demonstrating the primary endpoint: the proportion of patients who were free of exacerbation.
Subgroup Analyses
The primary outcome findings were unchanged if only those exacerbations included for reasons of persistent respiratory symptoms or only those with a raised CRP at 14 days were examined (see Table 2).
Table 2.
Summarizing the Subgroup Analysis of the Study Primary Outcome
| Reason for Inclusion | Hazard Ratio | Lower CI | Upper CI | P Value |
|---|---|---|---|---|
| Persistent symptoms (n = 128)* | 1.167 | 0.734 | 1.856 | 0.513 |
| CRP ≥8 mg/dl (n = 64) | 0.968 | 0.480 | 1.952 | 0.928 |
| No persistent symptoms but CRP ≥8 mg/dl (n = 16) | 0.667 | 0.073 | 6.06 | 0.719 |
Definition of abbreviations: CI = confidence interval; CRP = C-reactive protein.
The group with persistent symptoms included 48 participants with CRP ≥8 mg/dl.
There was a significant difference between the two major recruitment sites (Royal Brompton, London [n = 106] and Aintree, Liverpool [n = 25]), with a greater risk of a subsequent exacerbation at Aintree (HR, 2.14; P = 0.006).
Secondary Endpoints
Duration of symptoms after randomization
Data were available for 113 patients (78%); 25 participants failed to adequately complete diary cards and 6 participants continually recorded chronic symptoms. Symptoms persisted after retreatment for a median number of 3 days (IQR, 0–8; n = 56) with ciprofloxacin, and 4 days (IQR, 0–9; n = 57) with placebo (Mann-Whitney P = 0.703).
Exacerbation rate during follow-up
Over 90 days, there were slightly more exacerbations in the ciprofloxacin group (median = 1 [IQR, 0–1]; mean = 0.86 [SD = 0.91]) than in the placebo group (median = 1 [IQR, 0–1]; mean = 0.74 [SD = 0.90]). In an unadjusted analysis, there was no difference in exacerbation rate between the two arms, with an incident rate ratio for ciprofloxacin compared with placebo of 1.14 (95% CI, 0.78–1.66; P = 0.498).
Lung function changes
There were no significant between-group differences in changes in FEV1, FVC, or the FEV1/FVC ratio at any time point (Figure 4 and Table E2 in the online supplement). Once data that were collected after the second exacerbation (occurring in the 90-day study period) were excluded, significant improvements were seen in FEV1 and FVC, but not in the FEV1/FVC ratio, in both treatment groups after the index exacerbation (Figure E4). However, there were no significant differences between treatment groups in this analysis.
Figure 4.
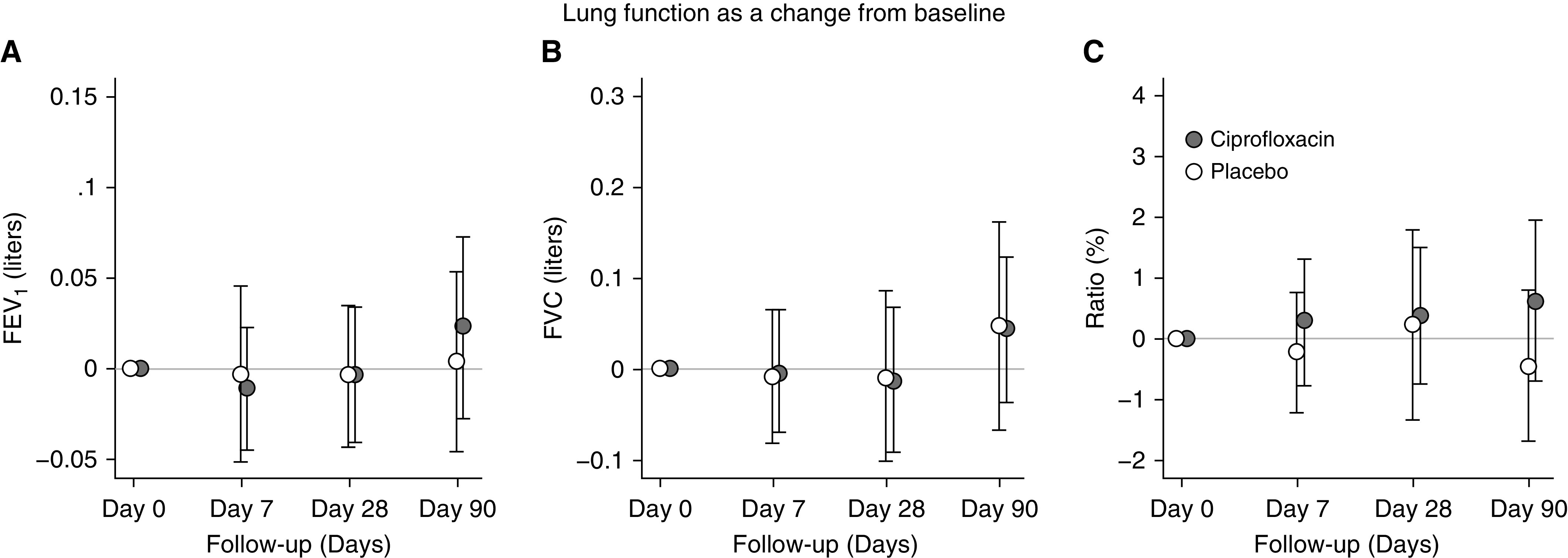
Effect of the intervention on changes in spirometry by treatment group. (A–C) Changes in FEV1 (A), FVC (B), and FEV1/FVC ratio (C).
Quality-of-life questionnaire assessments
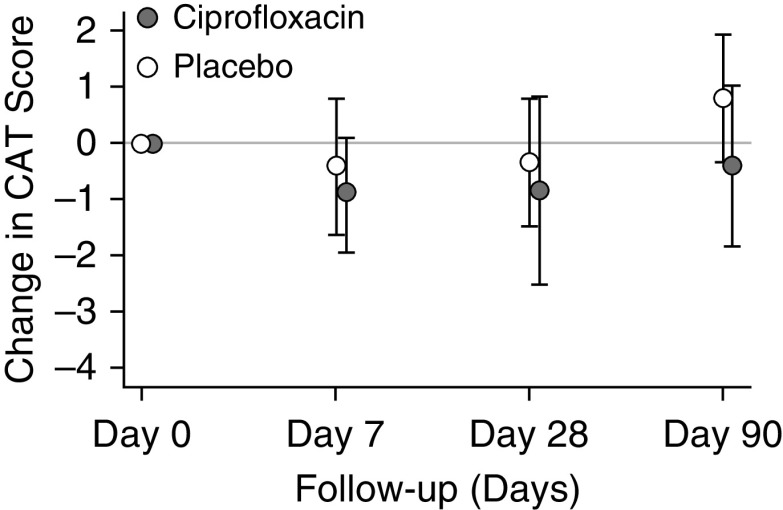
In a comparison of treatment arms, there was no difference in change in total or component SGRQ scores between randomization and Day 90. (Table 3). The CAT scores also did not differ between ciprofloxacin and placebo (0.77 higher for ciprofloxacin vs. placebo; 95% CI, −1.71 to 3.27; P = 0.540) (Figure 5).
Table 3.
Changes in Total, Impact, Activity, and Symptom Scores in the SGRQ between Visits 1 and 4
| Visit 1 (71 Placebo/69 Cipro) | Visit 4 (64 Placebo/63 Cipro) | Visit 4 − Visit 1 (63 Placebo/61 Cipro) | P Value (Visit 4 − Visit 1, by Treatment) | |
|---|---|---|---|---|
| SGRQ total, placebo | 52.4 (17.2) | 47.8 (16.0) | −3.37 (11.5) | |
| SGRQ total, cipro | 53.4 (18.1) | 50.5 (17.8) | −1.93 (9.1) | 0.440 |
| SGRQ impact, placebo | 40.8 (20.0) | 38.5 (18.4) | −1.97 (12.8) | |
| SGRQ impact, cipro | 39.0 (20.0) | 35.8 (17.8) | −0.74 (10.8) | 0.560 |
| SGRQ activity, placebo | 68.2 (21.8) | 65.1 (20.2) | −1.74 (17.1) | |
| SGRQ activity, cipro | 67.3 (21.7) | 67.8 (22.5) | 1.17 (12.8) | 0.283 |
| SGRQ symptoms, placebo | 66.4 (15.3) | 54.3 (19.3) | −11.3 (20.1) | |
| SGRQ symptoms, cipro | 68.9 (16.6) | 55.7 (22.0) | −11.5 (21.5) | 0.949 |
Definition of abbreviations: cipro = ciprofloxacin; SGRQ = St. George’s Respiratory Questionnaire.
Data are shown as mean (SD).
Figure 5.
Effect of the intervention on changes in chronic obstructive pulmonary disease assessment test (CAT) scores by treatment group.
CRP
CRP was significantly lower at V2 and V3 than at randomization, but there were no differences between treatment groups. All 144 patients with exacerbations were treated with antibiotics; 117 were also treated with oral prednisolone and 27 were treated with antibiotics alone. The prednisolone-treated patients with exacerbations had a CRP of 8 mg/dL (IQR, 3–20; n = 26), compared with 7.8 mg/dl (IQR, 3–9.9; n = 115) for those with antibiotic-only–treated exacerbations (Mann-Whitney P = 0.35). CRP was not recorded for three exacerbations.
Index exacerbation characteristics
At randomization, 46 out of 144 subjects (31.9%) provided sputum for bacterial culture, which yielded a positive result in 45.7%. Ciprofloxacin-resistant organisms were not present in sputum cultures that were subsequently collected from any participants receiving ciprofloxacin (Tables 4 and 5).
Table 4.
Changes in CRP Seen in the Ciprofloxacin and Placebo Groups at Study Visits
| Visit 1 (72 Placebo/71 Ciprofloxacin) | Visit 2 (65 Placebo/66 Ciprofloxacin) | Visit 3 (64 Placebo/63 Ciprofloxacin) | Visit 4 (63 Placebo/63 Ciprofloxacin) | |
|---|---|---|---|---|
| CRP placebo | 15.1 (17.2) | 11.0 (17.2) | 9.8 (17.7) | 9.0 (15.2) |
| CRP ciprofloxacin | 10.9 (12.3) | 9.8 (13.1) | 7.0 (17.7) | 15.2 (46.9) |
| As change from baseline | — | 63 placebo/66 ciprofloxacin | 62 placebo/62 ciprofloxacin | 61 placebo/62 ciprofloxacin |
| CRP placebo | — | −5.1 (22.1) | −5.3 (17.0) | −6.4 (20.1) |
| CRP ciprofloxacin | — | −1.7 (14.7) | −4.6 (12.4) | 3.9 (48.0) |
| P value, t test | — | 0.310 | 0.815 | 0.124 |
| Median (IQR) | ||||
| CRP placebo | — | −2 (−13 to 0) | −1.75 (−9 to 0.1) | −2 (−11.5 to 1) |
| CRP ciprofloxacin | — | 0 (−5 to 2) | −1 (−5 to 1) | −1 (−4 to 0) |
| P value, Mann-Whitney test | — | 0.030 | 0.741 | 0.195 |
Definition of abbreviations: CRP = C-reactive protein; IQR = interquartile range.
Data are shown as mean (SD) unless otherwise noted.
Table 5.
Summary of Bacterial Cultures of Spontaneous Sputum Samples
| Ciprofloxacin [n (%)] | Placebo [n (%)] | |
|---|---|---|
| Baseline | ||
| Number of patients with a sputum sample | 25 (34.7) | 22 (30.5) |
| Number of bacterial cultures positive for a pathogen | 15 (20.8) | 12 (16.6) |
| H. influenzae | 6 (8.3) | 4 (5.5) |
| S. pneumoniae | 1 (1.4) | 2 (2.8) |
| P. aeruginosa | 2 (2.8) | 3 (4.2) |
| M. catarrhalis | 3 (4.2) | 1 (1.4) |
| S. aureus | 0 | 1 (1.4) |
| Other gram-negative bacteria | 3 (4.2) | 1 (1.4) |
| Cultures with ciprofloxacin-resistant bacteria | 1 (1.4) | 0 |
| Day 90 | ||
| Number of patients with a sputum sample | 16 (22.2) | 17 (23.6) |
| Number of patients with bacterial culture positive for a pathogen | 7 (9.7) | 7 (9.7) |
| Number of patients with newly acquired ciprofloxacin resistance | 0 | 1 (1.4) |
Definition of abbreviations: H. influenzae = Haemophilus influenzae; M. catarrhalis = Moraxella catarrhalis; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus; S. pneumoniae = Streptococcus pneumoniae.
Adverse events
There were no clinically important between-group differences in adverse events (Tables 6 and 7).
Table 6.
Summary of Adverse Events
| Adverse Events | Ciprofloxacin | Placebo |
|---|---|---|
| Gastrointestinal, n | ||
| Nausea | 1 | 1 |
| Vomiting | 0 | 1 |
| Diarrhea | 5 | 1 |
| Dyspepsia | 1 | 0 |
| Abdominal colic/pain | 2 | 2 |
| Miscellaneous, n | ||
| Pruritis/rash | 0 | 2 |
| Dry mouth | 1 | 0 |
| Ankle pain/tendinitis | 2 | 0 |
| Tremor | 0 | 1 |
Table 7.
Summary of Serious Adverse Events
| Serious Adverse Events | Ciprofloxacin | Placebo |
|---|---|---|
| Fatal, n | ||
| Gastrointestinal | 0 | 1 |
| Respiratory | 1 | 0 |
| Nonfatal, n | ||
| Cardiovascular | 0 | 1 |
| Gastrointestinal | 0 | 2 |
| Psychological | 0 | 1 |
| Oncology | 1 | 1 |
| Respiratory | 0 | 4 |
Two patients (one from each treatment arm) died within the 90-day follow-up window. One died of an AECOPD and the other died of bowel ischemia and multiorgan failure; these deaths were not considered to be related to the trial interventions (Table 7). During the 90 days of follow-up, 5 of 72 patients (6.9%) with available data in the ciprofloxacin group and 6 of 72 patients (8.3%) with available data in the placebo group were hospitalized.
Discussion
This is the first study to examine retreatment with antibiotics of incompletely recovered AECOPD. We observed no effect of retreatment with ciprofloxacin compared with placebo on the primary outcome of time to the next exacerbation. There were also no effects on lung function, quality of life, CRP, or the duration of symptoms after the index exacerbation. The intervention was well tolerated, with no significant differences in the frequency of adverse events.
Although most COPD exacerbations last for approximately 10 days, some may last longer, and at 5 weeks, 25% may not have fully recovered (10). Another feature of COPD exacerbations is the increased risk of developing another exacerbation within an 8-week period (9, 14), and we have previously reported that a raised serum CRP concentration measured 14 days after a first exacerbation is predictive of a second, suggesting that failure to normalize the inflammatory response may predispose to another (recurrent) exacerbation (14). Exacerbations are associated with heterogeneous inflammatory processes, mainly neutrophilic, and usually triggered by respiratory viral infections followed by a secondary bacterial infection (19, 20) or primary bacterial infection. For these reasons, we aimed in this study to test an innovative approach for using antibiotics to prevent recurrent COPD exacerbations: the targeted retreatment of patients after a first exacerbation who have incomplete symptom or inflammatory recovery.
The lack of an effect of ciprofloxacin in this trial would suggest that by Day 14, the persistent symptoms and elevated CRP were driven predominantly by a residual airway inflammatory load without a significant bacterial infective component, and this is reinforced by the low rates of bacterial sputum culture positivity in these patients. Further studies are required to study the mechanisms of exacerbation recovery so that potential antiinflammatory therapies can be targeted. A recent study examined the use of an oral macrolide (azithromycin) at hospitalization and for 3 months after discharge (21, 22). Although the trial was underrecruited, a trend favored the azithromycin intervention over placebo for preventing subsequent AECOPD (treatment failure 49% vs. 60%; P = 0.0526). Taking our observations into account, this finding could be the result of antiinflammatory immunomodulatory effects of macrolides rather than antimicrobial action (23).
The time to next exacerbation is a commonly used outcome measure in clinical trials (24). In this study, all exacerbations were moderate to severe, and many of the patients were treated or hospitalized by independent physicians. The CAT scores and CRP concentrations of patients when they initially presented with exacerbation were similar to those reported at exacerbation presentation in other studies (21, 25, 26). The retreatment antibiotic, ciprofloxacin, was selected as a reasonable second-line antibiotic choice based on global guidelines for bacterial respiratory infection (17, 27, 28). It is important to acknowledge that the dose selected was not optimized to cover for active Pseudomonas infection (usually 750 mg/12 h for 14 d). However, we observed that only five participants (3.5%) yielded a positive bacterial culture for Pseudomonas, which suggests that this was not a major contributor to reexacerbation. It is also possible that the immunomodulatory effects of macrolides would have had a greater effect. The recruitment target was met, but even if the sample size had been larger, it is unlikely that a beneficial effect would have been detected. Importantly, the observed rate of a subsequent exacerbation was lower than anticipated by the power calculation. Our study anticipated a large effect size (HR, 0.46), which we believed would be meaningful to patients but created the possibility of type II error, or failure to detect a smaller beneficial effect of retreatment when a beneficial effect exists. However, the study recruited to target, and the absence of any trend favoring treatment makes this less likely. It is possible that a divergence in outcome between the ciprofloxacin and placebo groups might have developed had the participants been followed for 12 months rather than the 90 days we specified. However, previous work from our group suggested that a further exacerbation occurred in 36% of cases by 50 days (14). Furthermore, patients were recruited from emergency and outpatient clinic departments rather than an established longitudinal cohort, so commitment to a 12-month follow-up period could have increased the difficulty of recruitment. Thus, 90 days was considered a reasonable length of time to expect individuals to participate and mitigate the loss to follow-up.
We chose CRP as a familiar clinical parameter that can be measured with a relatively inexpensive point-of-care device. The utility of point-of-care testing in the context of AECOPD is also emerging. Butler and colleagues showed that at AECOPD onset, rapid detection of CRP can successfully guide antibiotic use (15). In this study, we used point-of-care CRP testing at Day 14 to assess nonrecovery. However, CRP cannot distinguish between a bacteria-triggered exacerbation and a virus-triggered one. Approximately half of all AECOPDs (22–64%) are triggered by a respiratory virus, particularly human rhinovirus (20). Empirical antibiotics do still offer a benefit (7), as recent work suggests that rhinovirus directly influences the lung’s innate immunity to impair bacterial phagocytosis, allowing bacterial outgrowth (20, 29).
Spirometry data collected at the V2–V4 time points may have been influenced by secondary exacerbations. However, once we excluded lung function measures collected after a second exacerbation within the 90-day study period, we observed significant improvements in FEV1 and FVC, but not the FEV1/FVC ratio, in both treatment groups after the index exacerbation. This is in line with exacerbation recovery but there were no significant differences between treatment groups.
Another way to improve the targeted retreatment would be to obtain a bacterial sputum culture; however, as observed in our study and in others with similar cohort demographics (21), many patients do not spontaneously expectorate 14 days after an index exacerbation. In our study, only 44 individuals (31.9%) were able to provide sputum, and a bacterial positive yield was obtained in 18.7% (20.8% active, 16.6% placebo).
During recruitment for this study, 38 out of 80 patients (48%) were excluded at the screening visit because they had recovered. This fits with our understanding of the normal time course of AECOPD (10). However, a notable finding was that 29% of individuals who had been identified as suitable for the study were excluded because they had already commenced antibiotic retreatment before screening at Day 14. This suggests that a sizable subpopulation of individuals with AECOPD experience treatment failure or require treatment intensification, a step-up in hospital care, or readmission early. The results of this study would suggest that there is scope to reduce unnecessary antibiotic retreatment.
The strengths of this study include the facts that it involved multiple centers and met the prespecified recruitment target. We found that the duration of symptoms after randomization as one of the secondary outcomes could not be determined easily because patients poorly recorded symptoms on the diary cards. Among the 144 participants, 43 did not record any increase in symptoms on Day 1 and Day 2 after randomization, 6 patients continuously recorded symptoms during the whole follow-up period, and 25 participants never recorded any symptoms. This was surprisingly poor compliance compared with our experience with the London COPD cohort, and could be explained by the participants’ inexperience with daily diary cards, as well as the fact that they comprised a postexacerbation cohort rather than stable cohort.
Conclusions
This randomized placebo-controlled trial showed no benefit from treatment with ciprofloxacin in patients with exacerbations and persistent respiratory symptoms or a raised CRP (≥8 mg/dl) at 14 days after exacerbation onset. This suggests that nonrecovered exacerbations, which may lead to hospital readmission, potentially need to be targeted with antiinflammatory therapies, and further research is now warranted to investigate exacerbation recovery.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the independent members of the trial steering committee, including Dr. Angshu Bhowmik (Chair), Prof. Rob Stockley, Prof. Marc Lipman, Dr. Daniel Jackson (statistical input), and Joan McCarthy (lay member). The authors also thank Dr. William Man and Dr. Namrata Syngal for their help in recruiting participants for the study.
Footnotes
Supported by the National Institute for Health Research under the Program Grants for Applied Research program (RP-PG-0109-10056). The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Author Contributions: S.E.B., B.H.V., J.P.A., L.A.-M., P.P.W., G.C.D., P.M.A.C., and J.A.W. contributed to the study design, protocol, and materials. A.I.R., S.E.B., B.H.V., L.J.F., J.P.A., L.A.-M., D.J.W., P.P.W., E.B., S.L.E., and P.M. contributed to patient recruitment and collection of study data at the participating centers. M.L. designed the statistical plan and performed prestudy power calculations. G.C.D. performed the statistical analysis. A.I.R. and S.E.B. wrote the first draft of the manuscript. All authors contributed to interpretation of the data and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-2058OC on April 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 4.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7:214–228. doi: 10.3109/15412555.2010.481697. [DOI] [PubMed] [Google Scholar]
- 7.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 8.Roede BM, Bresser P, Bindels PJ, Kok A, Prins M, ter Riet G, et al. Antibiotic treatment is associated with reduced risk of a subsequent exacerbation in obstructive lung disease: an historical population based cohort study. Thorax. 2008;63:968–973. doi: 10.1136/thx.2008.095349. [DOI] [PubMed] [Google Scholar]
- 9.Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:369–374. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 10.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 11.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57:137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Müller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 13.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Müllerova H, Donaldson GC, et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29:527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 15.Butler CC, Gillespie D, White P, Bates J, Lowe R, Thomas-Jones E, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381:111–120. doi: 10.1056/NEJMoa1803185. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie A, Brill SE, Vlies BH, Finney LJ, Allinson JP, Alves-Moreira L, et al. Presented at the 29th Annual Congress of the European Respiratory Society. Sept 28–Oct 2, Madrid, Spain. p. PA3386.
- 17.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo MB, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George SN, Garcha DS, Mackay AJ, Patel ARC, Singh R, Sapsford RJ, et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44:87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 21.Vermeersch K, Gabrovska M, Aumann J, Demedts IK, Corhay J-L, Marchand E, et al. Azithromycin during acute chronic obstructive pulmonary disease exacerbations requiring hospitalization (BACE): a multicenter, randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2019;200:857–868. doi: 10.1164/rccm.201901-0094OC. [DOI] [PubMed] [Google Scholar]
- 22.Wedzicha JA, Ritchie AI, Martinez FJ. Can macrolide antibiotics prevent hospital readmissions? Am J Respir Crit Care Med. 2019;200:796–798. doi: 10.1164/rccm.201905-0957ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal LN, Clemente JC, Wu BG, Wikoff WR, Gao Z, Li Y, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax. 2017;72:13–22. doi: 10.1136/thoraxjnl-2016-208599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathioudakis AG, Moberg M, Janner J, Alonso-Coello P, Vestbo J. Outcomes reported on the management of COPD exacerbations: a systematic survey of randomised controlled trials. ERJ Open Res. 2019;5:00072-2019. doi: 10.1183/23120541.00072-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185:1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]
- 26.Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 27.Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. European Respiratory Society; European Society of Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–1180. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 28.Balter MS, La Forge J, Low DE, Mandell L, Grossman RF. Canadian guidelines for the management of acute exacerbations of chronic bronchitis. Can Respir J. 2003;10(Suppl B):3b–32b. doi: 10.1155/2003/486285. [DOI] [PubMed] [Google Scholar]
- 29.Finney LJ, Belchamber KBR, Fenwick PS, Kemp SV, Edwards MR, Mallia P, et al. Human rhinovirus impairs the innate immune response to bacteria in alveolar macrophages in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;199:1496–1507. doi: 10.1164/rccm.201806-1095OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.