To the Editor:
Coronavirus disease (COVID-19) is a rapidly expanding global pandemic. In March 2020, models forecasted imminent exhaustion of regional ventilator supply in New York (1). In response, we developed a novel ventilator sharing strategy to support two patients simultaneously with one ventilator. This report details our initial ventilator sharing experience among patients with COVID-19–associated acute respiratory distress syndrome (ARDS).
Methods
Ventilator sharing commenced as a public health preparedness initiative approved by the hospital leadership and ethics committee and by New York State (2). The clinical protocol and detailed methods are provided in the online supplement and https://protocols.nyp.org.
Patient selection
Respiratory physiology, infectious status, and clinical stability were evaluated to identify compatible patient pairs using prespecified criteria (Table 1). The protocol prioritized minimizing between-patient difference in driving pressure given the importance of protective tidal ventilation in ARDS (3).
Table 1.
Recommended Initial Patient Compatibility Criteria
| Parameter | Acceptable Limit in Either Patient | Acceptable Difference between Patients (Patient A − Patient B) |
|---|---|---|
| Anticipated time needing invasive ventilation, h | 72 or higher | — |
| Vt, ml/kg PBW | 4–8 | — |
| Driving pressure (∆P = plateau pressure − PEEP), cm H2O | 5–16 | 0–6* |
| Respiratory rate, breaths/min | 12–30 | 0–8 |
| PEEP, cm H2O | 5–18 | 0–5 |
| FiO2, % | 21–60 | — |
| pH | 7.30 or higher | — |
| Oxygen saturation, % | 92–100 | — |
| Ventilator titration | No recent major changes as judged clinically | — |
| Neuromuscular blockade | No contraindication to initiation if not already receiving | — |
| Respiratory infectious status | Both patients have same respiratory pathogen | None |
| Asthma or COPD | No severe baseline disease nor current exacerbation | — |
| Hemodynamic stability | No rapid vasopressor increase | — |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PBW = predicted body weight; PEEP = positive end-expiratory pressure.
If patients do not meet all criteria, pairing them on a single ventilator is not recommended. Further details are provided in the full protocol (see the online supplement). PBW denotes predicted body weight in kg, calculated for males as PBW = 50 + 2.3 [height (inches) – 60] and for females as PBW = 45.5 + 2.3 [height (inches) – 60].
Acceptable differences for between-patient parameters were specified only for driving pressure, respiratory rate, PEEP, and respiratory infectious status.
Between-patient difference in driving pressure is the most important parameter to minimize in assessing potential compatibility of two patients.
In eligible patient pairs, deep sedation and neuromuscular blockade were initiated. Ventilators were set to pressure control with the initial driving pressure and inspiratory time adjusted to maintain each patient’s baseline Vt. Then, driving pressure, inspiratory time, respiratory rate, and positive end-expiratory pressure (PEEP) were adjusted per protocol (see the online supplement), typically using the average value between patients unless otherwise indicated, to achieve identical settings for both patients. Inspired oxygen was adjusted as needed to maintain SaO2 of 95% or higher. Thereafter, a safety check confirmed that Vt, minute volume, auto-PEEP, and arterial blood gas results remained within acceptable ranges. After demonstrating tolerance to identical settings on separate ventilators, patients were transitioned to a shared ventilator with the same settings.
Circuit configuration
Circuit configuration safety features included redundant antimicrobial filters and patient-specific monitoring of Vt, airway pressure, and capnography (see the online supplement). Proper dual-circuit functioning was confirmed with test lungs before transitioning patients.
Patient management
Neuromuscular blockade and the pressure control mode were used during ventilator sharing to prevent respiratory effort or mechanical changes in one patient from affecting the other. Additional patient care considerations were followed per protocol. Patient care was directed by the patients’ usual clinical team with support and assistance as needed from the ventilator sharing consult. The consult consisted of either of two intensivists (J.R.B. or A.M.M.) intimately familiar with the protocol, who alternated around-the-clock call whenever patients were on a shared ventilator. Ventilator sharing was predetermined not to exceed 2 days unless ventilator supply was exhausted.
Statistical analysis
Data were recorded during and for 48 hours before and after ventilator sharing. Time-series plots were created in SAS v9.4 (SAS Institute) without imputing missing data.
Results
Six patients (three pairs) with ARDS from COVID-19 underwent ventilator sharing during 1 week in March 2020 (Table 2).
Table 2.
Characteristics of Patients Undergoing Ventilator Sharing
| Characteristic | Patient 1A | Patient 1B | Patient 2A | Patient 2B | Patient 3A | Patient 3B |
|---|---|---|---|---|---|---|
| Age, yr | 62 | 74 | 58 | 73 | 43 | 59 |
| Sex | Female | Male | Female | Female | Male | Male |
| Height, cm | 162.6 | 182.9 | 157.5 | 175.0 | 165.1 | 190.5 |
| Weight, kg | 68.0 | 98.9 | 122.5 | 85.0 | 80.0 | 105.9 |
| Body mass index, kg/m2 | 25.7 | 29.6 | 49.4 | 27.8 | 29.3 | 29.2 |
| Predicted body weight, kg | 54.8 | 77.8 | 50.1 | 66.1 | 61.6 | 84.7 |
| Vasopressor-dependent shock before sharing | Yes | Yes | Yes | Yes | Yes | Yes |
| Modified SOFA score before sharing* | 10 | 10 | 12 | 13 | 10 | 11 |
| Days hospitalized before intubation | 1.0 | 3.1 | 1.1 | 1.0 | 1.0 | 6.1 |
| Days intubated before ventilator sharing | 5.6 | 2.5 | 0.7 | 7.9 | 1.8 | 3.6 |
| Respiratory parameters prior to matching | ||||||
| Ventilator mode | Volume control | Volume control | Volume control | Volume control | Volume-targeted pressure control | Volume-targeted pressure control |
| Vt, ml (ml/kg PBW) | 330 (6.0) | 480 (6.2) | 400 (8.0) | 490 (7.4) | 375 (6.1) | 370 (4.4) |
| Driving pressure, cm H2O | 14 | 16 | 24 | 22 | 19 | 20 |
| Peak inspiratory pressure, cm H2O | 31 | 25 | 30 | 34 | 37 | 30 |
| Plateau pressure, cm H2O | 28 | 24 | 34 | 32 | 31 | 30 |
| Respiratory rate, breaths/min | 26 | 19 | 24 | 25 | 26 | 28 |
| PEEP, cm H2O | 14 | 8 | 10 | 10 | 12 | 10 |
| FiO2 | 0.5 | 0.6 | 0.7 | 0.5 | 0.3 | 0.6 |
| Respiratory system compliance, ml/cm H2O (ml/kg PBW/cm H2O) | 24 (0.43) | 30 (0.39) | 17 (0.33) | 22 (0.34) | 20 (0.32) | 19 (0.22) |
| Minute volume, L/min | 8.6 | 9.1 | 9.6 | 12.3 | 9.8 | 10.4 |
| PaO2/FiO2† | 148 | 152 | 76 | 110 | 180 | 266 |
| |
|
|
||||
| Duration of sharing, h | 48.25 |
47.5 |
47.0 |
|||
| Time from start of ventilator sharing to last follow-up, d | 36 | 56 | 39 | 33 | 42 | 55 |
| Status at last follow-up | Discharged to SAR, nocturnal CPAP with no daytime support | Discharged to SAR, free from ventilator support | Discharged to SAR, free from ventilator support | Discharged to LTAC, nocturnal PSV with no daytime support | Discharged home, free from ventilator support | Discharged to SAR, free from ventilator support |
Definition of abbreviations: CPAP = continuous positive airway pressure; LTAC = long-term acute care hospital; PBW = predicted body weight; PEEP = positive end-expiratory pressure; PSV = pressure support ventilation; SAR = subacute rehabilitation hospital; SOFA = sequential organ failure assessment.
The original SOFA does not consider phenylephrine or vasopressin in the cardiovascular subscore. Patients receiving either of these vasopressors were assigned a SOFA-cardiovascular subscore of 3 unless other vasopressors warranted a higher score.
Lowest value in 24 hours prior to ventilator sharing. All patients met diagnostic criteria for acute respiratory distress syndrome.
Across patients, median Vt and pH were 5.7 ml/kg predicted body weight (interquartile range, 5.1–6.7) and 7.33 (interquartile range, 7.29–7.38) during ventilator sharing versus 6.0 ml/kg predicted body weight (interquartile range, 5.6–6.6) and 7.39 (interquartile range, 7.30–7.43) in the 48 hours before and after sharing (Figure 1 and Table E1 in the online supplement).
Figure 1.
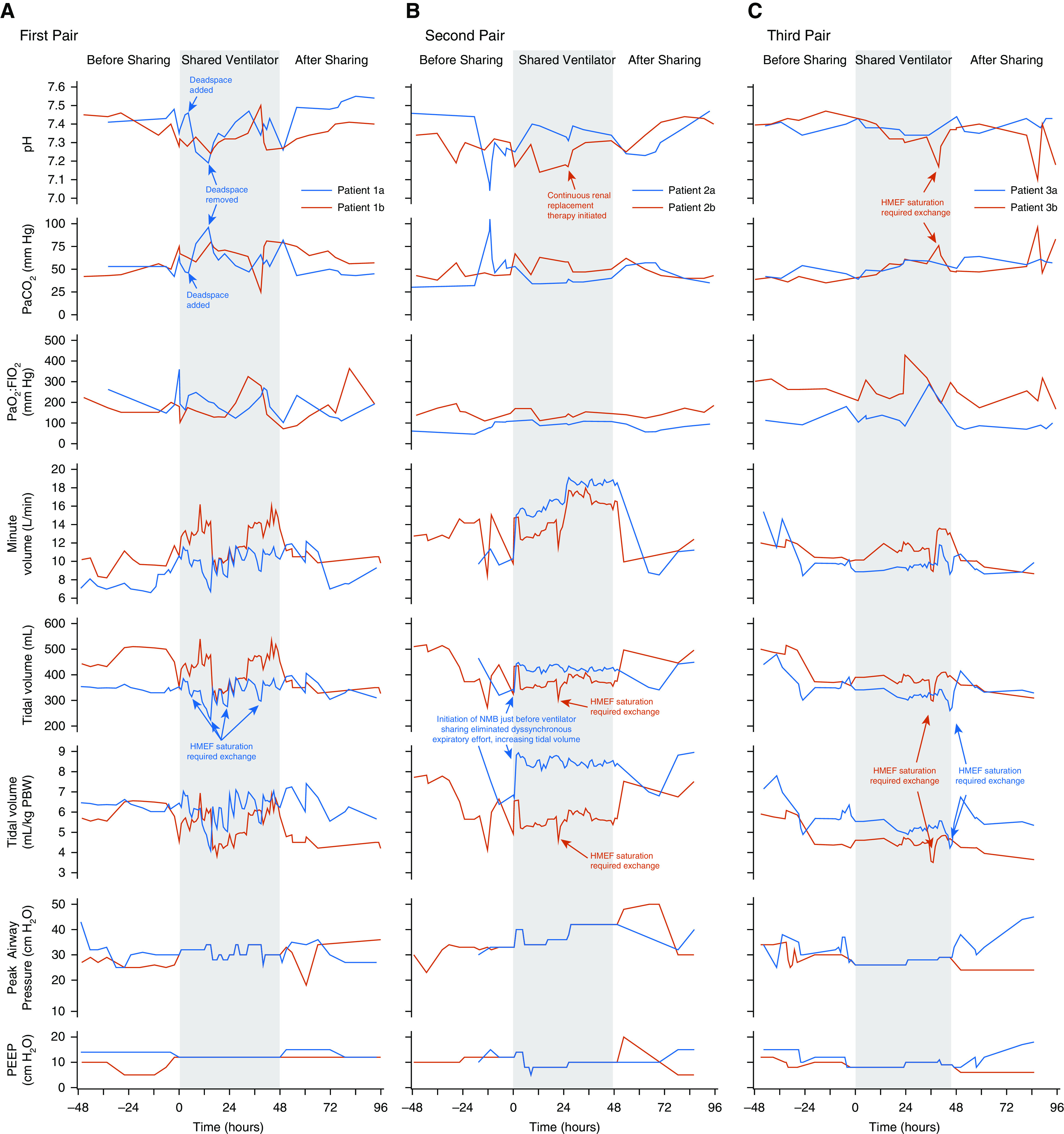
Clinical course of patients during ventilator sharing and for 48 hours preceding and afterward. (A) First pair. Patients shared a repurposed anesthesia machine. Approximately 4.5 hours after initiating ventilator sharing, patient 1a became alkalemic (pH 7.46), whereas patient 1b remained acidemic (pH 7.28). To treat alkalemia, deadspace tubing was added to the circuit of patient 1a, but resulting pH was lower than intended; with removal of this deadspace tubing, acidemia promptly improved. The HMEF had to be changed frequently for both patient circuits as CO2 absorbent-related moisture buildup increased resistance, an effect most pronounced in patient 1a. (B) Second pair. Patients shared a full-feature ICU ventilator. Patient 2a’s course illustrated the importance of ensuring steady-state ventilator requirements and reconfirming compatibility on neuromuscular blockade before initiating sharing. Patient 2a was intubated for 16 hours prior to ventilator sharing. During compatibility assessment, ventilator settings were matched and well tolerated but compatibility not reconfirmed after starting neuromuscular blockade in patient 2a; the patient exhibited overt, dyssynchronous expiratory effort before paralysis, and eliminating respiratory muscle activity substantially increased Vt for a given driving pressure. Patient 2b was initiated on renal replacement therapy at Hour 28 for renal failure, which promptly increased pH. The patient’s renal failure and plan for renal replacement predated ventilator sharing. (C) Third pair. Patients shared a full-feature ICU ventilator. Vt and acid–base balance were well controlled during ventilator sharing, reflecting cumulative experience and protocol refinement with incorporation of lessons learned. Patient 3b experienced a transient decrease in Vt and pH and increase in PaCO2 around Hour 36 owing to HMEF oversaturation that promptly resolved with its exchange. HMEF = heat and moisture exchanging filter; NMB = neuromuscular blockade; PBW = predicted body weight; PEEP = positive end-expiratory pressure.
All patients tolerated the prescribed 2-day sharing period without adverse events and subsequently survived to hospital discharge. Video E1 presents circuit configuration and monitoring of two patients undergoing ventilator sharing. Lessons learned for each patient pair are as follows.
First pair
Patients shared an anesthesia machine using the pressure control mode of ventilation. Use of an anesthesia machine introduced several challenges.
Several staff lacked experience with anesthesia machines.
The CO2 absorbent exhausted rapidly with two febrile patients with ARDS and was changed multiple times daily. Water produced from the absorbent’s chemical reaction created excess circuit humidity that quickly saturated the heat and moisture exchanging filter, increasing circuit resistance and requiring the heat and moisture exchanging filter be changed every 8–12 hours.
The patients’ combined minute volume (>20 L/min) exceeded the allowable range of alarm limits on the anesthesia machine. Audible alarms were notably subtler than on traditional ICU ventilators. Patient-specific respiratory monitors with alarms mitigated these issues.
The anesthesia machine’s bulk and configuration necessitated circuit extension tubing, whose high compliance complicated ventilator titration.
Second pair
Patients shared a full-feature ICU ventilator using the pressure control mode of ventilation. Patient 2a was intubated 16 hours before initiating ventilator sharing and exhibited overt, dyssynchronous active expiratory effort during screening, causing overestimation of subsequent mechanical support needs. Compatibility reevaluation after initiating neuromuscular blockade would have prevented this issue.
Third pair
Patients shared a full-feature ICU ventilator using the pressure control mode of ventilation without issue. A screening tool created in the electronic health record displayed key respiratory parameters for all invasively ventilated patients, facilitating rapid identification of compatible pairs.
With this experience, hospital leadership were confident ventilator sharing could be implemented at scale and adopted the protocol into the COVID-19 surge response plan.
Discussion
Prior ventilator splitting studies have included bench experiments with test lungs (4–6), animal models (7), and noninvasive ventilation in healthy volunteers (8). None have tested feasibility or safety in patients with lung injury.
Our protocol was designed with several patient safety features: patient compatibility criteria, restriction to two patients per ventilator, patient-specific monitoring, use of the pressure control mode of ventilation to ensure mechanical changes in one patient do not harm the other, use of medical-grade supplies, instructions for transitioning on and off the shared ventilator, management of divergent patient courses, and maintenance of an unoccupied rescue ventilator within each cluster of ventilator-sharing patients.
The greatest danger of ventilator sharing is wrongly equating the simplicity of its plumbing with ease of safe implementation. Patient selection and management require considerable expertise to ensure safety. Therefore, we recommend a regional referral model wherein ventilator sharing is restricted to expert centers, and patients and ventilators move throughout the region accordingly.
Alternative circuit configurations might add patient-specific PEEP or flow control valves to individualize support, or one-way valves to prohibit gas sharing. However, introducing new hardware to externally modify ventilator support during peak surge in patient volume—with non-ICU staff reassigned to ICU patient care in improvised clinical spaces (9)—is a setup for catastrophic human error. Even without human error, valve failure could result in asphyxiation. The ideal engineering solution is not the best clinical solution in this context.
Ventilator sharing does not obviate the need for more ventilators. Its greatest benefit may be when sufficient ventilators exist elsewhere and time is required to relocate ventilators or transfer patients. When resource and patient relocation are not options, ventilator sharing still may increase the number of patients supported and lives saved. However, it cannot double the number of supported patients. Some single-patient ventilators must be reserved for weaning and individualized support.
Limitations
Safety cannot be ascertained definitively from this small case series. Generalizability is untested, although our protocol’s step-by-step instructions strengthen safety and reproducibility. This protocol requires neuromuscular blockade to ensure passive ventilation, which appears safe for 48 hours (10) but holds unclear risk if prolonged. The weaning strategy proposed in the protocol is untested but could become necessary if ventilator sharing is prolonged. Identification of compatible patient pairs is essential to safe implementation of ventilator sharing and is likeliest in high-volume centers with many candidate patients.
Conclusions
This report demonstrates feasibility of ventilator sharing for COVID-19–associated ARDS. Following a rigorous clinical protocol, carefully selected patient pairs receiving continuous neuromuscular blockade tolerated ventilator sharing for 2 days without adverse events. In acute ventilator shortages, after exhausting alternatives, ventilator sharing is a reasonable stopgap to support potentially rescuable patients for at least 2 days in centers with appropriate expertise. This approach may be most useful when additional time is needed to relocate ventilators or patients to match supply with demand. The safety and utility of prolonged ventilator sharing, when ventilators or patients cannot be relocated, is unknown.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients and families who consented to participate in this public health initiative; their selflessness and generosity is likely to help save lives. The authors also express sincere gratitude to the many healthcare workers and other essential personnel who have responded to this crisis at their hospital, across New York, and throughout the world.
Footnotes
Supported in part by NIH grants K23HL133489 and R21HL145506 (J.R.B.).
Author Contributions: J.R.B., D.B., and L.L.H. conceived of the ventilator sharing initiative. J.R.B. designed the initial protocol. J.R.B., A.M.M., R. Kallet, R. Kacmarek, D.H., R.B., M.O., B.P., D.B., L.L.H., and B.T.T. revised the protocol. J.R.B., A.M.M., M.O., I.G., D.S.W., J.H., O.P., D.B., and L.L.H. implemented the protocol. J.R.B. extracted data and performed analyses. J.R.B. and A.M.M. prepared the initial manuscript draft. All authors revised the draft for substantive content. All authors approve of the final submission.
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1586LE on June 9, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Feuer A, Rosenthal BM.Coronavirus in N.Y.: ‘astronomical’ surge leads to quarantine warning New York Times 2020 [created 2020 Mar 24; accessed 2020 Mar 24]. Available from: https://www.nytimes.com/2020/03/24/nyregion/coronavirus-new-york-apex-andrew-cuomo.html
- 2.Cuomo A.New York State governor’s daily coronavirus press briefing 2020[created 2020 Mar 29; accessed 2020 Mar 29]. Available from: https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-state-scouting-new-sites-temporary
- 3.Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 4.Neyman G, Irvin CB. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006;13:1246–1249. doi: 10.1197/j.aem.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branson RD, Blakeman TC, Robinson BR, Johannigman JA. Use of a single ventilator to support 4 patients: laboratory evaluation of a limited concept. Respir Care. 2012;57:399–403. doi: 10.4187/respcare.01236. [DOI] [PubMed] [Google Scholar]
- 6.Tonetti T, Zanella A, Pizzilli G, Irvin Babcock C, Venturi S, Nava S, et al. One ventilator for two patients: feasibility and considerations of a last resort solution in case of equipment shortage. Thorax. 2020;75:517–519. doi: 10.1136/thoraxjnl-2020-214895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paladino L, Silverberg M, Charchaflieh JG, Eason JK, Wright BJ, Palamidessi N, et al. Increasing ventilator surge capacity in disasters: ventilation of four adult-human-sized sheep on a single ventilator with a modified circuit. Resuscitation. 2008;77:121–126. doi: 10.1016/j.resuscitation.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Smith R, Brown JM. Simultaneous ventilation of two healthy subjects with a single ventilator. Resuscitation. 2009;80:1087. doi: 10.1016/j.resuscitation.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip N.It’s almost like learning how to be a doctor again: how one New York ICU reinvented itself to treat COVID-19 patients Time 2020 [created 2020 Apr 9; accessed 2020 Apr 13]. Available from: https://time.com/5818835/coronavirus-doctor-new-york-icu
- 10.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


