Abstract
Rationale: The role of inspiratory effort still has to be determined as a potential predictor of noninvasive mechanical ventilation (NIV) failure in acute hypoxic de novo respiratory failure.
Objectives: To explore the hypothesis that inspiratory effort might be a major determinant of NIV failure in these patients.
Methods: Thirty consecutive patients with acute hypoxic de novo respiratory failure admitted to a single center and candidates for a 24-hour NIV trial were enrolled. Clinical features, tidal change in esophageal pressure (ΔPes), tidal change in dynamic transpulmonary pressure (ΔPl), expiratory Vt, and respiratory rate were recorded on admission and 2–4 to 12–24 hours after NIV start and were tested for correlation with outcomes.
Measurements and Main Results: ΔPes and ΔPes/ΔPl ratio were significantly lower 2 hours after NIV start in patients who successfully completed the NIV trial (n = 18) compared with those who needed endotracheal intubation (n = 12) (median [interquartile range], 11 [8–15] cm H2O vs. 31.5 [30–36] cm H2O; P < 0.0001), whereas other variables differed later. ΔPes was not related to other predictors of NIV failure at baseline. NIV-induced reduction in ΔPes of 10 cm H2O or more after 2 hours of treatment was strongly associated with avoidance of intubation and represented the most accurate predictor of treatment success (odds ratio, 15; 95% confidence interval, 2.8–110; P = 0.001 and area under the curve, 0.97; 95% confidence interval, 0.91–1; P < 0.0001).
Conclusions: The magnitude of inspiratory effort relief as assessed by ΔPes variation within the first 2 hours of NIV was an early and accurate predictor of NIV outcome at 24 hours.
Clinical trial registered with www.clinicaltrials.gov (NCT 03826797).
Keywords: acute respiratory distress syndrome, respiratory failure, noninvasive mechanical ventilation, transpulmonary pressure, esophageal pressure swings
At a Glance Commentary
Scientific Knowledge on the Subject
Noninvasive mechanical ventilation (NIV) is increasingly used to assist spontaneous breathing during acute hypoxic de novo respiratory failure (AHRF), even though its potential therapeutic effect in this setting is controversial. Reported data show that NIV is used in 15% of patients with acute respiratory distress syndrome, irrespective of the severity of respiratory failure, and it seems to be associated with higher mortality in the case of failure. Several predictors of NIV failure in AHRF have been investigated but were found to be insufficient to aid the timing of endotracheal intubation. Thus, there is a need for an early robust predictor of NIV failure to avoid intubation delay.
What This Study Adds to the Field
Our exploratory study shows that, in patients with moderate-to-severe AHRF who were candidates for a 24-hour NIV trial, the magnitude of inspiratory effort relief was an early and accurate predictor of NIV failure. Our study suggests that monitoring of esophageal pressure might assist clinicians in the timing of intubation for patients with AHRF undergoing a NIV trial.
The role of assisted spontaneous breathing (SB) in patients with acute hypoxic de novo respiratory failure (AHRF) is still controversial. When acute lung injury is mild, SB is desirable to preserve respiratory muscle function, improve the ratio and regional ventilation (1), and reduce sedation and days of invasive mechanical ventilation (MV) (2). On the other hand, recent studies have suggested that SB might be a potential mechanism for lung damage if acute respiratory distress is severe (3). In recent years, noninvasive MV (NIV) has been increasingly used to assist SB in the intensive care setting, even though its potential therapeutic effect in AHRF is still debated. It has been reported that NIV is used in 15% of patients with acute respiratory distress syndrome (ARDS), irrespective of the severity of respiratory failure, and it seems to be associated with higher mortality when the PaO2/FiO2 ratio is <150 mm Hg (4). Moreover, some studies have shown that NIV failure is associated with increased mortality in patients with AHRF (4, 5); however, when NIV treatment is successful, it might considerably reduce the risk of death and length of ICU stay in this subset of patients (5).
Despite the fact that several potential factors associated with NIV failure have been investigated in patients with hypoxia, there are no robust predictors that might alert the intensivist to the need for endotracheal intubation (ETI) within the very first hours of ventilation (6). Although the mechanisms behind the association between NIV failure and poorer survival remain unclear, a potential role for SB might be hypothesized. When SB is preserved during AHRF, the intensity of inspiratory effort may follow a critical increase in respiratory drive, thus producing uncontrolled tidal change in dynamic transpulmonary pressure (ΔPl) that would increase the risk of injury to the dependent lung and predispose the patient to the onset of self-inflicted lung injury (SILI) (6). The underlying mechanisms of SILI are heterogeneous and include the pendelluft phenomenon, increased transvascular pressure gradient aggravating alveolar damage, excessive diaphragmatic loading with impaired systemic oxygen delivery, and muscle injury (3, 7–9).
In this study, we explore the hypothesis that, in patients with moderate or severe AHRF undergoing a NIV trial, the excessive spontaneous effort of the patients, measured with tidal change in esophageal pressure (ΔPes), may be a major determinant of NIV failure at 24 hours.
Methods
Study Population
This prospective observational cohort study was performed in a single eight-bed respiratory ICU (RICU) at the University Hospital of Modena, Italy, after approval from the Ethics Committee Area Vasta Emilia Nord (registered protocol number 4485/C.E., document 266/16). After testing our study hypothesis in four patients (pilot data not included in the analysis) during the period from October 2016 to December 2018, the study was registered retrospectively on clinicaltrials.gov (NCT 03826797). Thirty consecutive patients were then enrolled in between February and October of 2019. Written informed consent to participate in the study and to analyze and divulgate clinical data was obtained from all patients admitted.
Inclusion criteria were age > 18 years; the presence of AHRF with a PaO2/FiO2 ratio < 200 mm Hg, despite high-flow nasal oxygen with the flow set at 60 L/min; and candidate’s approval for receiving a NIV trial by the attending RICU staff, whose decision was made upon clinical conditions being blinded to the purpose of the study. Patients were excluded in the case of a previously established diagnosis of chronic obstructive pulmonary disease, pulmonary embolism, neuromuscular disease, cardiogenic acute pulmonary edema, interstitial lung disease, or chest-wall deformities or the need for immediate ETI as represented by any of the following: cardiopulmonary arrest, respiratory arrest, loss of consciousness with respiratory pauses, psychomotor agitation requiring sedation, pH < 7.20, neurological deterioration or massive secretions, hemodynamic instability or major electrocardiographic abnormalities, pregnancy, intolerance to NIV, hypercapnic respiratory failure of any etiology (PaCO2 > 45 mm Hg), home long-term oxygen therapy, or denial of informed consent.
General Measures
Demographics and relevant comorbidities were assessed on admission. Clinical severity as assessed by the Kelly Scale, the Acute Physiology and Chronic Health Evaluation II score, the Simplified Acute Physiology Score, the Subsequent Organ Failure Assessment score, and the Heart Rate, Acidosis, Consciousness, Oxygenation and Respiratory Rate (HACOR) score were assessed and recorded on admission and after 2, 4, 12, and 24 hours. Arterial blood gases (PaO2 and PaCO2), pH, PaO2/FiO2 ratio, respiratory rate (RR), and blood lactate values were recorded before NIV start and 2, 4, 12, and 24 hours later. A chest X-ray was taken on admission and 24 hours after NIV start.
Physiological Measurements
A multifunctional nasogastric tube with a dedicated pressure transducer (NutriVent; Sidam Group) was placed before starting NIV. The nasogastric tube was connected to a dedicated monitoring system (OptiVent; Sidam Group) to record ΔPes and ΔPl. To avoid using absolute values for Pes and Pl, we always refer to ΔPes and ΔPl from the end-expiratory amounts (10). Appropriate catheter position was confirmed by visualization of cardiac artifacts on Pes traces and radiopaque markers on chest X-rays, and validation of Pes measurements was obtained through dynamic occlusion tests (11, 12). ΔPes was calculated as the negative deflections of Pes from the onset of inspiratory effort. ΔPl was calculated as airway pressure minus Pes (10).
ΔPes, ΔPl, and ΔPes/ΔPl ratios were assessed on admission and 2, 4, 12, and 24 hours after NIV start. Initial measurements were performed at each prespecified time point while the patient was breathing spontaneously through the ventilator circuit. Data were sampled at 100 Hz and processed on a dedicated data acquisition system (OptiVent; Sidam Group) (12). Data sampling was numerically stored and downloaded via a universal-serial-bus stick at each time of assessment. Offline breath-by-breath analysis was then performed for each measurement and then averaged by a specific software (Flux View Respiratory Mechanics Monitor [Medical Graphics]). For all the measurements, the beginning of the inspiratory phase was identified at the instant of Pes initial decay, whereas the end of inspiration was considered as the point of Pes at which 25% of the time from maximum deflection to return to baseline had elapsed.
Respiratory flow was measured by an external heated Fleisch No. 2 pneumotachograph (Fleisch) inserted between the patient’s oronasal facemask (Bluestar; KOO Medical Equipment) and a connector with a side port for mechanical measurement. Expiratory Vt (Vte) was obtained by numerical integration of the flow signal. Vte was then adjusted to the predicted body weight (PBW) to derive Vte/kg of PBW. Vte/kg of PBW was assessed on admission and 2, 4, 12 and 24 hours after NIV start. e was calculated as the product of Vte and RR and assessed on admission and 2, 4, 12 and 24 hours after NIV start. The Vte/ΔPL ratio was further measured at each predefined time point.
Leaks from the oronasal facemask were computed using dedicated ventilator-integrated software (GE Healthcare Engstrom Carestation; GE Healthcare) on the basis of the following equation: leaks (L/min) = (inspiratory Vt − Vte) × RR.
All measurements were performed during a stable SB pattern of 5 minutes, and results were averaged for each assessment step.
NIV Treatment
After Nutrivent placement, NIV was started and set by a respiratory physician with expertise in respiratory intensive care. Patients were connected via a conventional circuit with an appropriately sized oronasal facemask equipped with a dedicated output for probes (Bluestar; KOO Medical Equipment) to a high-performance ventilator (GE Healthcare Engstrom Carestation; GE Healthcare) in pressure-support preset mode. A heat and moisture exchanger (Hygrobac; DAR) was placed to the ventilator circuit’s Y-piece. Positive end-expiratory pressure (PEEP) was initially set at 6 cm H2O and subsequently fine-tuned (4–8 cm H2O) to target a SaO2 > 92% with a delivered FiO2 < 70%. Pressure support was set at 10 cm H2O and then progressively modified according to Vt (Vte/kg of PBW) to target a Vte/kg of PBW < 9.5 ml/kg of PBW and an RR < 30 breaths/min (bpm). The oronasal facemask was finely adjusted to target a leak flow < 20 L/min. The inspiratory trigger was set at 3 L/min, and respiratory cycling was set at 25% of the inspiratory peak flow. Great care was taken by the nurses in charge of NIV, who were blinded to the protocol, to avoid any possible air leaks. The inspiratory Fo2 delivered (FiO2) was increased to target a transcutaneous oxyhemoglobin saturation of 88–94%. The setting was adjusted by the attending physician blinded to the study purpose on the basis of blood gases and/or continuous oxymetry assessment. Patients receiving NIV treatment were not sedated. The decision as to whether to proceed to ETI at 24 hours after NIV start was made according to best clinical practice by the attending RICU staff, who were blinded to the results of the physiological assessment acquired through the Optivent monitor only at each predefined time point. NIV failure was defined by the onset of the need for ETI or by death. Criteria for ETI included 1) PaO2/FiO2 ratio unchanged or worsened or <150 mm Hg, 2) the need to protect airways because of neurological deterioration or massive secretions, 3) hemodynamic instability or major electrocardiographic abnormalities, and 4) unchanged or worsened dyspnea and persistence of respiratory distress (RR > 35 bpm, gasping for air, psychomotor agitation requiring sedation, or abdominal paradox).
Outcome Measures
The influence of ΔPes on NIV failure or success at 24 hours was prespecified as a primary outcome. The impact of ΔPl, ΔPes/ΔPl ratio, PaO2/FiO2 ratio, RR, Vte/kg of PBW, Vte/ΔPl ratio, e, and the HACOR score on NIV outcome at 24 hours and the correlation between ΔPes and radiographic changes on chest X-ray within the first 24 hours after NIV start were assessed as secondary outcomes. Radiographic changes on chest X-ray within the first 24 hours after admission were assessed by a radiologist with expertise in chest X-ray who was blinded to the purpose of the study. Changes were classified as follows: relevant worsening, worsening, mild worsening, unmodified, relevant improvement, improvement, and mild improvement.
Statistical Analysis
The statistical package GraphPad Prism 8.0 (GraphPad Software) was used for statistical analysis. Because of the exploratory nature of the study, no sample size calculation was performed. Descriptive statistics were used to characterize the study population as a whole and according to primary outcome. The nonparametric Mann-Whitney and Student’s t tests were used for the comparison of continuous variables. Comparison between dichotomous variables was performed by the χ2 test or Fisher exact test, when appropriate. The time course of ΔPes, ΔPl, ΔPes/ΔPl ratio, PaO2/FiO2 ratio, Vte/kg of PBW, Vte/ΔPl ratio, RR, e, and HACOR score according to NIV outcome within the first 24 hours of treatment was assessed through ANOVA analysis. Then, a post hoc Bonferroni-Dunn multiple test was used to perform the pairwise comparison of means for each analyzed variable at the prespecified time points. The correlation between baseline values of ΔPes and PaO2/FiO2 ratio, Vte, RR, HACOR score, Vte/ΔPl ratio, and the chest X-ray radiographic categories was assessed through Pearson correlation coefficient. The impact of ΔPes within 2 hours after NIV start and the baseline value of Vte/ΔPl ratio on NIV outcome was assessed through a logistic regression model. A receiver operating characteristic (ROC) analysis was then performed to identify the best predictive cutoff for ΔPes within 2 hours after NIV start and for baseline Vte/ΔPl ratio. The association between the best cutoff value of ΔPes after 2 hours of NIV and baseline Vte/ΔPl ratio, Vte > 9.5 ml/kg of PBW, RR > 30 bpm, PaO2/FiO2 ratio < 150 mm Hg, and HACOR score > 5 within 2 hours after NIV start on NIV failure at 24 hours was then tested through univariate logistic regression analysis. ROC analysis was used to assess the accuracy in predicting NIV failure at 24 hours for all the analyzed variables at prespecified cutoffs. Then, at 30 days, survival analysis was performed through a log-rank test for ΔPes within 2 hours after NIV start. A P value < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
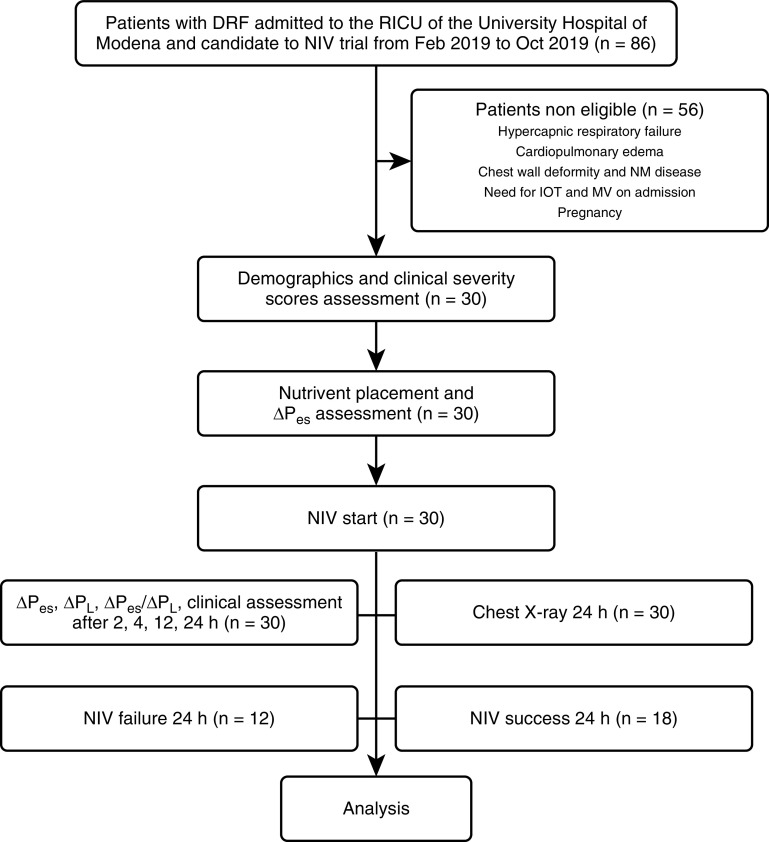
Over the study period, 30 out of 86 consecutive patients admitted for AHRF to the RICU of the University Hospital of Modena (Italy) and who were candidates to receive a NIV trial were enrolled in this study. Of these, 12 patients (40%) experienced NIV failure within 24 hours after NIV start. Those patients for whom the need for ETI was defined at 24 hours as the “alert” criterion of our internal guideline were thereafter intubated by the RICU staff. Of those who were successful in the 24-hour trial (60%), none were further intubated during their RICU stay. The flowchart for patients in this study is shown in Figure 1.
Figure 1.
Flowchart for patients in this study. ΔPes = tidal change in esophageal pressure; ΔPl = tidal change in transpulmonary pressure; DRF = de novo respiratory failure; IOT = intubation (orotracheal); MV = mechanical ventilation; NIV = noninvasive MV; NM = neuromuscular; RICU = respiratory ICU.
The general features and clinical characteristics of the whole population at baseline and according to NIV outcome at 24 hours are presented in Table 1. None of the features assessed were significantly different between the two groups of patients (NIV failure vs. NIV success) at baseline. In particular, the overall population presented an average PaO2/FiO2 ratio value of 125 (interquartile range [IQR], 101–170) mm Hg, which did not differ significantly according to NIV outcome at 24 hours (100 [118–141] mm Hg and 111 [132–173] mm Hg, respectively; P = 0.5). All patients with ARDS (n = 15) presented pulmonary ARDS. In 10 patients, the etiology was identified as infectious (bacterial, n = 4; fungal, n = 2; and viral, n = 4), whereas, for five patients, no etiological diagnosis was made. Patients with pneumonia had unilateral lung consolidation, and nine of them presented a bacterial infectious cause (Streptococcus pneumoniae, n = 4; intracellular pathogens, n = 4; and Hemophilus influenzae, n = 1). The presence of pneumonia and ARDS was equally distributed between the two groups (42% vs. 44%, P > 0.9 and 58% vs. 44%, P = 0.7, respectively).
Table 1.
Baseline Features of the Study Population Presented as a Whole or as NIV Outcome at 24 Hours
| Feature | Overall | NIV Failure | NIV Success | P Value |
|---|---|---|---|---|
| Number of patients | 30 | 12 | 18 | — |
| Age, yr | 71 (66–81) | 69 (62–80) | 71 (68–81) | 0.7 |
| Sex, M | 20 (67) | 8 (67) | 12 (67) | >0.9 |
| BMI, kg/m2 | 23 (19–27) | 22.5 (18–26) | 24 (21–27) | 0.3 |
| Charlson index score | 4 (3–5.5) | 4 (3–5) | 4.5 (3–6) | 0.6 |
| Pneumonia | 13 (23) | 5 (42) | 8 (44) | >0.9 |
| ARDS | 15 (50) | 7 (58) | 8 (44) | 0.7 |
| Kelly scale score | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.4 |
| APACHE II score | 27 (21–38) | 24.5 (19–45) | 28 (25–37) | 0.8 |
| SAPS II score | 36 (26–41) | 36 (31–38) | 36 (25–44) | 0.6 |
| SOFA score | 6 (4–8.8) | 5.5 (3–8) | 6.5 (4–9) | 0.6 |
| PaO2/FiO2 ratio, mm Hg | 125 (101–170) | 118 (100–141) | 133 (111–144) | 0.5 |
| pH | 7.48 (7.44–7.51) | 7.49 (7.46–7.52) | 7.48 (7.44–7.5) | 0.2 |
| PaCO2, mm Hg | 35 (30–40) | 34 (30–37) | 36 (30–42) | 0.2 |
| Blood lactate, mg/dl | 27 (14–40) | 30 (18–40) | 25 (12–40) | 0.7 |
| Serum creatinine, mg/dl | 0.68 (0.5–0.9) | 0.6 (0.5–0.7) | 0.8 (0.65–0.8) | 0.4 |
| PEEP, cm H2O | 8 (6.5–10) | 8 (7.5–10) | 8 (6–10) | 0.7 |
| PS, cm H2O | 10 (10–14) | 11 (10–12) | 11 (10–14) | 0.3 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ARDS = acute respiratory distress syndrome; BMI = body mass index; NIV = noninvasive mechanical ventilation; PEEP = positive end-expiratory pressure; PS = pressure support; SAPS II = Simplified Acute Physiology Score; SOFA = Subsequent Organ Failure Assessment.
Data are presented as n (%) for dichotomous values or median (interquartile range) for continuous values unless otherwise specified.
Physiological Measurements and NIV Outcome
Table 2 shows the physiological dynamic respiratory mechanics for the whole population at baseline and in the NIV outcome subgroups at baseline and after 2 hours of NIV. At baseline, the median (IQR) value of ΔPes was 34 (26–40) cm H2O. Of note, none of the physiological features analyzed were significantly different at baseline between the two groups. After 2 hours of NIV, the median (IQR) value of ΔPes was significantly lower for those patients who had a successful outcome in the 24-hour NIV trial compared with patients who had NIV failure: 11 (8–15) cm H2O versus 31.5 (30–36) cm H2O (P < 0.0001). Moreover, these latter patients presented a significantly increased value of ΔPl once NIV had started compared with patients who experienced NIV success at 24 hours (median [IQR], 39.5 [37.5–42–3] cm H2O vs. 30.5 [28–43.5] cm H2O; P = 0.04).
Table 2.
Clinical and Physiological Features of the Study Population at Baseline and after 2 Hours of NIV
| Feature | Overall | NIV Failure | NIV Success | P Value |
|---|---|---|---|---|
| Baseline RR, bpm | 36 (27–44) | 34 (27–42) | 36 (27–45) | 0.8 |
| RR after 2 h of NIV, bpm | 30 (24–37) | 31 (25–37) | 30 (24–37) | 0.6 |
| Baseline ΔPl (ΔPes), cm H2O | 35 (26–40) | 38 (32–42) | 32.5 (24–39) | 0.1 |
| ΔPes after 2 h of NIV, cm H2O | 19.5 (12.5–31) | 31.5 (30–36) | 11 (8–15) | <0.0001 |
| ΔPl after 2 h of NIV, cm H2O | 37 (30–43) | 39.5 (37.5–42.3) | 30.5 (28–43.5) | 0.04 |
| Baseline e, L/min | 28.1 (25.6–34.7) | 28.3 (25.8–32.3) | 27.4 (22.2–28.9) | 0.6 |
| e after 2 h of NIV, L/min | 23.3 (18.2–27.3) | 27.2 (25–27.8) | 19.8 (16.5–25) | 0.07 |
| Baseline Vte, ml/kg of PBW | 11 (9–12) | 11 (9.5–12.3) | 10.9 (9–11.2) | 0.7 |
| Vte after 2 h of NIV, ml/kg of PBW | 11 (10–12) | 11 (10–12.3) | 10.8 (8.5–12) | 0.5 |
| Baseline Vte/ΔPL ratio, ml/kg/cm H2O | 0.32 (0.28–0.57) | 0.31 (0.29–0.57) | 0.33 (0.27–0.4) | 0.3 |
| Vte/ΔPL ratio after 2 h of NIV, ml/kg/cm H2O | 0.31 (0.25–0.39) | 0.36 (0.21–0.44) | 0.29 (0.26–0.31) | 0.1 |
| HACOR score | 6 (5–8) | 6.5 (4.8–8) | 6 (6–7) | 0.5 |
| HACOR score after 2 h of NIV | 6 (5–6) | 6 (4.8–6.5) | 5.5 (4–6) | 0.4 |
Definition of abbreviations: bpm = breaths/min; ΔPes = tidal change in esophageal pressure; ΔPl = tidal change in dynamic transpulmonary pressure; HACOR = Heart Rate, Acidosis, Consciousness, Oxygenation and Respiratory Rate; NIV = noninvasive mechanical ventilation; PBW = predicted body weight; RR = respiratory rate; Vte = expiratory Vt.
Data are presented as median (interquartile range).
Figure 2A shows ΔPes from baseline within the first 2 hours of NIV for the whole population and according to NIV outcome at 24 hours. ΔPes decreased significantly after 2 hours of NIV for the whole population and for those patients who were successful in the NIV trial, whereas it did not change for patients who experienced NIV failure. Moreover, only these latter patients presented a significant increase in ΔPl after 2 hours of NIV (Figure 2B).
Figure 2.
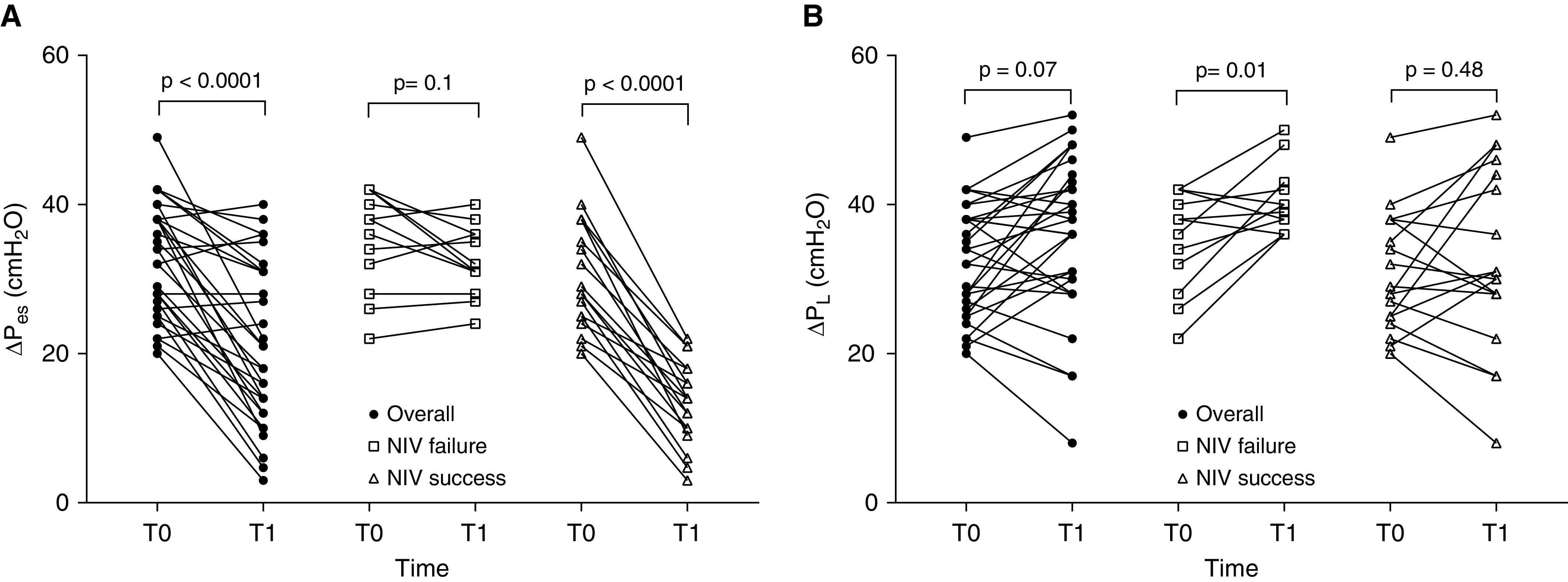
(A) ΔPes changes from baseline within the first 2 hours of NIV for the whole population and according to NIV outcome at 24 hours. (B) ΔPl changes from baseline within the first 2 hours of NIV for the whole population and according to NIV outcome at 24 hours. ΔPes = tidal change in esophageal pressure; ΔPl = tidal change in transpulmonary pressure; NIV = noninvasive mechanical ventilation.
Waveform analysis of ΔPl and ΔPes 2 hours after NIV start is displayed in Figure 3 for a patient who had NIV failure in the 24-hour trial (Figures 3A and 3C) and for a patient who had NIV success (Figures 3B and 3D). The time course of the physiological and clinical variables (ΔPes, ΔPl, ΔPes/ΔPl ratio, RR, PaO2/FiO2 ratio, HACOR score, Vte/kg of PBW, Vte/ΔPl ratio, and e) in the two categories of patients according to NIV outcome showed a significant improvement over time in patients who were successful in the NIV trial. Moreover, only ΔPes significantly decreased earlier (2 h after NIV start) in those patients who were successful in the NIV trial compared with those who failed (P < 0.0001, see Figure E1A in the online supplement). The ratio between ΔPes and ΔPl was significantly different 2 hours after NIV start between the two groups (P < 0.0001, Figure E1C), whereas ΔPl (P = 0.04, Figure E1B), Vte/kg of PBW, e, Vte/ΔPl ratio (P = 0.01, P = 0.01, and P = 0.001; Figure E2A–E2C, respectively), RR, PaO2/FiO2 ratio, HACOR score (P = 0.02, P < 0.0001, and P = 0.0; Figure E3A–E3C, respectively) were all significantly different more than 2 hours after NIV start.
Figure 3.
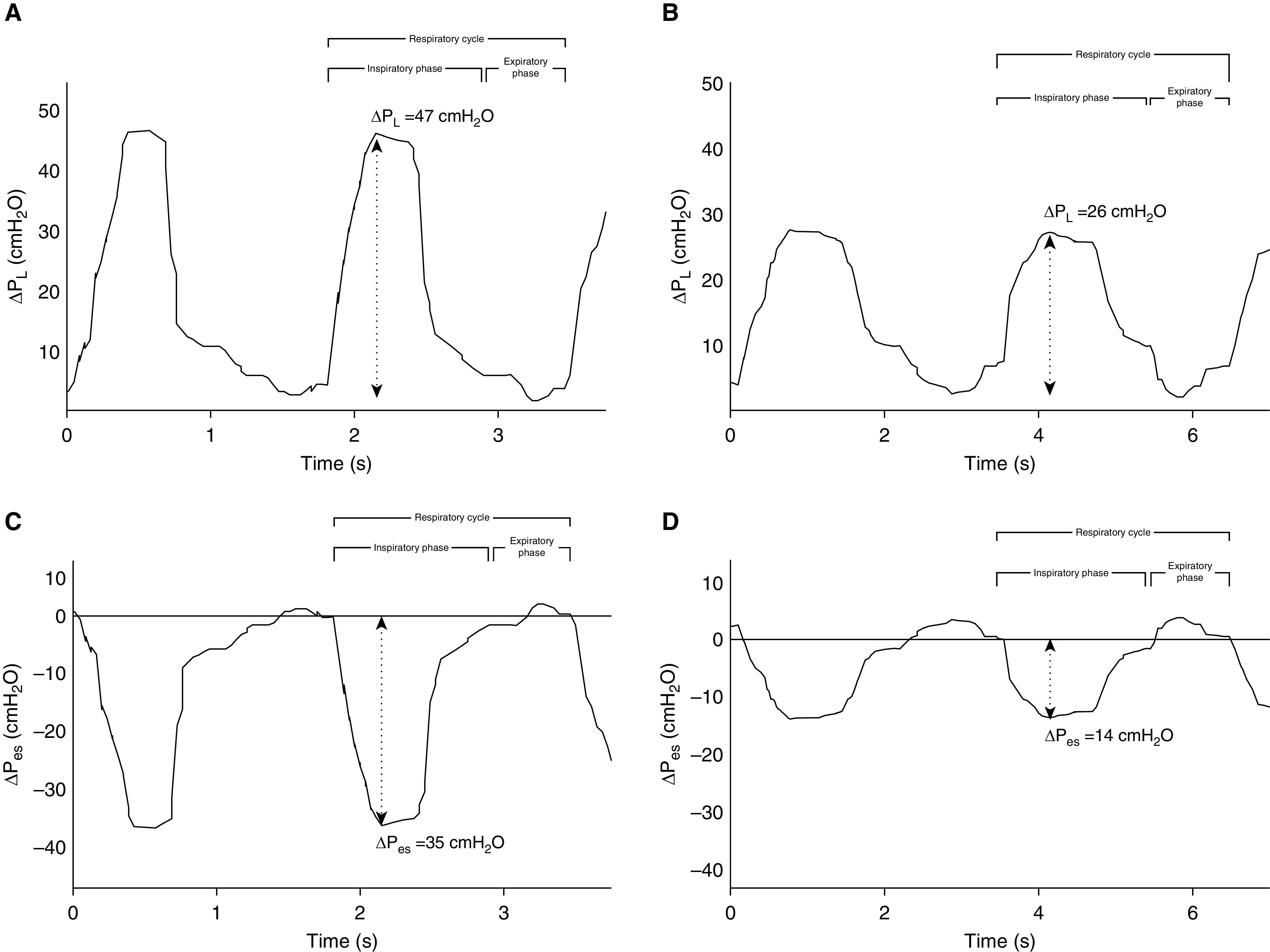
Graphical representation of ΔPl and ΔPes waveform swings after 2 hours of NIV for (A and C) a patient who had failure in the noninvasive mechanical ventilation trial at 24 hours and for (B and D) a patient who had success. The beginning of the inspiratory phase was identified at the time of Pes initial decay, whereas the end of inspiration was considered at the point of Pes that elapsed 25% of time from its maximum deflection to return to baseline. ΔPes = tidal change in esophageal pressure; ΔPl = tidal change in dynamic transpulmonary pressure.
Significant inverse correlation was found between baseline ΔPes and Vte/ΔPl ratio (r = −077, P < 0.0001, Figure E4). No significant correlation was found between baseline ΔPes and PaO2/FiO2 ratio (r = −0.01, P = 0.9, Figure E5A), RR (r = 0.23, P = 0.2, Figure E5B), HACOR score (r = 0.05, P = 0.8, Figure E5C), and Vte/kg of PBW (r = −0.05, P = 0.8, Figure E5D).
Radiological Changes and Inspiratory Effort
The correlation analysis performed for radiographic changes showed that patients with a greater reduction in ΔPes 2 hours after NIV start experienced more consistent improvements on chest X-ray at 24 hours, whereas patients with a limited reduction of ΔPes were those who showed a deterioration on chest X-ray (Figure E6).
Inspiratory Effort and Clinical Outcome
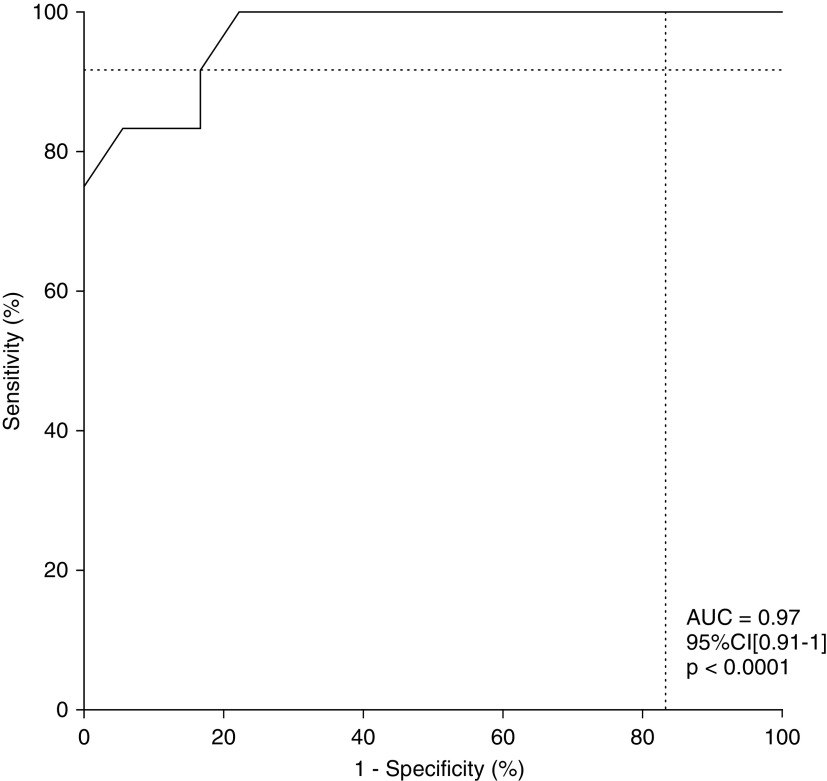
In the logistic regression model, ΔPes changes within the first 2 hours of NIV showed a significant association with NIV failure at 24 hours (odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2–3; P = 0.01), whereas the baseline Vte/ΔPl ratio was not significantly associated with NIV outcome (P = 0.03). From ROC analysis, ΔPes changes < 10 cm H2O gave the most accurate cutoff value for prediction of NIV failure (sensitivity, 0.91; 95% CI, 0.65–1 and specificity, 0.83; 95% CI, 0.61–0.94; likelihood ratio, 5.5; positive predictive value, 0.79; 95% CI, 0.52–0.92; negative predictive value, 0.94; 95% CI, 0.72–1; Table E1); a Vte/ΔPl ratio < 0.33 ml/kg/cm H2O showed the best cutoff value for prediction of NIV failure (sensitivity, 0.67; 95% CI, 0.40–0.86 and specificity, 0.5; 95% CI, 0.29–0.71; likelihood ratio, 1.3; positive predictive value, 0.47; 95% CI, 0.26–0.7; negative predictive value, 0.7; 95% CI, 0.42–87; Table E2). When univariate logistic regression was applied to the prespecified potential predictors of NIV failure, ΔPes < 10 cm H2O showed the highest association with NIV failure at 24 hours (OR, 15; 95% CI, 2.8–110; P = 0.001). Among the other predictors tested, Vte > 9.5 ml/kg of PBW and HACOR > 5 after 2 hours of NIV were significantly associated with NIV failure at 24 hours (OR, 7.9; 95% CI, 1.5–72; P = 0.02 and OR, 6.3; 95% CI, 0.9–49; P = 0.046, respectively), whereas RR > 30 bpm, PaO2/FiO2 ratio < 150 mm Hg, and Vte/ΔPl ratio < 0.33 ml/kg/cm H2O, although strongly associated, did not reach statistical significance (Table 3). From ROC analysis, ΔPes < 10 cm H2O within the first 2 hours after NIV start showed higher accuracy in predicting NIV failure (area under the curve [AUC], 0.97; 95% CI, 0.91–1; P < 0.0001) (Figure 4) than baseline Vte > 9.5 ml/kg of PBW(AUC, 0.88; 95% CI, 0.76–0.99; P = 0.0005), HACOR score > 5 (AUC, 0.85; 95% CI, 0.71–0.99; P = 0.00), RR > 30 bpm (AUC, 0.83; 95% CI 0.67–0.98; P = 0.003) PaO2/FiO2 ratio < 150 mm Hg (AUC, 0.74; 95% CI, 0.56–0.92; P = 0.03), and Vte/ΔPl ratio < 0.33 ml/kg/cm H2O (AUC, 0.58; 95% CI, 0.37–0.8; P = 0.44).
Table 3.
Association between Physiological and Clinical Variables and NIV Failure at 24 Hours
| Feature | OR | 95% CI | P Value |
|---|---|---|---|
| ΔPes < 10 cm H2O post 2 h NIV | 15 | 2.8–110 | 0.001 |
| Vte > 9.5 ml/kg of PBW | 7.9 | 1.5–72 | 0.02 |
| HACOR score >5 post 2 h NIV | 6.3 | 0.9–49 | 0.046 |
| RR > 30 bpm | 5.5 | 0.8–112 | 0.14 |
| PaO2/FiO2 ratio < 150 mm Hg | 2 | 0.5–9.8 | 0.4 |
| Vte/ΔPl ratio < 0.33 ml/kg/cm H2O | 2 | 0.4–9.8 | 0.36 |
Definition of abbreviations: bpm = breaths/min; CI = confidence interval; ΔPes = tidal change in esophageal pressure; ΔPl = tidal change in dynamic transpulmonary pressure; HACOR = Heart Rate, Acidosis, Consciousness, Oxygenation and Respiratory Rate; NIV = noninvasive mechanical ventilation; OR = odds ratio; PBW = predicted body weight; RR = respiratory rate; Vte = expiratory Vt.
Figure 4.
Receiver operating characteristic analysis. Tidal change in esophageal pressure changes < 10 cm H2O within the first 2 hours of noninvasive mechanical ventilation showed a high accuracy in predicting noninvasive mechanical ventilation failure (AUC, 0.97; P < 0.0001). AUC = area under the curve; CI = confidence interval.
Kaplan-Meier curves showed a significant increase in 30-day mortality among patients with ΔPes reduction < 10 cm H2O within the first 2 hours after NIV start compared with patients with a more consistent early improvement (hazard ratio, 4.5; 95% CI, 1.01–17.9; P = 0.048; Figure E7).
Discussion
In this exploratory study, patients with moderate-to-severe AHRF undergoing a NIV trial presented a median baseline value for ΔPes of 34 cm H2O that was significantly reduced within the first 2 hours of ventilation in patients who were successful in the NIV trial, whereas those patients failing the NIV trial did not have a significantly reduced ΔPes. This study therefore shows that a significant ΔPes reduction within the first 2 hours of NIV start was an early and accurate predictor of NIV outcome and was significantly correlated with radiographic changes after 1 day of NIV. Moreover, the magnitude of inspiratory effort at baseline did not show a significant correlation with the severity of respiratory failure, Vt, RR, or HACOR score on admission.
Physiological Measurements and NIV Outcome
Early prediction of NIV failure in AHRF
The application of NIV in treating patients with AHRF is a controversial issue and it is currently used in clinical practice, irrespective of the severity of the PaO2/FiO2 ratio. Despite the initial promising results on the effectiveness of NIV in patients with hypoxic respiratory failure (13, 14), more recent studies focusing on patients with AHRF and excluding underlying chronic respiratory diseases or cardiogenic pulmonary edema warn of the increased mortality rates once ETI is delayed (5, 15, 16). Despite the fact that failure rates can exceed 60% in patients with more severe AHRF, successful application of NIV is independently associated with survival and shorter length of ICU stay (5). Giving these assumptions, it seems of critical interest to identify early predictors of NIV failure to avoid deleterious intubation delay in this subset of patients.
Previous studies have shown that several factors (i.e., higher severity score on admission, older age, ARDS or pneumonia as the etiology for acute respiratory failure, or a lack of improvement in blood gas exchange within 1 h of treatment) are associated with NIV failure in patients with AHRF, although these were insufficient to influence ETI timing (17). In our study, none of these factors were different in patients who had 24-hour NIV failure compared with patients who had NIV success. In a recent single-center study, Duan and coworkers (18) developed and validated the HACOR score for prediction of NIV failure in patients with AHRF, showing that patients with a HACOR score > 5 after the first hour of NIV were at greater risk for NIV failure and, if switched to invasive MV within the first 12 hours, presented reduced in-hospital mortality. In our study, the HACOR score was significantly associated with increased NIV failure but not as early as ΔPes. Moreover, both groups of patients presented a HACOR score > 5 after the first 2 hours of NIV. Two recent studies have demonstrated that moderate-to-severe hypoxemia significantly affects NIV outcome in patients with ARDS-induced AHRF (19, 20). Our study presented a carefully selected population of patients with moderate-to-severe AHRF, whose average PaO2/FiO2 ratio was 132 mm Hg and in whom significant differences between those who had success in the NIV trial and those who were subjected to ETI did not become evident until 12 hours after the start of NIV. Of interest, the inspiratory effort at baseline as expressed by ΔPes did not show a significant correlation with the severity of respiratory failure. These findings are in line with data reported in a recent physiological study by Grieco and colleagues (21), in which ΔPes was unrelated to oxygenation impairment during helmet NIV and high-flow oxygen treatment. Our data further underline the inability of PaO2/FiO2 ratio alone to identify patients with harmful respiratory drive.
In a recent trial, Carteaux and coworkers (22) showed that a Vte value > 9.5 ml/kg was independently associated with NIV failure in patients with AHRF, suggesting a role of high Vte as a potential predictor of NIV failure in this setting (19). The results from our study are in line with their reported data, although significant differences in Vte between patients who had NIV treatment failure and those who had treatment success became evident 12 hours after NIV start. Moreover, the magnitude of inspiratory effort was not correlated with average Vte at baseline. Considering these data, the inability to apply protective ventilation should be considered a critical mechanism of NIV failure in this subset of patients.
The main result from our study was that a change in ΔPes < 10 cm H2O within the first 2 hours after NIV start was an early and accurate predictor of NIV failure at 24 hours when compared with other variables, such as PaO2/FiO2 ratio, Vte, HACOR, and RR. From a clinical point of view, these data might suggest that, in patients with moderate-to-severe AHRF, the effectiveness of a NIV trial should be related to the reduction in the patient’s inspiratory effort, which is quantifiable through esophageal manometry. The consequences of this reduction translate into a subsequent significant reduction of Vte, a decrease in RR, and an improvement in PaO2/FiO2 ratio with a few hours’ latency. Moreover, the correlation analysis showed that ΔPes on admission was not associated with the baseline value of other predictors of NIV failure.
Radiological Changes and Inspiratory Effort
Inspiratory effort and SILI during NIV
Our results showed a significant correlation between ΔPes changes within the first 2 hours of NIV and radiographic progression at 24 hours. Despite being less accurate than a computed tomography scan, chest X-rays showed good sensitivity in detecting lung alteration in patients with ARDS (23) and might be considered reliable in the evaluation of the extent and distribution of lung opacities, once a diagnosis has already been made (24).
The results of our study support the hypothesis that inspiratory effort might be a potential mechanism of lung damage enhancement if acute respiratory distress is severe. Although data from animal models indicate ΔPl as a major determinant of SILI, experimental studies conducted on normal trained subjects during exhausting endurance exercise demonstrated that potentially injurious values of ΔPl (up to 52 cm H2O) did not translate into lung mechanical changes (25, 26). To understand this, we have to consider that in normal fluid-like lung, the inspiratory swing in pleural pressure produced by inspiratory effort is homogeneously distributed across the pleural surface. In contrast, in injured solid-like lung, the inspiratory pleural swing is not uniformly dissipated, resulting in a more negative deflection in the dependent lung zones with tidal overrecruitment and local overstretch (6). More recently, two trials investigating the role of assisted SB in mechanically ventilated patients showed that SB was not associated with a poorer outcome when compared with controlled MV (27, 28), but they lacked an assessment of the inspiratory effort. Our results might suggest that a major determinant in generating lung stress lies in the dynamic component of the inspiratory effort rather than in the absolute value of the pressure generated. Interestingly, within the first 2 hours of NIV, the ΔPes/ΔPl ratio was different in those who were successful in the NIV trial compared with those who failed it. This ratio might express to what extent dynamic ΔPl is affected by the patient’s respiratory drive and might introduce a new insight in the understanding of SILI. In particular, for the same value of ΔPl, patients who presented higher values of ΔPes experienced a higher NIV failure rate. This mechanism, alongside a Vt > 6 ml, high breathing frequency, and elevated mechanical power, should be considered critical for SILI. These results highlight the potential role of the pendelluft phenomenon and negative pressure alveolar edema in determining SILI. Recently, in a rat model of acute lung injury, Henzler and coworkers (29) showed that ΔPl was more important than inspiratory effort in generating ventilator-associated lung injury during partial ventilatory support. These results are apparently contradictory to those reported in our study, but some issue might have influenced the conclusions. First, the experimental PEEP was set at 5 cm H2O, which, in a murine model, is comparable to higher amounts in larger animals, producing a sort of recruitment favoring a fluid-like behavior of the lung and reducing the harmful role of SB (25). Second, the animals ventilated with a lower degree of support presented hypercapnic acidosis that might have mitigated the ventilator-associated lung injury. Furthermore, in our study, we have assessed the Vte/ΔPl ratio as a surrogate of lung compliance to explore the concept of baby lung during NIV. Data show an inverse linear correlation between Vte/ΔPl ratio and inspiratory effort (Figure E4). Moreover, the time course of this index resulted in differences between those who succeeded the 24-hour NIV trial and those who failed (Figure E2C). Thus, this might justify the discrepancies in the behavior of Vte and inspiratory effort. Although not significantly associated with NIV failure, this index deserves further investigations in larger physiological trials.
Limitations of the Study
Our study has several limitations. First, the number of patients might have underpowered the results obtained. In particular, the value of ΔPes changes < 10 cm H2O should be confirmed in larger trials. Second, our study population was highly selected, influencing the generalization of our results. In particular, none of the patients who were successful in the 24-hour trial required further intubation, thus indicating that patients were enrolled very early in the course of the disease. Third, we did not carry out any assessment of inflammatory biomarkers. The determination of cytokine concentrations might clarify the role of vigorous inspiratory effort in exaggerating lung injury. Moreover, as patients were studied during SB, what we measured was dynamic Pl; thus, the influence of the inspiratory and expiratory resistances on the measured pressures should be considered. Furthermore, we did not perform gastric pressure assessment, so ΔPes values may have been overestimated in the case of expiratory muscle recruitment. Finally, despite the fact that our study identifies ΔPes changes as the major and early physiological predictor of NIV failure, the evaluation of a composite parameter that takes into account the various components of the respiratory drive (including e, RR, inspiratory flow rate, and airway occlusion pressure) as a bundle, might be of relevant clinical importance and should be assessed in further multicenter trials. At this time, we believe that this technique produces highly reliable data if managed in centers with expertise in esophageal manometry. Notwithstanding this, an increase in its use should raise the degree of confidence in daily clinical practice.
Conclusions
Even with the limitations described, our study highlights new concepts that can be summarized as follows: 1) patients with severe AHRF undergoing NIV may achieve harmful Pl amounts; 2) the magnitude of inspiratory effort during NIV is the earliest and most accurate parameter that predicts failure; 3) the amount of inspiratory effort is not correlated with oxygenation, and the PaO2/FiO2 ratio therefore cannot be used as a surrogate of ΔPes; and 4) the significant correlation between ΔPes changes within the first 2 hours of NIV and radiographic progression at 24 hours suggest that SILI might be a potential mechanism of lung damage in these patients.
In the hypothesis of SILI as a critical factor affecting NIV failure in patients with AHRF, we found that the magnitude of inspiratory effort as assessed by ΔPes variation within the first 2 hours of NIV treatment is an early and accurate predictor of outcome at 24 hours. The clinical implications of our study suggest that monitoring Pes might help clinicians in the decision-making process (airway intubation) for patients with AHRF undergoing a NIV trial. Because of the exploratory nature of this study, findings should be confirmed in multicenter clinical trials.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank professional editor Colin Woodham (Alpha Science Editors) for language editing.
Footnotes
Author Contributions: R.T. and A.M. designed the study, enrolled the patients, analyzed data, and wrote the paper. R.F., L.T., I.C., and L.P. made substantial contributions to the literature review, data collection, and writing of the paper. M.R.P., G.D.C., and M.G. reviewed the literature, analyzed data, wrote the manuscript, and produced the figures. R.D’A. made a substantial contribution to the statistical analysis and reviewed and edited the manuscript. S.N. and E.M.C. designed the study and reviewed and edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2512OC on April 23, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:1241–1248. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- 2.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. LUNG SAFE Investigators; ESICM Trials Group. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 5.Demoule A, Girou E, Richard JC, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–1765. doi: 10.1007/s00134-006-0324-1. [DOI] [PubMed] [Google Scholar]
- 6.Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS, et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med. 2018;197:1285–1296. doi: 10.1164/rccm.201706-1244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. 2012;40:1578–1585. doi: 10.1097/CCM.0b013e3182451c40. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med. 2013;41:536–545. doi: 10.1097/CCM.0b013e3182711972. [DOI] [PubMed] [Google Scholar]
- 9.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 10.Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20:142. doi: 10.1186/s13054-016-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine) The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 12.Mojoli F, Iotti GA, Torriglia F, Pozzi M, Volta CA, Bianzina S, et al. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care. 2016;20:98. doi: 10.1186/s13054-016-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure: a randomized comparison with conventional therapy. Chest. 1995;107:761–768. doi: 10.1378/chest.107.3.761. [DOI] [PubMed] [Google Scholar]
- 15.Hraiech S, Alingrin J, Dizier S, Brunet J, Forel JM, La Scola B, et al. Time to intubation is associated with outcome in patients with community-acquired pneumonia. PLoS One. 2013;8:e74937. doi: 10.1371/journal.pone.0074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, et al. oVNI Study Group; REVA Network (Research Network in Mechanical Ventilation) Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016;42:82–92. doi: 10.1007/s00134-015-4087-4. [DOI] [PubMed] [Google Scholar]
- 17.Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med. 2001;27:1718–1728. doi: 10.1007/s00134-001-1114-4. [DOI] [PubMed] [Google Scholar]
- 18.Duan J, Han X, Bai L, Zhou L, Huang S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43:192–199. doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 19.Frat JP, Ragot S, Coudroy R, Constantin J-M, Girault C, Prat G, et al. REVA Network. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018;46:208–215. doi: 10.1097/CCM.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 20.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 21.Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2019;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 22.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 24.Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011;87:612–622. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 25.Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in normal subjects. J Clin Invest. 1969;48:564–573. doi: 10.1172/JCI106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581:1309–1322. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Haren F, Pham T, Brochard L, Bellani G, Laffey J, Dres M, et al. Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) Investigators. Spontaneous breathing in early acute respiratory distress syndrome: insights from the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE study. Crit Care Med. 2019;47:229–238. doi: 10.1097/CCM.0000000000003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henzler D, Schmidt A, Xu Z, Ismaiel N, Zhang H, Slutsky AS, et al. Increased effort during partial ventilatory support is not associated with lung damage in experimental acute lung injury. Intensive Care Med Exp. 2019;7:60. doi: 10.1186/s40635-019-0272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.