Abstract
Rationale: Adverse events have limited the use of bronchial thermoplasty (BT) in severe asthma.
Objectives: We sought to evaluate the effectiveness and safety of using 129Xe magnetic resonance imaging (129Xe MRI) to prioritize the most involved airways for guided BT.
Methods: Thirty subjects with severe asthma were imaged with volumetric computed tomography and 129Xe MRI to quantitate segmental ventilation defects. Subjects were randomized to treatment of the six most involved airways in the first session (guided group) or a standard three-session BT (unguided). The primary outcome was the change in Asthma Quality of Life Questionnaire score from baseline to 12 weeks after the first BT for the guided group compared with after three treatments for the unguided group.
Measurements and Main Results: There was no significant difference in quality of life after one guided compared with three unguided BTs (change in Asthma Quality of Life Questionnaire guided = 0.91 [95% confidence interval, 0.28–1.53]; unguided = 1.49 [95% confidence interval, 0.84–2.14]; P = 0.201). After one BT, the guided group had a greater reduction in the percentage of poorly and nonventilated lung from baseline when compared with unguided (−17.2%; P = 0.009). Thirty-three percent experienced asthma exacerbations after one guided BT compared with 73% after three unguided BTs (P = 0.028).
Conclusions: Results of this pilot study suggest that similar short-term improvements can be achieved with one BT treatment guided by 129Xe MRI when compared with standard three-treatment-session BT with fewer periprocedure adverse events.
Keywords: asthma, volumetric computed tomography, feasibility studies, hyperpolarized xenon
At a Glance Commentary
Scientific Knowledge on the Subject
The use of bronchial thermoplasty in severe asthma has been limited by periprocedure adverse events.
What This Study Adds to the Field
In this pilot study, we demonstrate that a single treatment guided by 129Xe magnetic resonance imaging to the most involved airways can have improvements in quality of life 12 weeks after bronchial thermoplasty similar to those in a standard three-treatment bronchial thermoplasty but with fewer periprocedure adverse events.
There has been a significant increase in the number of therapeutic options for severe asthma since bronchial thermoplasty (BT) obtained U.S. Food and Drug Administration approval in 2010 (1). To date, all of the approved therapeutics are aimed at the type 2 inflammation high (T2-high) phenotype mediated by IL-4, IL-5, and IL-13 cytokines secreted by T-helper 2 cells. BT may be a well-suited alternative for T2-low disease or for those who have failed biologic therapy. However, published guidelines from 2014 recommended restricting its use to clinical trials and registries because of the concern for adverse events (AEs) in the periprocedure period and uncertainty over long-term safety (2). Long-term safety and efficacy have now been demonstrated out to 5 years (3); however, the short-term complications remain an important concern.
Currently, BT is performed over three treatment sessions with all airways subject to treatment with thermal energy. Each treatment session, which requires conscious sedation or general anesthesia, increases the risk of an asthma exacerbation that requires hospitalization by 3% (4). Airway remodeling in asthma is a heterogeneous process; therefore, conventional BT may be treating airways that are largely uninvolved (5).
Hyperpolarized 129Xe magnetic resonance imaging (129Xe MRI) is a pulmonary imaging technique that has been used in assessing regional heterogeneity of ventilation in numerous pulmonary diseases (6–10). Ventilation defect percentage (VDP), the volume of nonventilated lung in relation to total lung volume, correlates with several clinical parameters, including spirometry, severity, and control, and predicts severe exacerbations (11–14). In addition, it is a repeatable measurement (15–17). Mucous plugging and airway remodeling have both been demonstrated by computed tomography (CT) in the airways, leading to ventilation defects (18, 19), and both entities theoretically could improve based on histological modifications after BT (20–22).
An initial feasibility trial by our group (23) demonstrated that ventilation defects improved over time after BT. This led us to hypothesize that this imaging technique may be useful in guiding a single treatment to the most involved airways, thereby reducing the number of procedures and AEs. Therefore, we sought to evaluate the effectiveness and safety of using hyperpolarized gas MRI to prioritize the most involved airways for guided BT. Some of the results of these studies have been previously reported in the form of abstracts (17, 24).
Methods
Design
The study was a double-blind (treatment planner/outcome assessor and participant), parallel assignment with a 1:1 randomization to guided BT versus standard treatment at a single institution (clinical trial number NCT01832363). All participants provided written informed consent. The study was approved by our institutional review board and was conducted in accordance with the Health Insurance Portability and Accountability Act.
Subjects
All subjects had severe asthma that met American Thoracic Society/European Respiratory Society workshop criteria (2) and were scheduled for a clinically indicated BT by their treating physician. Expanded details regarding all methods can be found in the online supplement.
Randomization and Blinding
At the first study visit, and before each MRI study, Asthma Quality of Life Questionnaire (AQLQ), Asthma Control Test (ACT), and spirometry were performed (Figure 1). Prior to randomization, subjects underwent a baseline CT scan and two baseline hyperpolarized 129Xe imaging sessions separated by 3 weeks in a characterization phase to assess the temporal stability and repeatability of regional ventilation. In addition, at each MRI visit throughout the study, we performed two ventilation studies to assess within-visit repeatability. Xenon ventilation was quantitated, and the outcomes assessor developed a guided treatment plan.
Figure 1.

Bronchial thermoplasty (BT) guided by 129Xe magnetic resonance imaging (MRI) study timeline. All subjects underwent routine testing prior to BT including baseline CT and PFT. Two baseline MRI sessions separated by 3 weeks were performed to assess for stability of ventilation defects. Subjects underwent the first BT and were allowed 12 weeks of recovery prior to a third MRI. Then, the second and third BTs were completed. The fourth MRI was performed after 12 weeks of recovery. Subjects were followed for 1 year. CT = computed tomography; PFT = pulmonary function testing.
Subjects were then randomized in a double-blinded fashion to either the guided group or standard (unguided) treatment group. The proceduralist performing the BT was unblinded and had no role in developing the treatment plan or evaluating outcomes. After the first BT, subjects were allowed 12 weeks of recovery and then underwent a third MRI. All subjects had the remaining two BT treatments performed as detailed below, and a fourth MRI was performed 12 weeks after the last BT. Follow-up visits by phone or in person were performed at 3, 6, 9, and 12 months after the last MRI (post-treatment period). The outcome’s assessor remained blinded until the last subject completed 1 year of follow-up.
Outcomes
The primary outcome was change in AQLQ from baseline 12 weeks after one BT for the guided group compared with AQLQ 12 weeks after three BTs for the unguided treatment group. Secondary outcomes were change in ACT, xenon metrics of ventilation, lung function, and AEs. One year follow-up data included AQLQ, ACT, exacerbations, oral/inhaled corticosteroid dose, and lung function. The minimal clinically important difference (MCID) for AQLQ and ACT is 0.5 and 3, respectively (25, 26). BT responders are defined as subjects who meet the MCID of 0.5 for AQLQ.
Imaging
Clinical volumetric CT imaging was performed at TLC preceding BT using a standard protocol (27) to evaluate the structural anatomy. Proton (1H) and hyperpolarized 129Xe MRI were performed on a 1.5T Siemens Avanto (Siemens Healthcare). Hyperpolarized 129Xe gas (MagniXene; Xemed LLC) was produced using a commercial polarizer system, and subjects inhaled 1 L 129Xe from FRC using a Tedlar bag. Two xenon three-dimensional gradient echo MRI ventilation images were acquired at 6 mm isotropic resolution during separate breath holds, separated by at least 2 minutes. All images were performed without prior treatment with bronchodilator or corticosteroids.
Image Analysis
Segmentation of the lung segments was performed on the CT images using Apollo software (VIDA Diagnostics). A thoracic cavity mask was created from the proton MR images using a custom-trained convolutional neural network based on the U-Net model (28). The xenon ventilation images were bias corrected and segmented into four ventilation categories representing nonventilated, poorly ventilated, normal, and well-ventilated lung (29, 30). Three-dimensional deformable registration was used to align the CT and MRI images, allowing transfer of the segmental and ventilation masks into a common image space (Figure 2) (31). A separate ventilation quantification technique, VDP, was performed by labeling voxels with a signal intensity <60% of the mean whole-lung 129Xe signal as defect, overall and for each lung segment (23). The mean VDP from up to four baseline MR images were used to determine which airways to treat in the guided group. All ventilation images were reviewed for inclusion by a panel of three experts in the field (J.C.W., R.P.T., and J.D.Q.) and scored for quality.
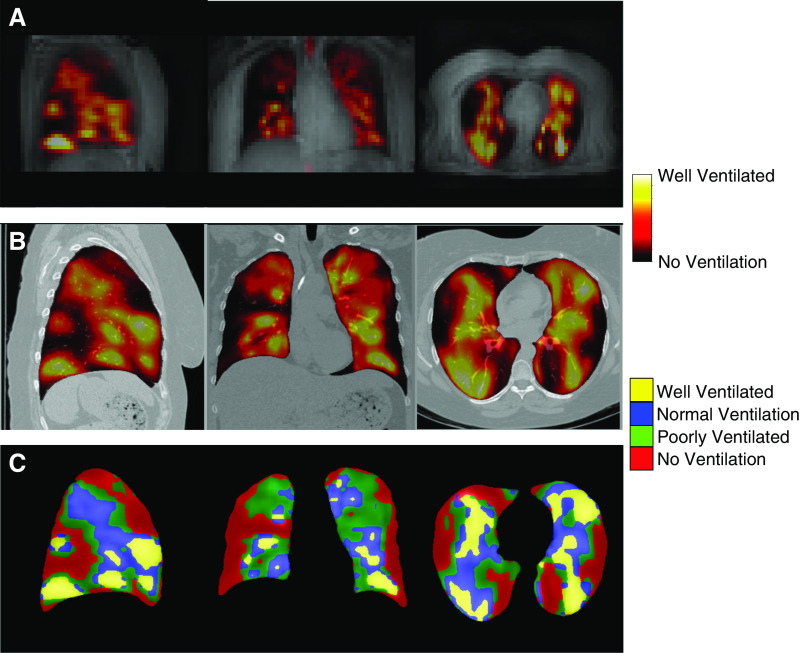
Figure 2.
129Xe magnetic resonance imaging (MRI) images in severe asthma. (A) Low-resolution proton MRI with ventilation image overlay of a patient with severe asthma prior to bronchial thermoplasty. (B) High-resolution volumetric computed tomography with MRI ventilation image overlay after registration to the computed tomography space. (C) The continuous ventilation image is segmented into four categories (no ventilation, poor ventilation, normal ventilation, and well ventilated).
Bronchial Thermoplasty
BT was performed using the Alair Bronchial Thermoplasty System (Boston Scientific). Subjects were randomized to two treatment options for BT: half underwent a standard treatment regimen, and the other half underwent a guided protocol in which the six segments with the largest ventilation defects as determined by quantitative 129Xe image analysis were targeted in the first session (could include right middle lobe). The remaining airways were grouped by region for treatment during the second and third treatment sessions. All patients underwent a standard 5-day corticosteroid burst starting 3 days prior to BT, with alternative dosing only performed if clinically indicated.
Statistical Methods
This study was powered at 80% (26 subjects) to detect a minimum difference in change in AQLQ since baseline (Δ) between treatment groups of 1.0 (twice the MCID) and an SD of 0.9. Δ AQLQ, and ACT outcomes were analyzed using a linear mixed model approach. Treatment, BT # (1 or 3), and their interaction were included as fixed effects in the models, the subject was treated as a random effect to account for repeated measurements, and the outcome at baseline was used as a covariate. The model-adjusted means corresponding to the primary (Δ guided BT #1 vs. Δ unguided BT #3) and secondary hypotheses (guided BT #1 vs. guided baseline, unguided BT #1 vs. Unguided baseline) were assessed with approximate t tests derived from the mixed model (PROC MIXED, SAS version 9.4; SAS Institute).
Similarly, changes in MRI outcomes since baseline were analyzed using linear mixed models. Treatment and baseline ventilation were included as fixed effects, and the subject was treated as a random effect to account for multiple airway segments per subject at each visit. Separate analyses were conducted for the primary (Δ guided BT #1 vs. Δ unguided BT #3) and secondary hypothesis tests (Δ guided BT #1 vs. Δ unguided BT #1; Δ guided BT#3 vs. Δ unguided #3). MRI outcomes are reported as percentages in the results. To better meet model assumptions of normality and homoscedasticity, VDP was analyzed as a square root–transformed proportion. For MRI outcomes after the first BT, only targeted airways are included in the analyses. After all three BTs, all airways are included in the model.
To test for differences in baseline characteristics between the treatment groups, t tests, chi-square tests, or Fisher’s exact tests were used as appropriate. Healthcare use outcomes at baseline were analyzed using negative binomial regression. All 1-year follow-up models were analyzed using analysis of covariance where the baseline value was included as a covariate. P values < 0.05 were considered significant.
Results
Study Cohort
Thirty-four subjects were screened, and 30 were randomized to the guided BT group (15 subjects) or unguided BT group (15 subjects) (Figure 3). One subject did not tolerate the MRI procedure owing to an anxiety attack and was dropped before randomization. The primary endpoint was reached in 15 guided and 14 unguided BT subjects (1 lost to follow-up after second BT). All airways identified as targets were accessible by BT. Nine out of 15 subjects in the guided group had at least one segment from the right middle lobe treated. As planned in the study protocol, the median time between the first BT (guided or unguided) and the third MRI (primary endpoint for guided group) was 13 weeks (quartile 1 [Q1], 12.1; quartile 3 [Q3], 15.6; min, 11.1; max, 32.7). Fourteen subjects from each group completed 1 year of follow-up. Baseline characteristics were well paired between groups (Table 1). Subjects had severe (mean FEV1 ± SD, 69.7 ± 24.2% predicted; 63% on chronic oral corticosteroids and 43% on omalizumab) and uncontrolled (mean ACT ± SD, 9.6 ± 3.6; mean AQLQ ± SD, 2.9 ± 0.9) asthma. In the unguided group, there was a trend toward more subjects on chronic oral corticosteroids (unguided = 8/15 vs. guided = 3/15; P = 0.058) and omalizumab (unguided = 9/15 vs. guided = 4/15; P = 0.07). When compared with the AIR2 (Asthma Intervention Research 2) trial (4), our subjects had more airflow obstruction and lower quality of life with worse asthma control at baseline.
Figure 3.
Bronchial thermoplasty (BT) guided by 129Xe magnetic resonance imaging (MRI) study flowchart. Thirty-four subjects underwent screening. Two subjects were deemed inappropriate for BT, and one subject refused treatment. Thirty-one subjects entered the characterization phase, which includes baseline MRIs. One subject did not tolerate the MRI procedure and was dropped from the study. A total of 30 subjects were randomized to either guided or unguided BT. Fifteen subjects in the guided and 14 in the unguided treatment group completed three BT treatments. One subject was lost to follow-up after the second BT in the unguided treatment group. Fourteen subjects from each arm completed 1 year of follow-up. f/u = follow-up; LLL = left lower lobe; RLL = right lower lobe.
Table 1.
Subject Demographics and Baseline Characteristics
| Characteristics | Guided BT (n = 15) | Unguided BT (n = 15) | P Value |
|---|---|---|---|
| Age at enrollment, yr* | 45.46 ± 10.19 (15) | 45.11 ± 9.39 (15) | 0.92 |
| Age at diagnosis, yr* | 19.13 ± 17.15 (15) | 18.4 ± 16.01 (15) | 0.90 |
| Duration of asthma, yr* | 26.33 ± 15.9 (15) | 26.71 ± 14.06 (15) | 0.94 |
| Sex† | 0.65 | ||
| Male | 2/15 (13) | 4/15 (27) | |
| Female | 13/15 (87) | 11/15 (73) | |
| Race† | >0.99 | ||
| White | 14/15 (93) | 14/15 (93) | |
| Nonwhite | 1/15 (6.7) | 1/15 (6.7) | |
| BMI, kg/m2* | 34.73 ± 5.53 (15) | 35.59 ± 9.34 (15) | 0.76 |
| Asthma Control Test score* | 8.8 ± 3.59 (15) | 10.47 ± 3.54 (15) | 0.21 |
| Asthma Quality of Life Questionnaire score* | 2.79 ± 0.78 (15) | 3.05 ± 1.1 (15) | 0.45 |
| Baseline FEV1, L* | 2.15 ± 0.71 (15) | 2.27 ± 0.86 (15) | 0.68 |
| FEV1% predicted* | 70.09 ± 25.64 (15) | 69.21 ± 23.65 (15) | 0.92 |
| Bronchodilator FEV1 reversibility % improvement* | 16.12 ± 21 (15) | 12.87 ± 9.88 (15) | 0.59 |
| PC20, mg/ml‡ | 0.51 ± 1.12 (11) | 1.11 ± 1.32 (8) | 0.38 |
| IgE‡ | 60.88 ± 4.5 (15) | 82.58 ± 4.08 (13) | 0.64 |
| Blood eosinophils ≥ 300 cells/ml† | 8/15 (53) | 5/14 (36) | 0.34 |
| Sputum eosinophils ≥ 3% | 4/9 (44) | 2/6 (33) | >0.99 |
| At least one positive allergy skin test† | 9/10 (90) | 7/8 (88) | >0.99 |
| Medication use | |||
| Chronic oral corticosteroids† | 3/15 (20) | 8/15 (53) | 0.06 |
| Oral corticosteroid dose* | 18.33 ± 2.89 (3) | 16.88 ± 7.99 (8) | 0.77 |
| Inhaled corticosteroids dose* | 1,991.47 ± 560.06 (15) | 1,881.07 ± 783.33 (15) | 0.66 |
| Number of pulses of oral or i.v. corticosteroid in the past year* | 6.4 ± 5.44 (15) | 4.93 ± 3.33 (15) | 0.32 |
| Omalizumab therapy† | 4/15 (26.7) | 9/15 (60) | 0.07 |
| Unscheduled visits in past year* | 3.6 ± 6.12 (15) | 2.47 ± 2.64 (15) | 0.49 |
| ER visits in the past year* | 1.2 ± 1.37 (15) | 2.8 ± 3.78 (15) | 0.09 |
| Hospitalizations in past year* | 0.6 ± 0.83 (15) | 1.13 ± 2.2 (15) | 0.39 |
| VDP, %* | |||
| Whole lung | 14.5 ± 12.6 (10) | 10.1 ± 9.4 (11) | 0.37 |
| First BT | 23.7 ± 22.8 (10) | 11.9 ± 15.9 (11) | 0.18 |
| Poor + no ventilation, %* | |||
| Whole lung | 47.8 ± 16.5 (10) | 45.9 ± 15.7 (11) | 0.79 |
| First BT | 58.8 ± 25.3 (10) | 42.0 ± 15.8 (11) | 0.08 |
Definition of abbreviations: BMI = body mass index; BT = bronchial thermoplasty; ER = emergency room; first BT = ventilation defect of segments at baseline treated in first BT session; i.v. = intravascular; PC20 = provocation concentration causing a 20% fall in FEV1; VDP = ventilation defect percentage.
Data are mean ± SD (number of subjects).
Data are numbers (%); for categorical variables, percentages reported are based on total nonmissing sample size.
Statistics presented as back-transformed geometric mean ± SD (number of subjects).
Primary Outcome
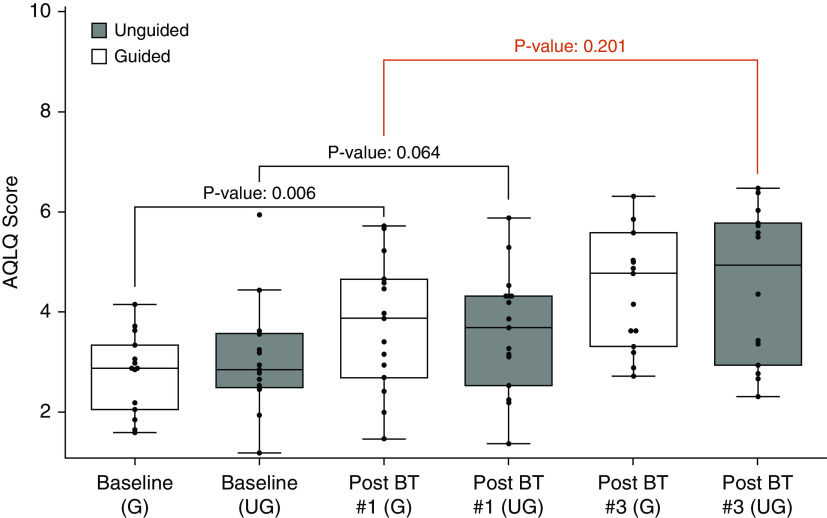
There was no statistical difference (P = 0.201) in AQLQ improvement from baseline after one guided treatment (adjusted mean Δ, 0.91 [95% confidence interval (CI), 0.28–1.53]; P = 0.006) when compared with three unguided BT treatments (adjusted mean Δ, 1.49 [95% CI, 0.84–2.14]; P = <0.001) (Figure 4). This remained true when controlling for the differences in oral corticosteroid use and anti-IgE therapy at baseline. The improvement after one treatment was clinically (Δ AQLQ > 0.5) and statistically significant (P = 0.006) for the guided group (adjusted mean Δ, 0.91 [95% CI, 0.28–1.53]), whereas the unguided treatment group had a smaller and nonsignificant improvement after one treatment (adjusted mean Δ, 0.59 [95% CI, −0.04 to 1.22]; P = 0.064). Eleven out of 15 (73%) subjects in the guided group were responders after one treatment, and in comparison, 10 out of 14 (71%) were responders in the unguided group after three treatments. Nine out of 15 (60%) subjects in the unguided group had an improvement in AQLQ >0.5 after one treatment. After three treatments, the guided group lost 1 initial responder and gained 3 unique responders for a total of 13 out of 15 (87%).
Figure 4.
Primary endpoint: Change in quality of life after bronchial thermoplasty—guided and unguided. Boxplots are shown demonstrating AQLQ score at baseline, after one treatment, and after three treatments by group. The red brackets represent the primary endpoint. AQLQ = Asthma Quality of Life Questionnaire; BT = bronchial thermoplasty; G = guided; UG = unguided.
Overall, there was no significant difference in the number of BT activations between groups (see Table E1 in the online supplement), as prior data suggest a relationship between the number of activations with improved asthma control after BT (32). However, during the first BT, the guided group had significantly more activations than the unguided group (guided [mean ± SD] = 142 ± 31, unguided [mean ± SD] = 114 ± 35, P = 0.03). There was no association between the number of BT activations and the change in AQLQ after the first BT (P = 0.77).
Asthma Control
The adjusted mean improvement in ACT after one guided and one unguided treatment from baseline was 1.67 (95% CI, −0.71 to 4.04) and 1.54 (95% CI, −0.83 to 3.91), respectively. The mean ACT improvement from baseline after three unguided treatments (adjusted mean Δ, 5.73 [95% CI, 3.27–8.2]; P = <0.001) was significantly higher (P = 0.022) than one guided treatment (adjusted mean Δ, 1.67 [95% CI, −0.71 to 4.04]; P = 0.165). The guided group had further improvement in ACT after completing the final two treatments compared with a single treatment, with an additional mean improvement of 2.8 (95% CI, 1.1–6.7; P = 0.06) (see Figure E1).
Ventilation
There was a total of 160 acceptable scans and 53 unacceptable scans for subjects who underwent BT (see Table E2). The median signal-to-noise ratio for acceptable scans was 38.2 (Q1, 21.1; Q3, 65.8), and for the unacceptable scans, the median was 16.8 (Q1, 5.6; Q3, 29.9). The large number of unacceptable scans were in large part due to coil problems experienced during the middle of the study. All results are limited to acceptable MR images.
Overall, there were similar distributions of ventilation defects in both groups by segments and whole lung (see Figure E2). Baseline MRIs, separated by a median of 3 weeks, did not show any statistically significant differences in ventilation (see Figure E3). Similarly, the mean differences between MRIs within visits were generally not different from zero across airway segments and visits (see Figures E4–E7).
After one BT, the guided group had a greater reduction in the percentage of poorly/nonventilated lung from baseline in treated airways when compared with unguided (adjusted mean Δ guided = −12.4% [95% CI, −21.1 to −3.6], adjusted mean Δ unguided = +4.9% [95% CI, −3.6 to 13.4], P = 0.009). Alternatively, the guided group had a 12.4% relative improvement in normal/well-ventilated lung compared with baseline. Using a second quantification technique, VDP, the guided group demonstrated a reduction (unadjusted mean Δ, −10.3%) compared with an increase (unadjusted mean Δ, 3.4%) in the unguided group after one treatment (P value from analysis of square root–transformed outcome = 0.018) (Figure 5A). The analysis controlled for baseline ventilation of treated airways.
Figure 5.
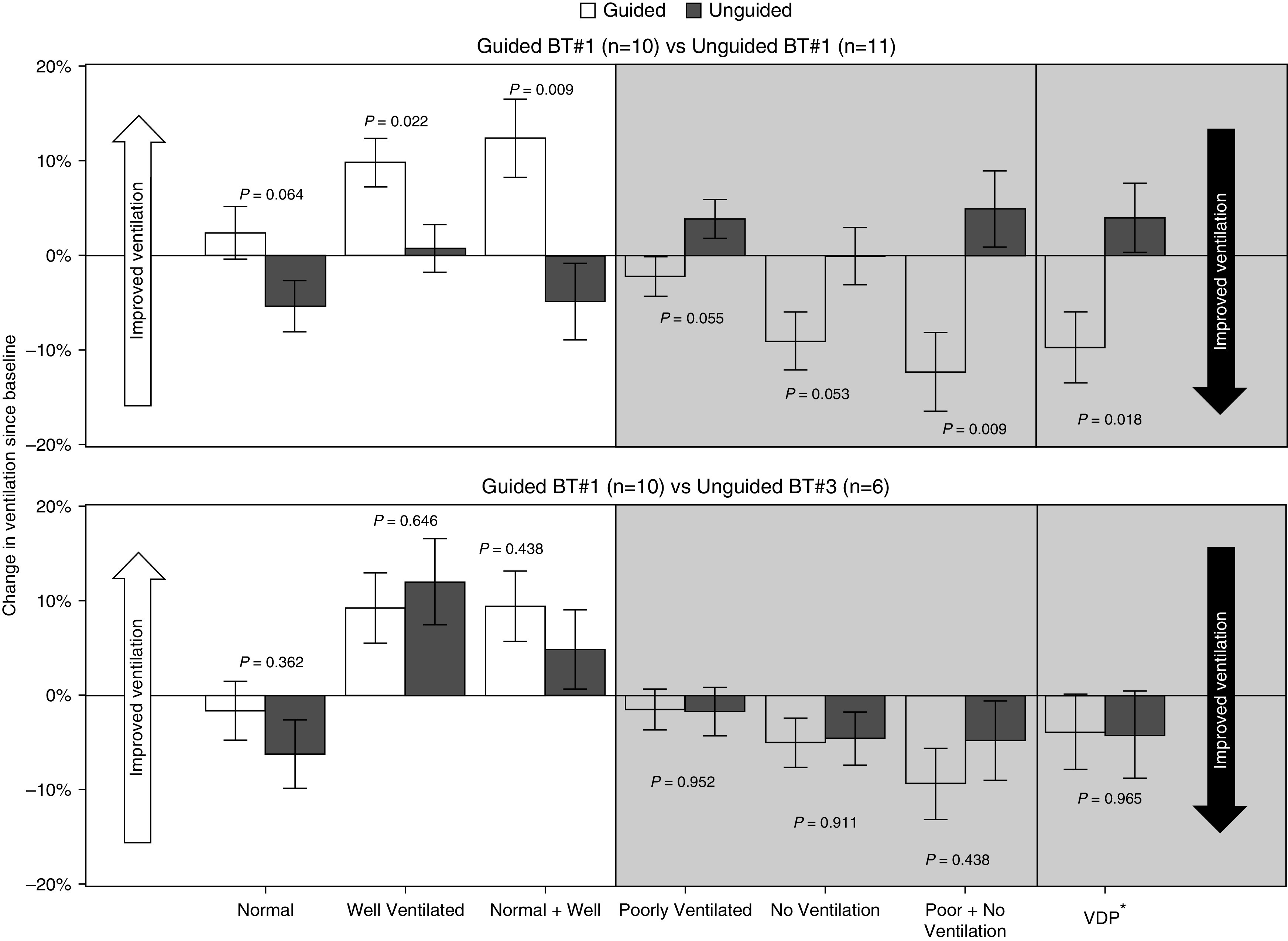
Secondary endpoint: Change in 129Xe magnetic resonance imaging ventilation after bronchial thermoplasty (BT)—guided and unguided. (Top) Mean adjusted ventilation change is shown in treated airways from baseline after one BT for both guided and unguided groups. The analysis controlled for baseline ventilation of treated airways. The guided group has significantly more improvement in the percentage of normal/well-ventilated lung as well as reduction in the amount of poorly/nonventilated lung. (Bottom) Mean adjusted ventilation change is shown in treated airways from baseline after one BT for the guided group compared with three BT treatments in the unguided group. There are no significant differences between the groups. Analysis is limited to images with acceptable quality. Error bars correspond to ± 1 SE. VDP* = square root–transformed ventilation defect percentage.
The ventilation changes from baseline in treated airways after one guided treatment compared with three unguided treatments were not different for any of the ventilation categories (Figure 5B). Similarly, after three BT treatments, there were no ventilation differences between the guided and unguided groups.
When the evaluation was performed on all MRI scans (including those with inadequate quality), we saw a similar trend in results (see Figure E8). Despite the lower sample size, our estimates were more precise when we dropped the low-quality scans. Figure 6 demonstrates the ventilation improvement in a subject treated with guided therapy.
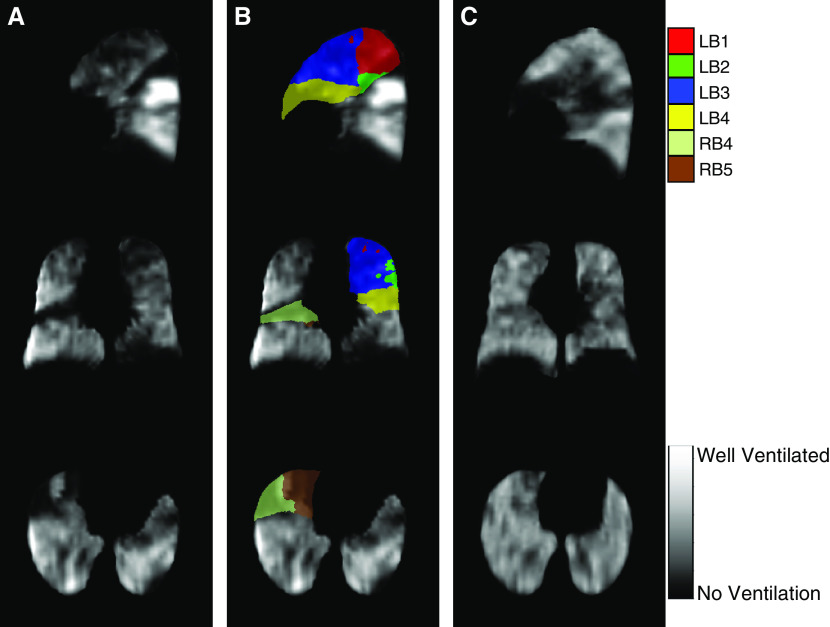
Figure 6.
129Xe magnetic resonance imaging ventilation images before and after bronchial thermoplasty. (A) Grayscale ventilation images from a patient with severe asthma before guided bronchial thermoplasty. (B) Ventilation image with color mask overlay of the six segments treated with bronchial thermoplasty. (C) Grayscale ventilation images from the same patient after guided bronchial thermoplasty with improved ventilation in the treated areas. Colors denote the Boyden classification of bronchi. LB1 and LB2 = apicoposterior segment of left upper lobe; LB3 = anterior segment of left upper lobe; LB4 = superior lingular segment of left upper lobe; RB4 = lateral segment of right middle lobe; RB5 = medial segment of right middle lobe.
Adverse Events
Of the 89 treatments, the guided group had fewer asthma-related AEs/severe adverse events (SAEs) in the peritreatment period (guided = 13, unguided = 23) (Table 2). Thirty-three percent experienced asthma exacerbations after one guided BT compared with 73% after three unguided BT (P = 0.028). Only one severe exacerbation occurred in the guided group after a single BT compared with six in the unguided group after three treatments. After three treatments, four severe exacerbations occurred in the guided group and six occurred in the unguided group. After 1 year of follow-up, 11 subjects in each group had asthma exacerbations; however, the guided group had more exacerbation events in the post-treatment period (guided = 52 vs. unguided = 23) (see Table E3). Total AEs/SAEs throughout the duration of the study can be found in Table E4.
Table 2.
Secondary Outcomes: Adverse Events and Serious Adverse Events in the Periprocedure and Post-treatment Period by Group
| Guided BT | Unguided BT | |
|---|---|---|
| Peri-BT period | ||
| BT 1 | ||
| AE | ||
| Asthma exacerbation | 4/15 (26.7) | 7/15 (46.7) |
| Other | 2/15 (13.3) | 1/15 (6.7) |
| SAE | ||
| Asthma exacerbation | 1/15 (6.7) | 2/15 (13.3) |
| Other | 1/15 (6.7) | 0/15 (0) |
| Pneumonia | 1/15 (6.7) | 0/15 (0) |
| BT 2 | ||
| AE | ||
| Asthma exacerbation | 1/15 (6.7) | 5/15 (33.3) |
| Other | 1/15 (6.7) | 2/15 (13.3) |
| Sinusitis | 0/15 (0) | 1/15 (6.7) |
| Upper respiratory infection | 0/15 (0) | 1/15 (6.7) |
| SAE | ||
| Asthma exacerbation | 1/15 (6.7) | 1/15 (6.7) |
| Pneumonia | 0/15 (0) | 1/15 (6.7) |
| BT 3 | ||
| AE | ||
| Asthma exacerbation | 4/15 (26.7) | 5/14 (35.7) |
| Other | 0/15 (0) | 1/14 (7.1) |
| Pneumonia | 0/15 (0) | 1/14 (7.1) |
| Sinusitis | 1/15 (6.7) | 0/14 (0) |
| Upper respiratory infection | 0/15 (0) | 1/14 (7.1) |
| SAE | ||
| Asthma exacerbation | 2/15 (13.3) | 3/14 (21.4) |
| Post-treatment period | ||
| AE | ||
| Acute upper respiratory infection | 5/15 (33.3) | 2/14 (14.2) |
| Asthma exacerbation | 11/15 (73.3) | 10/14 (71.4) |
| Bronchitis | 2/15 (13.3) | 1/14 (7.1) |
| Sinusitis | 2/15 (13.3) | 2/14 (14.3) |
| Other | 3/15 (20) | 1/14 (7.1) |
| SAE | ||
| Asthma exacerbation | 3/15 (20) | 1/14 (7.1) |
| Other | 2/15 (13.3) | 0/14 (0) |
| Any AE or SAE | 11/15 (73.3) | 12/14 (85.7) |
Definition of abbreviations: AE = adverse event; BT = bronchial thermoplasty; peri-BT = day of BT up to 3 weeks after BT; post-treatment = magnetic resonance imaging 4 through 1-year follow-up visit; SAE = severe adverse event requiring hospitalization.
AEs/SAEs are reported as subject AE/SAE category. If an AE transitioned to an SAE, then only the SAE is reported. Other AEs include chest pain, dizziness and giddiness, nausea and vomiting, sciatica, tachycardia, unspecified fracture of ankle (closed), unspecified local infection of skin, subcutaneous tissue, central retinal vein occlusion, urinary tract infection, diaphragmatic hernia, and gastroenteritis. Data are shown as numbers (%).
Transient dizziness and giddiness, lasting a few seconds, was the most common AE experienced during the MRI. There were no SAEs related to the MRI or xenon inhalation. Patients also experienced other mild nonspecific symptoms related to xenon inhalation (see Table E5).
One-Year Follow-up
At 1 year, both quality of life and asthma control improved from baseline (ΔAQLQ [mean ± SD] = 1.50 ± 1.63, P = <0.001; ΔACT [mean ± SD] = 5.61 ± 5.24, P < 0.001). There was no significant difference in AQLQ or ACT scores between treatment groups at 1 year (AQLQ guided [mean ± SD] = 4.29 ± 1.6, unguided [mean ± SD] = 4.64 ± 1.11, P = 0.51; ACT guided [mean ± SD] = 14.14 ± 6.46, unguided [mean ± SD] = 16.64 ± 4.96, P = 0.26). The change in healthcare use at 1 year compared with baseline was similar between groups; however, the unguided group had a greater reduction in the number of unscheduled visits (guided [mean ± SD] = −0.64 ± 5.24, unguided [mean ± SD] = −1.43 ± 2.71, P = 0.033). Both groups had a reduction in the oral and inhaled corticosteroid dose at 1 year, but the unguided group had a significant reduction in the need for pulse dose corticosteroids. Changes in spirometry measures since baseline were not different between the treatment groups at 1 year (Table 3).
Table 3.
Secondary Outcomes: One-Year Outcome Changes from Baseline by Group
| Guided BT (n = 14) | Unguided BT (n = 14) | P Value | |
|---|---|---|---|
| Medication use | |||
| OCS dose* | −5 ± 13.23 (3) | −3.5 ± 6.02 (5) | 0.77 |
| ICS dose* | −328 ± 826.94 (14) | −439.14 ± 682.8 (14) | 0.61 |
| Oral corticosteroid pulses* | −1.86 ± 4.07 (14) | −2.5 ± 2.9 (14) | 0.045 |
| Asthma control | |||
| ACT* | 5.36 ± 5.31 (14) | 5.86 ± 5.35 (14) | 0.59 |
| MCID† | 10/14 (71) | 9/14 (64) | >0.99 |
| Quality of life | |||
| AQLQ* | 1.42 ± 1.62 (14) | 1.58 ± 1.7 (14) | 0.53 |
| MCID† | 9/14 (64) | 11/14 (79) | 0.68 |
| Healthcare use | |||
| ER visits*‡ | 0.07 ± 1.14 (14) | −2.29 ± 3.45 (14) | 0.053 |
| Unscheduled visits*‡ | −0.64 ± 5.24 (14) | −1.43 ± 2.71 (14) | 0.033 |
| Hospitalizations*‡ | 0.07 ± 1 (14) | −0.79 ± 2.26 (14) | 0.31 |
| Spirometry | |||
| FEV1* | 0.05 ± 0.31 (12) | 0.05 ± 0.34 (12) | 0.66 |
| FEV1% predicted* | 2.51 ± 10.73 (12) | 3.21 ± 9.61 (12) | 0.8 |
Definition of abbreviations: ACT = Asthma Control Test; AQLQ = Asthma Quality of Life Questionnaire; BT = bronchial thermoplasty; ER = emergency room; ICS = inhaled corticosteroid; MCID = minimal clinically important difference, ACT MCID ≥ 3, AQLQ MCID ≥ 0.5; OCS = oral corticosteroid.
Data reflect 1-year follow-up – baseline. Bold indicates a significant P value.
Data are mean ± SD (number of subjects).
Data are numbers (%); for categorical variables, percentages reported are based on total nonmissing sample size.
Events over the past year.
Discussion
In this pilot study, we demonstrated that a single BT treatment guided by 129Xe MRI provided similar quality-of-life improvements after 12 weeks as standard three-session BT. One of the most notable benefits of a single treatment is reduced periprocedure AEs, making it a more favorable option. However, the single treatment resulted in a lower short-term quality-of-life improvement than three treatments (though not statistically significant). The number of BT responders in the guided group after one treatment was similar to the number of responders in the unguided group after all three treatments (11/15 vs. 10/14).
There is limited information in assessing changes in ventilation and asthma outcomes after targeted BT. Determining the appropriate time point after BT to evaluate the subjects was based on prior work by Thomen and colleagues demonstrating that ventilation improvements are a function of time (23). We chose to allow 12 weeks of recovery based on these data, though this time point may not be optimal for detecting changes in asthma control and quality of life.
The optimal number and areas of the lung to be treated with BT has not been well studied. Donovan and colleagues (33) recently evaluated structure- and function-guided treatments using human lung specimens and computational methods to predict response to BT. They found that a single structure-guided treatment demonstrated significant improvements over the standard practice. This work built upon a prior discovery that the reduction in smooth muscle in centrally treated airways leads to improved flow patterns in the more distal small airways (34). They hypothesize that areas of low ventilation may be better targets for BT than complete defects when using function-guided therapy. Furthermore, our study design selected the six segments with the largest VDP for targeted treatment based on prior experience that patients could tolerate this number of segments being treated in one session. However, alternative strategies such as selecting for treatment only segments with at least a VDP of 5% or greater may result in improved outcomes. In addition, recent literature suggests that optimizing sputum cell counts prior to BT improves outcomes, which was not evaluated in our study (35). Doing so may optimize patient selection for those with primarily smooth muscle dysfunction as opposed to inflammatory ventilation defects (36). Prior studies have suggested that the number of activations plays an important role in asthma control improvements after BT (32). Although the guided group had more activations than the unguided group in the first treatment, there was no association with improvement in AQLQ. Both groups surpassed the minimum recommended activations that should be performed in a treatment session as determined by Langton and colleagues (32).
In the past 10 years, numerous therapies have come to market to aid in the treatment of severe asthma. This study adds to the growing list of studies that demonstrate the efficacy of BT as an additional treatment tool. Our cohort had more severe asthma and a lower baseline quality of life than the AIR2 trial subjects (mean baseline AQLQ in AIR2 ± SD = 4.30 ± 1.17) (4). Yet, the AQLQ improvements from baseline achieved at 1 year were similar (AIR2 [mean ± SD] = 1.35 ± 1.10, our study [mean ± SD] = 1.50 ± 1.63). Regardless of treatment group, the AQLQ improvements at 1 year compare favorably with the available biologic therapies (AQLQ omalizumab = 1.01, mepolizumab = 0.55, reslizumab = 1.08, benralizumab = 1.47, dupilumab = 1.28) (37–41). Single-session guided BT may represent a more cost-effective treatment when compared with lifelong biologic therapy in appropriate candidates; however, a direct comparison trial has not been performed.
The results of our pilot study of guided BT have several limitations. First, as BT was clinically indicated, we felt obligated to complete the final two treatments in the guided group. We wanted to provide as close to standard of care as possible, while still answering the research question. This makes comparing long-term outcomes between groups in the post-treatment period difficult, as all airways were treated in both groups. Second, 12 weeks after BT may not be the optimal time to evaluate quality of life and asthma control after a single BT, and longitudinal studies using only a single treatment are needed. In fact, we did see a greater improvement in asthma control than quality of life after all three unguided treatments in comparison with one guided treatment. However, this may be related to differences in duration of recall for the questionnaires. There will continue to be questions surrounding how many airways are appropriate to treat in a single guided treatment. It may be unnecessary to limit treatment to six airways if a smaller or larger number of segments have ventilation abnormalities. Third, we did not have a sham control group to compare outcomes as was previously done in the AIR2 trial (4). Fourth, there was a short time period in which we experienced MR coil problems, and the quality of images was affected. To help with quality assessment, we asked a panel of three experts in 129Xe MRI to independently score each ventilation image prior to unblinding and statistical assessment. Lastly, the reproducibility of ventilation abnormalities over time was another concern. In general, we did not see a significant difference in ventilation between the two baseline MRIs; however, there was segmental variability. To mitigate this issue, we targeted the segments with the largest mean VDP in up to four baseline ventilation images. In addition, image segmentation and registration errors could contribute to variability and target selection errors. When designing future trials, it may be worthwhile using post-bronchodilator images to decrease ventilation variability in segments.
Moving forward, it is important to verify these results with a larger multicenter randomized trial using a single treatment in the guided group. This would allow for more accurate comparisons of the AEs in the post-treatment period. As we powered the study to have an effect size that was twice the MCID, it is also important to verify the results with a larger sample size. In addition, there is need toward standardizing ventilation quantification in much the same way that the Radiological Society of North America Quantitative Imaging Biomarkers Alliance is doing for quantitative CT and other imaging biomarkers. This will facilitate reproducibility among centers. Relating the ventilation changes noted on 129Xe MRI to surrogate CT biomarkers of small airways disease (parametric response mapping, disease probability mapping, and < −850 Hounsfield units on expiration) or airway remodeling (wall thickness, wall area, and Pi10) may also facilitate the accessibility of guided BT.
Conclusions
In summary, this study demonstrated that a single BT treatment guided with 129Xe MRI provides a significant benefit over baseline after 12 weeks, yielding a mean benefit in quality of life similar to standard treatment. When comparing a single guided BT treatment with the standard three-treatment regimen, the mean percentage of responders (those who met the MCID of 0.5 for AQLQ) was nearly equivalent, with fewer patients experiencing periprocedure asthma exacerbations from the single guided session. We believe these promising results call for further studies of targeted BT to enable better quantification of the mean benefit achieved by a single guided treatment and the associated level of risk reduction.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge NVIDIA Corporation for donating the Titan Xp GPU used in this study and Boston Scientific Corporation for donating the Alair catheters.
Footnotes
Supported by NIH grants R44 HL112397, UL1 TR000448, T32 HL007317, and T32 AI106688.
Author Contributions: J.D.Q., J.C.W., I.C.R., F.W.H., and M.C. contributed to study design and data acquisition, interpretation, and analysis. J.K., L.G., and T.K. contributed to data acquisition. R.P.T., N.J.T., and J.P.M. contributed to image analysis. C.W.G., D.L., and K.B.S. contributed to statistical analysis. C.S.H. contributed to data acquisition, interpretation, and analysis, image analysis and interpretation, and drafted the initial manuscript. All authors reviewed, edited, and approved the final version for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201905-1021OC on June 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma Eur Respir J 201443343–373.[Published erratum appears in Eur Respir J 43:1216; Published erratum appears in Eur Respir J 52:1352020.] [DOI] [PubMed] [Google Scholar]
- 3.Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa e Silva JR, Shah PL, et al. Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295–1302. doi: 10.1016/j.jaci.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aysola R, de Lange EE, Castro M, Altes TA. Demonstration of the heterogeneous distribution of asthma in the lungs using CT and hyperpolarized helium-3 MRI. J Magn Reson Imaging. 2010;32:1379–1387. doi: 10.1002/jmri.22388. [DOI] [PubMed] [Google Scholar]
- 6.Virgincar RS, Cleveland ZI, Kaushik SS, Freeman MS, Nouls J, Cofer GP, et al. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR Biomed. 2013;26:424–435. doi: 10.1002/nbm.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros. 2017;16:275–282. doi: 10.1016/j.jcf.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner L, He M, Virgincar RS, Heacock T, Kaushik SS, Freemann MS, et al. Hyperpolarized 129Xenon magnetic resonance imaging to quantify regional ventilation differences in mild to moderate asthma: a prospective comparison between semiautomated ventilation defect percentage calculation and pulmonary function tests. Invest Radiol. 2017;52:120–127. doi: 10.1097/RLI.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenningsen S, Kirby M, Starr D, Leary D, Wheatley A, Maksym GN, et al. Hyperpolarized (3) He and (129) Xe MRI: differences in asthma before bronchodilation. J Magn Reson Imaging. 2013;38:1521–1530. doi: 10.1002/jmri.24111. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi A, Hall C, Hoffman EA, Woods JC, Gierada DS, Castro M. Using imaging as a biomarker for asthma. J Allergy Clin Immunol. 2017;139:1–10. doi: 10.1016/j.jaci.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenningsen S, Nair P, Guo F, McCormack DG, Parraga G. Is ventilation heterogeneity related to asthma control? Eur Respir J. 2016;48:370–379. doi: 10.1183/13993003.00393-2016. [DOI] [PubMed] [Google Scholar]
- 12.Altes TA, Mugler JP, III, Ruppert K, Tustison NJ, Gersbach J, Szentpetery S, et al. Clinical correlates of lung ventilation defects in asthmatic children. J Allergy Clin Immunol. 2016;137:789–796, e7. doi: 10.1016/j.jaci.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey K, Mummy D, Zha W, Evans M, Sorkness RL, Hall C, et al. Ventilation defect percent from hyperpolarized helium-3 MRI is predictive of asthma exacerbation severity [abstract] Am J Respir Crit Care Med. 2020;201:A6102. [Google Scholar]
- 14.Mummy DG, Kruger SJ, Zha W, Sorkness RL, Jarjour NN, Schiebler ML, et al. Ventilation defect percent in helium-3 magnetic resonance imaging as a biomarker of severe outcomes in asthma. J Allergy Clin Immunol. 2018;141:1140–1141, e4. doi: 10.1016/j.jaci.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niles DJ, Kruger SJ, Dardzinski BJ, Harman A, Jarjour NN, Ruddy M, et al. Exercise-induced bronchoconstriction: reproducibility of hyperpolarized 3He MR imaging. Radiology. 2013;266:618–625. doi: 10.1148/radiol.12111973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP, III, et al. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009;250:567–575. doi: 10.1148/radiol.2502080188. [DOI] [PubMed] [Google Scholar]
- 17.Hall C, Quirk JD, Goss C, Kozlowski J, Thomen RP, Woods JC, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty [abstract] Am J Respir Crit Care Med. 2018;197:A6382. [Google Scholar]
- 18.Mummy D, Dunican E, Lampkins T, Zha W, Evans M, Schiebler M, et al. Ventilation defects in asthma on hyperpolarized gas MRI are associated with airway mucus plugs on CT. Am J Respir Crit Care Med. 2020;201:A6376. [Google Scholar]
- 19.Svenningsen S, Kirby M, Starr D, Coxson HO, Paterson NA, McCormack DG, et al. What are ventilation defects in asthma? Thorax. 2014;69:63–71. doi: 10.1136/thoraxjnl-2013-203711. [DOI] [PubMed] [Google Scholar]
- 20.Chakir J, Haj-Salem I, Gras D, Joubert P, Beaudoin ÈL, Biardel S, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc. 2015;12:1612–1618. doi: 10.1513/AnnalsATS.201504-208OC. [DOI] [PubMed] [Google Scholar]
- 21.Pretolani M, Dombret MC, Thabut G, Knap D, Hamidi F, Debray MP, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190:1452–1454. doi: 10.1164/rccm.201407-1374LE. [DOI] [PubMed] [Google Scholar]
- 22.Haj Salem I, Gras D, Joubert P, Boulet LP, Lampron N, Martel S, et al. Persistent reduction of mucin production after bronchial thermoplasty in severe asthma. Am J Respir Crit Care Med. 2019;199:536–538. doi: 10.1164/rccm.201811-2064LE. [DOI] [PubMed] [Google Scholar]
- 23.Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, Szczesniak RD, et al. Regional ventilation changes in severe asthma after bronchial thermoplasty with (3)He MR imaging and CT. Radiology. 2015;274:250–259. doi: 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall C, Quirk JD, Goss CW, Lew D, Kozlowski J, Thomen RP, et al. Targeted bronchial thermoplasty guided by 129Xe MRI [abstract] Am J Respir Crit Care Med. 2019;199:A7355. doi: 10.1164/rccm.201905-1021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 26.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124:719–723, e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. MICCAI. 2015;9351:234–241. [Google Scholar]
- 29.Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tustison NJ, Avants BB, Flors L, Altes TA, de Lange EE, Mugler JP, III, et al. Ventilation-based segmentation of the lungs using hyperpolarized (3)He MRI. J Magn Reson Imaging. 2011;34:831–841. doi: 10.1002/jmri.22738. [DOI] [PubMed] [Google Scholar]
- 31.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langton D, Sha J, Ing A, Fielding D, Thien F, Plummer V. Bronchial thermoplasty: activations predict response. Respir Res. 2017;18:134. doi: 10.1186/s12931-017-0617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan GM, Elliot JG, Boser SR, Green FHY, James AL, Noble PB. Patient-specific targeted bronchial thermoplasty: predictions of improved outcomes with structure-guided treatment. J Appl Physiol (1985) 2019;126:599–606. doi: 10.1152/japplphysiol.00951.2018. [DOI] [PubMed] [Google Scholar]
- 34.Donovan GM, Elliot JG, Green FHY, James AL, Noble PB. Unraveling a clinical paradox: why does bronchial thermoplasty work in asthma? Am J Respir Cell Mol Biol. 2018;59:355–362. doi: 10.1165/rcmb.2018-0011OC. [DOI] [PubMed] [Google Scholar]
- 35.Svenningsen S, Lim HF, Goodwin S, Kjarsgaard M, Parraga G, Miller J, et al. Optimizing sputum cell counts prior to bronchial thermoplasty: a preliminary report. Can J Respir Crit Care Sleep Med. 2019;3:143–147. [Google Scholar]
- 36.Svenningsen S, Eddy RL, Lim HF, Cox PG, Nair P, Parraga G. Sputum eosinophilia and magnetic resonance imaging ventilation heterogeneity in severe asthma. Am J Respir Crit Care Med. 2018;197:876–884. doi: 10.1164/rccm.201709-1948OC. [DOI] [PubMed] [Google Scholar]
- 37.Chipps B, Buhl R, Beeh KM, Fox H, Thomas K, Reisner C. Improvement in quality of life with omalizumab in patients with severe allergic asthma. Curr Med Res Opin. 2006;22:2201–2208. doi: 10.1185/030079906X148643. [DOI] [PubMed] [Google Scholar]
- 38.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 40.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 41.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.