To the Editor:
Patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus frequently develop coronavirus disease (COVID-19)–related acute respiratory distress syndrome (ARDS). It has been advocated that ARDS related to COVID-19 is not “typical” ARDS (1) because patients have a better compliance of the respiratory system (Crs) that is discrepant to the amount of shunt. Later, it was specified that this relates specifically to “L”-type ARDS with a low elastance, low lung weight, and low / (2). Treatment recommendations that have been based on conceptional physiological models resulting from these observations go against long-standing evidence-based interventions such as low Vt ventilation and prone positioning (1, 2).
ARDS was first described over 50 years ago as a syndrome that presents with “acute onset of tachypnea, hypoxemia, and loss of compliance after a variety of stimuli; the syndrome did not respond to usual and ordinary methods of respiratory therapy.” This description is strikingly similar to the common presentation of patients with severe COVID-19 pneumonia. The mean Crs of intubated patients with COVID-19 ranged between 30 and 50 ml/cm H2O in two recent series (1, 3). These values are actually comparable with those reported in LUNG-SAFE, the largest observational cohort study to date (4). Though patients with non–COVID-19–related ARDS do frequently not show signs of diffuse alveolar damage (DAD) on autopsy (5), the available autopsy reports of patients who died from COVID-19 show DAD even in patients who never received mechanical ventilation (6). The available data indicate that severe COVID-19 pneumonia is similar to the original description of the syndrome and fits within the current consensus definition.
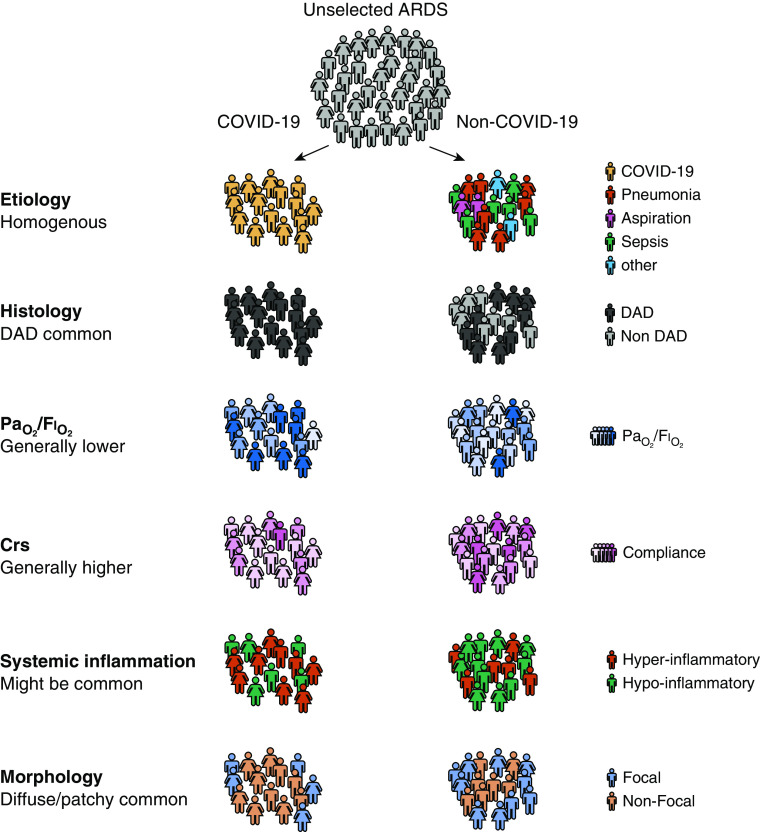
In recent years, the pulmonary critical care community has come to realize that ARDS can be split into subphenotypes (Figure 1) that might respond differently to interventions (7). Heterogeneity can be observed in 1) the etiology of lung injury, 2) physiological changes, 3) morphology of affected lung parenchyma, and 4) biological response. Based on post hoc analyses of randomized clinical trials, patients with systemic hyperinflammation might respond differently to higher end-expiratory pressure, restrictive fluid management, or immunomodulation with simvastatin treatment, whereas patients with a nonfocal lung morphology benefit more from recruitment than prone positioning (8, 9). However, no one is advocating for implementing these personalized approaches into clinical practice before they are validated in prospective clinical trials, despite a much stronger basis of evidence than is currently provided for COVID-19–related ARDS phenotypes.
Figure 1.
Subphenotypes of ARDS, stratified for the etiological subphenotype of COVID-19–related ARDS. ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; Crs = compliance of the respiratory system; DAD = diffuse alveolar damage.
Etiology is generally a minor determinant of the pathophysiological presentation of ARDS, meaning that many patients with a similar “hit” show different biological, physiological, and morphological patterns. COVID-19–related ARDS is an etiological subphenotype of ARDS with a particular set of characteristics: frequent DAD, (possibly) a higher than expected Crs, low PaO2/FiO2 values, frequent nonfocal morphology, and some suggestions of profound systemic inflammation (Figure 1). But are patients with COVID-19–related ARDS inherently different from “typical ARDS”? With appreciation of the heterogeneity within ARDS, we have come to realize that there is no “typical ARDS.”
Despite the described heterogeneity that is inherent to the syndromic definition of ARDS, low Vt ventilation was found to decrease mortality in an unselected population, and prone positioning was effective in patients with persistent hypoxemia. Yet, these interventions are the ones that are now challenged for the supportive treatment of COVID-19–related ARDS (2). Does subphenotyping of COVID-19–related ARDS require a different level of evidence before we adjust clinical practice? Or were we too strict in implementing subphenotype-based interventions in the pre–COVID-19 era? I would argue that we should maintain the highest standard to adjust our clinical practice and resist the temptation to jump to conclusions and provide alternative treatments that might harm our patients.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202004-1423LE on June 24, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome [letter] Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez J-MM, Aramburu J-AA, Peñuelas O, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med. 2013;187:761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 6.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott AHC, Calfee CS, Taylor B, Angus DC, Liu VX. Toward smarter lumping and smarter splitting : rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;112018:1–28. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2020;25:12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantin J-M, Jabaudon M, Lefrant J-Y, Jaber S, Quenot J-P, Langeron O, et al. AZUREA Network. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7:870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.