To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic that continues to wreak havoc on people’s lives and livelihoods. As of June 16, 2020, the number of coronavirus disease (COVID-19) cases surpassed 8 million, and the death toll stood at more than 400,000 (1). Although the majority of the patients developed mild symptoms and eventually recovered from this disease, a significant proportion suffered from serious pneumonia and developed acute respiratory distress syndrome, septic shock, and/or multiorgan failure (2, 3). The degree of the disease severity should result from direct viral damages on epithelial surface layer and the host immune response. SARS-CoV-2 infection may trigger a dysfunctional response leading to an overproduction of cytokines (cytokine storm) and the recruitment of more immune cells into the lungs, resulting in greater damages (4). However, the immune effectors that determine or influence the severity of the disease and the reason why immune response mediates recovery in some individuals (5), but not in others, are far from clear. In this study, we addressed these issues by analyzing the blood samples of patients with COVID-19 with varying degrees of disease severity and by collecting their clinical data over a period of more than 3 months. Our findings highlight the importance of T-cell immunity in COVID-19 recovery.
Methods
Longitudinal peripheral blood mononuclear cells from 12 patients with severe COVID-19 hospitalized at the First Affiliated Hospital, Guangzhou Medical University, (Guangzhou, China), 6 with regressing imaging scores (recovering group [R]: R1, R2, R3, R4, R5, and R6) and 6 with no improvements in imaging scores within 6 weeks after disease onset (severe persistence group [S]: S1, S2, S3, S4, S5, and S6), were analyzed (Ethics No. 202051).
The method used for scoring computed tomographic and X-ray images was similar to the previous report (6), in which one point was assigned to the presence of a single lesion observed in the lung. A score was marked up or down by 0.5 points when consolidation was increased or resolved, respectively. Flow cytometric analysis for T-cell immune effectors was done using a FACSAria III instrument (BD Bioscience) and analyzed with FlowJo software (Treestar). Cytokines were measured by using Cytometric Bead Array kits (BD Bioscience). Focus reduction neutralization test was performed to evaluate the levels of neutralizing antibodies (nAbs) using Vero E6 cells infected with SARS-CoV-2 and rabbit anti–SARS-CoV-2 nucleocapsid protein polyclonal antibody (Sino Biological). The foci were visualized by TrueBlue reagent and counted with an ELISPOT reader (CTL S6 Ultra).
Results
The clinical data and immune status of patients examined are shown in Table 1. The comparison of oxygenation indexes (PaO2/FiO2) shows that the R group was better than the S group, which includes two extracorporeal membrane oxygenation users (P = 0.03). Furthermore, the S group had significantly higher Sequential Organ Failure Assessment scores than the R group (P = 0.002). At Days 95–110 after disease onset, five patients from the S group remained hospitalized in the ICU, whereas all six patients from the R group had long been discharged.
Table 1.
Demographics of the COVID-19 Patient Cohort
| Group* | Patient No. | Age (yr) | Underlying Medical Disorders | SOFA at Last Detected Time Point | Sepsis† | Injury in Other Organs | Imaging Score of Radiological Findings |
Ventilation Days§ | Disease Outcome|| | Oxygenation Index at Last Detected Time Point (mm Hg) | Immune Effectors¶ |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum | Latest‡ | Activated CD8+ | Activated CD4+ | nAbs | ||||||||||
| R | R1 | Diabetes II, CHD | 2 | N | None | 5 | 3 | 34 | D | 395 | 9,687 | 30,036 | 357.0 | |
| R2 | HBV | 5 | Y | Myocardial | 6 | 3 | 30 | D | 284 | 12,597 | 32,307 | 553.8 | ||
| R3 | Diabetes, COPD | 2 | Y | Myocardial | 6.5 | 2.5 | 19 | D | 298 | 9,124 | 41,679 | 318.5 | ||
| R4 | Hypertension, diabetes, COPD | 4 | Y | Kidney | 6 | 2.5 | 38 | D | 101 | 6,728 | 16,286 | 780.0 | ||
| R5 | Pneumatocele, hepatic cyst, renal cyst, | 4 | N | Kidney | 6 | 4.5 | 34 | D | 255 | 3,958 | 22,798 | 586.7 | ||
| R6 | Diabetes II, coronary atherosclerotic heart disease, COPD | 9 | Y | Kidney, myocardial | 4 | 2 | 37 | D | 157 | 5,261 | 10,934 | 306.8 | ||
| Average | 59.0 | — | 4.3 | — | — | 5.6 | 2.9 | 32.0 | — | 248.3 | — | — | 483.8 | |
| S | S1 | None | 10 | Y | None | 6.5 | 6.5 | 71 | C | 107 | 3,993 | 14,516 | 324.0 | |
| S2 | None | 7 | Y | None | 7 | 6.5 | 22 | D | 108 | 2,048 | 4,722 | 493.0 | ||
| S3 | Renal cyst | 11 | N | None | 8 | 8 | 83 | C | 196 | 2,377 | 5,167 | 500.0 | ||
| S4 | Postoperation of intracranial tumor | 10 | Y | Myocardial | 7 | 7 | 87 | C | ECMO | 2,731 | 12,700 | 786.0 | ||
| S5 | HBV, sleep apnea syndrome | 14 | Y | Myocardial | 8 | 8 | 95 | C | ECMO | 2,678 | 8,869 | 371.8 | ||
| S6 | Hypertension II, diabetes II, hyperuricemia | 7 | Y | Myocardial | 7 | 5.5 | 92 | C | 183 | 22,112 | 33,879 | 74.8 | ||
| Average | 62.3 | 9.8 | — | — | 7.3 | 6.9 | 75.0 | — | 148.5 | — | — | 424.9 | ||
Definition of abbreviations: CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; ECMO = extracorporeal membrane oxygenation; HBV = hepatitis B virus; nAbs = neutralizing antibodies; R = recovering group; S = severe persistence group; SOFA = Sequential Organ Failure Assessment.
R group: six males; S group: two females and four males.
Y: sepsis; N: no sepsis.
Imaging scores observed within the 6 weeks after disease onset; regression of scores from “maximum to latest” was used as an indicator of grouping.
Days of ventilation from the initiation to May 8, including invasive ventilation and noninvasive ventilation less than 12 h/d.
D: discharged from hospital; C: continued hospitalization as of May 8.
Integral average of immune effectors within 5 weeks after disease onset. Activated CD8+ is defined by CD38+HLA-DR+CD8+ T cells (n/ml blood); activated CD4+ is defined by CD38+HLA-DR+CD4+ T cells (n/ml blood); and nAbs is defined by units of neutralizing antibodies in 1 ml blood.
Longitudinal changes in plasma levels of sTM (soluble thrombomodulin), syndecan-1, MMP2, and MMP9 were analyzed to evaluate the damages to the epithelial surface layer in SARS-CoV-2 infection. Meanwhile, cytokines IL-6, IL-8, IP-10, MCP-1, and MIG were measured as inflammatory injury markers (7). Our data showed that the levels of syndecan-1 and IL-6 were significantly higher in the S than the R group (Figure 1A), suggesting that these effectors could be used as potential severity markers.
Figure 1.
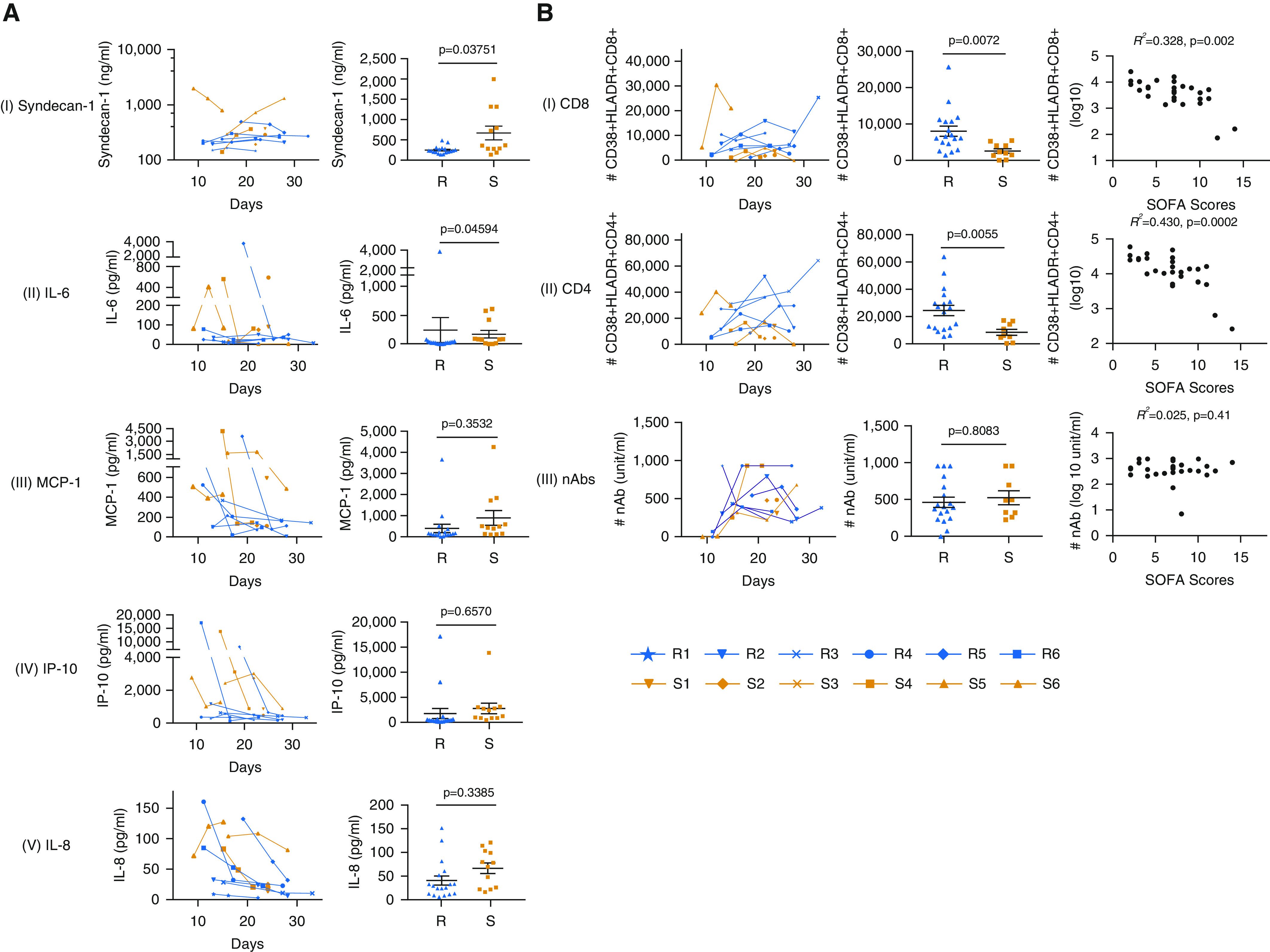
(A) (Left panel) The levels of representative lung injury and inflammation effectors in the blood plasma of the recovering (R) and severe persistence (S) groups of patients with coronavirus disease (COVID-19) at different days after disease onset. (Right panel) Comparison of the levels of syndecan-1, IL-6, MCP-1, IP-10, and IL-8 is shown. The data are presented as the mean ± SEM (18 measurements from the 6 patients in R group and 12 measurements from the 6 patients in S group). Because the data contain multiple measurements over a time period from individual patients, a linear mixed-effect model, which is commonly applied for this kind of data analysis (10, 11), was used to determine if the mean level of a biomarker was statistically distinct between the R and S groups. Two linear mixed-effect models, one of which included the classification of R and S groups as a predictor, were fitted with each biomarker data set, and a likelihood ratio test was then performed to examine if the former model was acceptable. This was based on a confidence level of 95%; that is, a P value less than 0.05 suggests that the mean biomarker level is statistically distinct between the R and S groups. The details of the statistical method, the data, and the R code are publicly available (https://github.com/wzhf1218/COVID19-Wang_etal.git). (B) (Left panel) The presence of CD38+HLA-DR+CD8+ T cells (I), CD38+HLA-DR+CD4+ T cells (II), and neutralizing antibodies (nAbs) (III) in the blood plasma of the R and S groups of the patients with COVID-19 at different time points. (Middle panel) Comparison of absolute numbers of CD38+HLA-DR+CD8+ T cells (I), CD38+HLA-DR+CD4+ T cells (II), and nAbs (III) in 1 ml blood samples is shown. The data are presented as the mean ± SEM (18 measurements from the 6 patients in R group and 9 measurements from the 5 patients excluding patient S6 in S group) and the P values were calculated using the aforementioned statistical method. (Right panel) Correlation analyses between immune effectors (CD38+HLA-DR+ double-positive CD8+/CD4+ T cells and nAb titers) and COVID-19 disease severity evaluated by SOFA (Sequential Organ Failure Assessment) scores was performed using the linear regression model.
To dissect immune recovery mechanisms in severe COVID-19 cases, the frequency of activated CD8+ and CD4+ T cells was analyzed based on the expression of CD38 and HLA-DR. nAbs were also measured at corresponding time points. The data in Table 1 showed that S6, who had the highest level of CD8+ activation among all the samples (22,112 CD38+HLA-DR+CD8+ cells/ml) and a very strong CD4+ activation (33,879 CD38+HLA-DR+CD4+ cells/ml), developed more severe disease. However, this patient also exhibited an extreme low level of nAbs (74.8 U, compared with 324.0–786.0 U in the rest of S group) (Table 1). Obviously, S6 whose immune response is distinctive from that of the others in the S group forms a separate category in terms of the T-cell and B-cell immunity and demands an independent assessment. As such, the data from S6 were not included in the subsequent analysis.
Marked differences between the R and S groups were seen for the number of CD38+HLA-DR+CD8+ (P = 0.0072) and CD38+HLA-DR+CD4+ (P = 0.0055), whereas no significant differences were observed for nAbs (Figure 1B, left and middle panels). Regression analyses show that activation of CD8+ (R2 = 0.328, P = 0.002) and CD4+ (R2 = 0.430, P = 0.0002) T cells are strongly and inversely correlated to the severity of COVID-19 in patients (Figure 1B, right panel).
Discussion
The key findings of this study are 1) the lung injury and inflammation effectors (syndecan-1 and IL-6) are associated with disease severity, and 2) CD8+ and CD4+ T cells play a major role in the recovery of patients with critical COVID-19 under the caveat that adequate amounts of nAbs must also be present. These are consistent with the observations made in the studies of other severe infections with emerging viruses such as Ebola and influenza A virus H7N9 (8, 9). The T-cell immunity and lung injury markers were analyzed at a relatively early stage of COVID-19 (within Day 33 after disease onset). The updated fact that 6/6 of the R group had long been discharged while 5/6 of S group still suffered acute respiratory distress syndrome and had a prolonged use of ventilators in ICU (Table 1) strongly suggests that T-cell immunity can be used as a prognostic marker for COVID-19. Nevertheless, because of the small sample size, our findings warrant further verifications with larger cohorts.
Importantly, our study emphasizes that a balance between T-cell immunity and neutralizing antibodies is required for the COVID-19 recovery. The variability of T-cell immunity in individuals suggests that patients with a different balance of immune activation may require tailored treatments. For example, convalescent serum antibody therapy may benefit those patients who have strong T-cell immunity but low levels of nAbs (as in the case of S6), whereas other patients with insufficient T-cell activation may need a T-cell immunity boost strategy and should be cautiously treated with corticosteroids to suppress the cytokine storm.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Ji Yang and Dr. Alexandra Corbett for critical review and preparing this manuscript.
Footnotes
Author Contributions: Designed experiments: Z.W. and P.R. Patient recruitment: Y.Z. and X.L. Performed experiments: X.Y., J.S., J. Zhang, X.M., J. Zhong, and J. Zhao. Analyzed experiments: Z.W., J. Zhao, and P.R. Wrote the manuscript: Z.W. and P.R.
Originally Published in Press as DOI: 10.1164/rccm.202005-1701LE on July 1, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Johns Hopkins University. Coronavirus resource center [updated 2020 Jun 16; accessed 2020 Jun 16]. Available from: https://coronavirus.jhu.edu/map.html.
- 2.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med (Torino) 2020;125:509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Zhang A, Wan Y, Liu X, Qiu C, Xi X, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci USA. 2014;111:769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Zhu L, Nguyen THO, Wan Y, Sant S, Quiñones-Parra SM, et al. Clonally diverse CD38+HLA-DR+CD8+ T cells persist during fatal H7N9 disease. Nat Commun. 2018;9:824. doi: 10.1038/s41467-018-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci USA. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng S, Diergaarde B, Picchi MA, Wilson DO, Gilliland FD, Yuan JM, et al. Gene promoter hypermethylation detected in sputum predicts FEV1 decline and all-cause mortality in smokers. Am J Respir Crit Care Med. 2018;198:187–196. doi: 10.1164/rccm.201708-1659OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


