To the Editor:
On the basis of recent correspondence (1) and an expert editorial (2), two phenotypes of severe coronavirus disease (COVID-19) pneumonia have been proposed: “Type L, characterized by Low elastance (i.e., high compliance), Low ventilation to perfusion ratio, Low lung weight and Low recruitability and Type H, characterized by High elastance, High right-to-left shunt, High lung weight and High recruitability” (2).
Features of the L phenotype are not typical of acute respiratory distress syndrome (ARDS) as defined by the Berlin criteria. Importantly, the authors suggest that recommended treatment strategies for severe COVID-19 pneumonia based on ARDS management (3) may lead to disease progression and excess harm (1, 2). The authors provide anecdotal evidence for their observations based on their combined experience of treating several hundred severe COVID-19 cases. As outlined by Singer and colleagues (4), we need a rational approach. Considering the potential importance for modifying the management of these patients and the growing volume of data available from China and Italy, quantitative data are needed to test this hypothesis. Balancing the trade-off between “learning” and “doing” in this pandemic is crucial (5). Large randomized controlled trials are not yet available, and observational data remain at high risk of bias. A number of predictive models have been described with severe methodological flaws (6). The appropriate use of emerging observational data requires collaborative input to improve understanding of treatment effects and complement the results of ongoing randomized controlled studies.
The wealth of data generated by critically ill patients and the complexity of covariate interactions make it challenging to use traditional statistical modeling to establish causal relationships. We aim to determine the causal pathway between the use of an ARDS management strategy for L-phenotype patients and subsequent harm using a directed acyclic graph (DAG) (Figure 1). The DAG achieves two things. First, we can construct a complex system of interacting baseline, clinical, and disease features, allowing explicit statement of prior knowledge before any data analysis. Second, we can use the DAG to determine a minimal adjustment set of variables to reliably estimate the direct effect of our exposure (ARDS ventilation strategy in COVID-19 L-phenotype patients) and outcome (ICU mortality).
Figure 1.
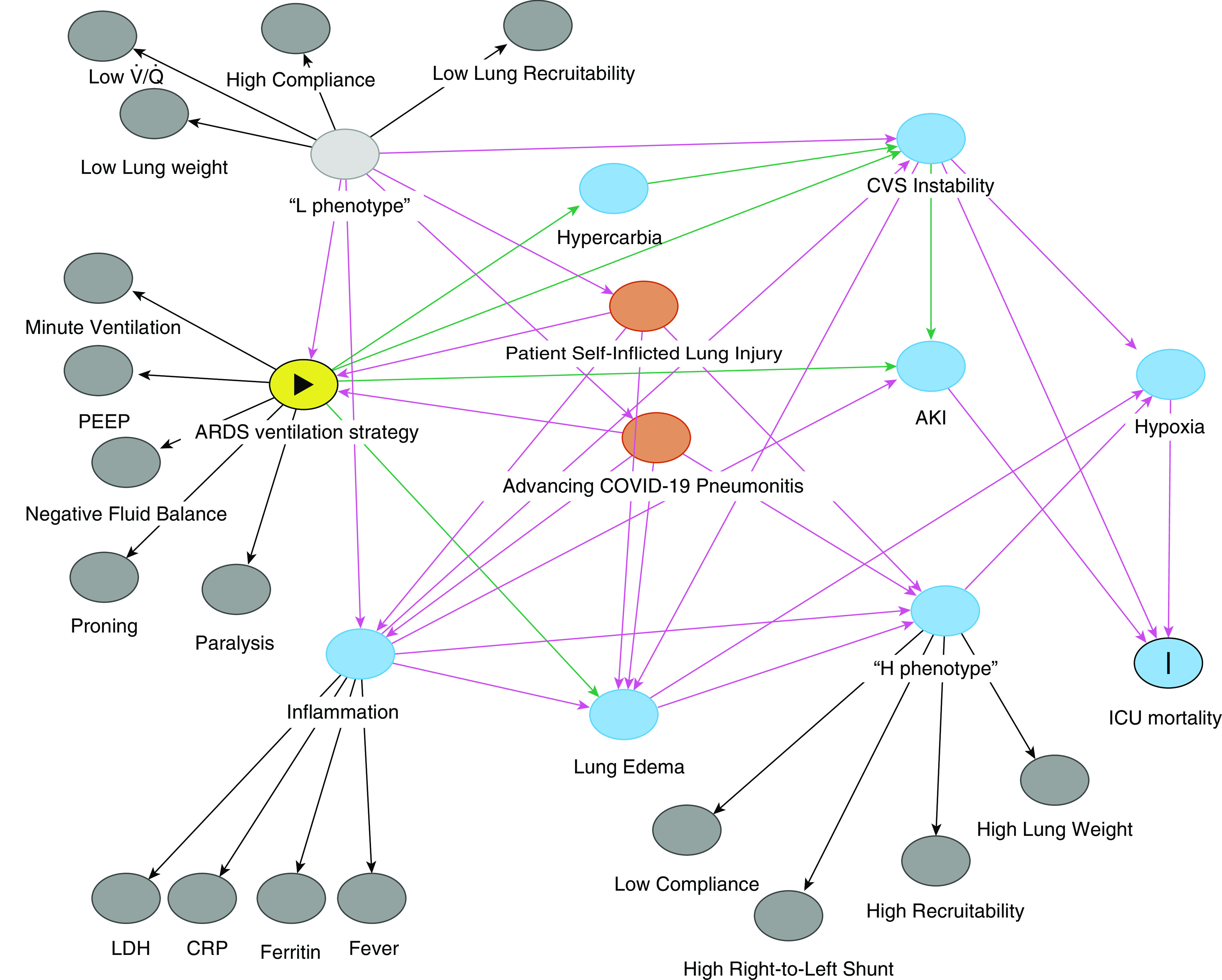
Proposed directed acyclic graph. Arrows represent proposed causal pathways. The solid triangle indicates exposure and the vertical line indicates outcome. AKI = acute kidney injury; ARDS = acute respiratory distress syndrome; COVID-19 = coronavirus disease; CRP = C-reactive protein; CVS = cardiovascular system; H = high elastance, high right-to-left shunt, high lung weight, and high recruitability; L = low elastance, low /, low lung weight, and low recruitability; LDH = lactic-acid dehydrogenase; PEEP = positive end-expiratory pressure.
The DAG was developed on the basis of the information in the expert editorial outlining the two phenotypes. In doing so, we have transformed the initial hypothetical construct into a testable mechanistic structure. Arrows represent proposed causal pathways, such as the link between a high positive end-expiratory pressure strategy of standard ARDS management and worsening edema and cardiovascular instability. Combined, these paths can be used to elucidate the appropriate adjustment set of variables. In this case, one adjustment set included cardiovascular instability, hypoxia, and acute kidney injury, all of which are readily measurable among intensive-care patients receiving treatment for COVID-19.
This approach has a number of limitations, including the fact that the evidence underpinning the structure is currently anecdotal. Without high-quality, unbiased evidence, it will be challenging to determine the true direct effect because of unmeasured confounders. Highlighting different phenotypes and different responses to treatment is a welcome approach that echoes the thoughts of some intensivists treating patients with COVID-19 and, if supported through the appropriate use of data, has the potential to reduce harm to future patients. The DAG allows easy inclusion of increasing knowledge as new findings emerge and provides an objective analytical framework to facilitate ongoing discussion. We welcome comments and encourage readers to examine the structure themselves by running the code (code freely available on request). We would also be interested to know the calculated effects if anyone wishes to test the hypothesis with appropriately collected data.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202004-1293LE on June 24, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome [letter] Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello DC, Caironi P, Busana M, Romitti F, Brazzi L.et alCOVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020461099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer BD, Jain M, Budinger GRS, Wunderink RG. A call for rational intensive care in the era of COVID-19. Am J Respir Cell Mol Biol. [online ahead of print] 21 Apr 2020; DOI: 10.1165/rcmb.2020-0151LE. [DOI] [PMC free article] [PubMed]
- 5.Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. [online ahead of print] 30 Mar 2020; DOI: 10.1001/jama.2020.4984.
- 6.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of COVID-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


