Abstract
Rationale: Acute rejection, manifesting as lymphocytic inflammation in a perivascular (acute perivascular rejection [AR]) or peribronchiolar (lymphocytic bronchiolitis [LB]) distribution, is common in lung transplant recipients and increases the risk for chronic graft dysfunction.
Objectives: To evaluate clinical factors associated with biopsy-proven acute rejection during the first post-transplant year in a present-day, five-center lung transplant cohort.
Methods: We analyzed prospective diagnoses of AR and LB from over 2,000 lung biopsies in 400 newly transplanted adult lung recipients. Because LB without simultaneous AR was rare, our analyses focused on risk factors for AR. Multivariable Cox proportional hazards models were used to assess donor and recipient factors associated with the time to the first AR occurrence.
Measurements and Main Results: During the first post-transplant year, 53.3% of patients experienced at least one AR episode. Multivariable proportional hazards analyses accounting for enrolling center effects identified four or more HLA mismatches (hazard ratio [HR], 2.06; P ≤ 0.01) as associated with increased AR hazards, whereas bilateral transplantation (HR, 0.57; P ≤ 0.01) was associated with protection from AR. In addition, Wilcoxon rank-sum analyses demonstrated bilateral (vs. single) lung recipients, and those with fewer than four (vs. more than four) HLA mismatches demonstrated reduced AR frequency and/or severity during the first post-transplant year.
Conclusions: We found a high incidence of AR in a contemporary multicenter lung transplant cohort undergoing consistent biopsy sampling. Although not previously recognized, the finding of reduced AR in bilateral lung recipients is intriguing, warranting replication and mechanistic exploration.
Keywords: lung transplantation, acute rejection, lymphocytic bronchiolitis
At a Glance Commentary
Scientific Knowledge on the Subject
Acute rejection, manifesting as lymphocytic inflammation in a perivascular (acute perivascular rejection [AR]) or peribronchiolar (lymphocytic bronchiolitis) distribution, is common in lung transplant recipients and increases the risk for chronic graft dysfunction. Despite the prognostic implications of AR and lymphocytic bronchiolitis, the precise incidence and clinical risk factors contributing to their occurrence remain unclear.
What This Study Adds to the Field
Using a present-day, multicenter cohort of 400 lung transplant recipients undergoing serial bronchoscopies (totaling 2,026 lung biopsies), we found over half of lung recipients experience at least one episode of AR during the first post-transplant year. Multivariable analyses accounting for center differences indicated receipt of a bilateral, as opposed to single, lung transplant significantly reduced AR risk, whereas four or more total human leukocyte antigen mismatches between the donor and the recipient increased AR risk. These data underscore the importance of frequent surveillance for AR in lung recipients and identifies new opportunities to better understand the complexity of the host response to the donor organ in the development of AR.
Lung transplantation is an established therapy for many end-stage pulmonary diseases. Despite contemporary immunosuppression strategies, lung recipients have higher rates of acute and chronic allograft rejection compared with recipients of other commonly transplanted solid organs. Importantly, acute lung rejection has been consistently identified as a risk factor for chronic lung allograft dysfunction (CLAD), a manifestation of chronic rejection that accounts for the majority of lung recipient deaths after the first post-transplant year (1–4).
Acute cellular lung rejection is diagnosed on the histological observation of lymphocytic infiltrates in a perivascular (acute perivascular inflammation [AR]) or peribronchiolar (lymphocytic bronchiolitis [LB]) distribution. Standardized criteria for the diagnosis and severity grading of AR and LB, based on the degree of lymphocytic infiltration, have been established by the International Society for Heart and Lung Transplantation (ISHLT) (5). ISHLT registry data reveal that approximately one-third of lung recipients will experience at least one episode of acute rejection during the first post-transplant year (1). However, these data are self-reported by centers and reflect wide variation in clinical practice over many eras.
Despite the prognostic implications of AR and LB, the precise incidence of these histologies in a consistently sampled modern-day lung transplant cohort has not been ascertained. In addition, the clinical risk factors contributing to their occurrence remain unclear (6). We leveraged a multicenter, prospective observational cohort of 400 lung transplant recipients who underwent over 2,000 allograft biopsies to study AR and LB during the first post-transplant year. Because LB occurred primarily concurrent with AR, we concentrated on assessing donor or recipient factors associated with the first occurrence of AR and investigated the impact of significant risk factors on AR frequency and severity during the first post-transplant year.
Methods
Cohort
The cohort was drawn from Clinical Trials in Organ Transplantation (CTOT)–20 (clinicaltrials.gov NCT02631720), a prospective observational study of lung transplant recipients at five North American centers. This analysis included the first 400 enrolled transplanted subjects with at least one biopsy performed within the first post-transplant year. Subjects were transplanted between December 2015 and June 2017. The study was approved by each center’s institutional review board.
Assessments
Recipients were managed according to center-specific practices (online supplement). Bronchoscopies were recorded as surveillance if performed in absence of a specific indication or for routine follow-up after an AR episode. Bronchoscopies were recorded as for cause if performed for evaluation of a specific symptom or sign. Biopsies were reviewed by an experienced pathologist at the enrolling center. Before study initiation, pathologists participated in a working group to share terminology and diagnostic approaches around lung allograft histology, including a review of representative cases. AR and LB were defined as the presence of perivascular or peribronchiolar lymphocytic inflammation, respectively, and scored for severity according to the ISHLT criteria (5). In addition, the presence of microorganisms was assessed on BAL or other lower respiratory tract specimens (e.g., sputum).
Analyses
Descriptive statistics were used to summarize cohort demographics. To determine factors associated with AR, Cox proportional hazards models were used to model the time to first AR occurrence in the first post-transplant year (through post-transplant Day 410, upper window of the 1 yr study visit). AR occurrence was defined as the presence of AR histology on biopsy, whether in isolation or concurrent with other histology. Center was incorporated into the Cox models as a random effect, which allowed us to control for center-level heterogeneity in the hazards of AR (Table E1 in the online supplement) and to estimate the influence of patient-level covariates that were strongly colinear with center (7). Time-independent covariates considered included donor age, sex, race, and cause of death; recipient age and sex; donor-recipient sex mismatch; native lung disease; transplant type; lung allocation score; induction immunosuppression; maintenance immunosuppression at hospital discharge; total number of HLA mismatches between the donor and recipient as determined by HLA genotyping; number of mismatches at the HLA A, B, or DR locus, respectively; donor-to-recipient predicted TLC ratio; primary graft dysfunction grade 3 within 72 hours (6); ischemic time; and pretransplant HLA sensitization. Induction immunosuppression was defined as the receipt of augmented immunosuppression (other than intravenous steroids alone) within 24 hours of transplantation. Time-dependent covariates included any positive fungal, bacterial, mycobacterial, or viral organism on respiratory specimen; cytomegalovirus (CMV) detection (defined as either a quantifiable concentration of CMV in the serum or histopathological evidence of CMV pneumonitis on biopsy); and receipt of augmented immunosuppression after postoperative Day 1. Time-dependent covariates were modeled as binary indicators with values switched from 0 to 1 at the first occurrence. Univariable and multivariable models were fit.
To control the number of covariates in the multivariable model, variables with substantial multicollinearity or low event frequencies were removed. We explored the influence of covariates significantly associated with AR on the cumulative burden of AR as indicated by the normalized AR score, which was calculated as the sum of AR grades over the first post-transplant year divided by the number of gradable biopsies. The normalized AR score was compared among subgroups of interest using the Wilcoxon rank-sum test. The Kaplan-Meier log-rank test was used to compare the time to the first AR occurrence. Analyses were performed using SAS version 9.4.
Results
Cohort Characteristics
Table 1 describes the cohort characteristics. The median (quartile 1–quartile 3) age at transplant was 59.5 (51.0–66.0) years. Over half were male (239/400, 59.8%), and the most common transplant indication was restrictive lung disease (217/400, 54.3%). A majority of patients underwent bilateral lung transplantation (303/400, 75.8%). Induction immunosuppression was given in 50.8% of cases, with the most common medication being basiliximab. At the time of post-transplant discharge, 300 patients (75.0%) were on tacrolimus-based maintenance immunosuppression, whereas the remaining 100 patients (25.0%) were on cyclosporine. In addition, nearly all patients were on a cell-cycle inhibitor, with the majority receiving mycophenolate. At 1 year post-transplant, 26 (7%) subjects had died or were retransplanted, 12 (3%) terminated for other reasons, and 362 (91%) remained in active follow-up.
Table 1.
Clinical Characteristics of the Study Cohort Presented Overall and Stratified by Patients Experiencing at Least One Episode of AR or LB
| Characteristics | Overall Study Cohort (N = 400) | Patients with AR (n = 213) | Patients with LB (n = 59) |
|---|---|---|---|
| Baseline characteristics | |||
| Age at transplant, median (quartile 1–quartile 3), yr | 59.5 (51.0–66.0) | 60.0 (50.0–66.0) | 62.0 (45.0–69.0) |
| Sex, n (%) | |||
| F | 161 (40.3) | 76 (35.7) | 22 (37.3) |
| M | 239 (59.8) | 137 (64.3) | 37 (62.7) |
| Race, n (%) | |||
| Black | 20 (5.0) | 10 (4.7) | 2 (3.4) |
| Other | 18 (4.5) | 8 (3.8) | 2 (3.4) |
| White | 362 (90.5) | 195 (91.5) | 55 (93.2) |
| Native lung disease (UNOS category), n (%) | |||
| Obstructive (A) | 112 (28.0) | 59 (27.7) | 11 (18.6) |
| Vascular/other (B) | 16 (4.0) | 5 (2.3) | 1 (1.7) |
| Cystic fibrosis (C) | 55 (13.8) | 29 (13.6) | 11 (18.6) |
| Restrictive (D) | 217 (54.3) | 120 (56.3) | 36 (61.0) |
| LAS at transplant, median (quartile 1–quartile 3) | 38.5 (34.4–46.5) | 38.3 (34.4–47.1) | 38.1 (34.4–44.9) |
| Transplant characteristics | |||
| Donor age, median (quartile 1–quartile 3), yr | 41.0 (27.5–54.0) | 38.0 (25.0–54.0) | 41.0 (23.0–53.0) |
| Donor sex, n (%) | |||
| F | 161 (40.3) | 87 (40.8) | 27 (45.8) |
| M | 239 (59.8) | 126 (59.2) | 32 (54.2) |
| Donor cause of death, n (%) | |||
| Intracranial hemorrhage/blunt injury | 227 (56.9) | 120 (56.3) | 39 (66.1) |
| Other/unknown | 172 (43.1) | 93 (43.7) | 20 (33.9) |
| Recipient/donor sex mismatch, n (%) | |||
| F/M | 60 (15.0) | 24 (11.3) | 7 (11.9) |
| M/F | 60 (15.0) | 35 (16.4) | 12 (20.3) |
| Matched | 280 (70.0) | 154 (72.3) | 40 (67.8) |
| Ratio predicted TLC, median (quartile 1–quartile 3) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Ischemic time, median (quartile 1–quartile 3), h | 6.4 (4.9–8.1) | 6.5 (5.3–8.5) | 5.0 (3.9–7.5) |
| Total HLA mismatches, n (%) | |||
| 0 | 1 (0.3) | 0 (0.0) | 1 (1.7) |
| 1 | 2 (0.5) | 1 (0.5) | 0 (0.0) |
| 2 | 9 (2.3) | 4 (1.9) | 1 (1.7) |
| 3 | 49 (12.3) | 16 (7.5) | 3 (5.1) |
| 4 | 104 (26.1) | 65 (30.7) | 24 (40.7) |
| 5 | 144 (36.2) | 74 (34.9) | 18 (30.5) |
| 6 | 89 (22.4) | 52 (24.5) | 12 (20.3) |
| A locus mismatches, n (%) | |||
| 0 | 22 (5.5) | 14 (6.6) | 4 (6.8) |
| 1 | 167 (42.0) | 82 (38.7) | 23 (39.0) |
| 2 | 209 (52.5) | 116 (54.7) | 32 (54.2) |
| B locus mismatches, n (%) | |||
| 0 | 10 (2.5) | 4 (1.9) | 2 (3.4) |
| 1 | 116 (29.1) | 58 (27.4) | 19 (32.2) |
| 2 | 272 (68.3) | 150 (70.8) | 38 (64.4) |
| DR locus mismatches, n (%) | |||
| 0 | 23 (5.8) | 9 (4.2) | 4 (6.8) |
| 1 | 158 (39.7) | 79 (37.3) | 23 (39.0) |
| 2 | 217 (54.5) | 124 (58.5) | 32 (54.2) |
| Recipient HLA-sensitized pretransplant, n (%) | 169 (42.3) | 94 (44.1) | 24 (40.7) |
| Transplant type, n (%) | |||
| Bilateral | 303 (75.8) | 148 (69.5) | 32 (54.2) |
| Single | 97 (24.3) | 65 (30.5) | 27 (45.8) |
| PGD grade 3 within 72 h, n (%) | 65 (16.3) | 34 (16.0) | 5 (8.5) |
| Induction immunosuppression, n (%) | |||
| Basiliximab | 178 (44.5) | 87 (40.8) | 22 (37.3) |
| Antithymocyte globulin | 25 (6.3) | 6 (2.8) | 7 (11.9) |
| None | 197 (49.3) | 120 (56.3) | 30 (50.8) |
| Maintenance immunosuppression, n (%)* | |||
| Tacrolimus | 300 (75.0) | 158 (74.2) | 47 (79.7) |
| Cyclosporine | 100 (25.0) | 55 (25.8) | 12 (20.3) |
| Cell-cycle inhibitor, n (%)* | |||
| Mycophenolate mofetil | 337 (84.3) | 182 (85.4) | 51 (86.4) |
| Azathioprine | 43 (10.8) | 20 (9.4) | 4 (6.8) |
| Other | 20 (5.0) | 11 (5.2) | 4 (6.8) |
| Azithromycin, n (%)* | 120 (30.0) | 53 (24.9) | 26 (44.1) |
| Biopsy characteristics | |||
| Biopsies per patient, median (quartile 1–quartile 3) | 5.0 (4.0–6.0) | 6.0 (5.0–7.0) | 5.0 (3.0–7.0) |
| Time to first biopsy, median (quartile 1–quartile 3), d | 30.0 (22.0–38.0) | 28.0 (21.0–35.0) | 27.0 (21.0–37.0) |
| Time to first AR or LB event, median (quartile 1–quartile 3), d | — | 43.0 (27.0–100.0) | 48.0 (24.0–104.0) |
Definition of abbreviations: AR = acute perivascular rejection; LAS = lung allocation score; LB = lymphocytic bronchiolitis; PGD = primary graft dysfunction; UNOS = United Network for Organ Sharing.
Medication use assessed at time of discharge from the transplant hospitalization.
Occurrence of AR and LB
In total, 2,026 lung biopsies were performed over the first post-transplant year (median, 5.0 [quartiles 1–3, 4.0–6.0] biopsies per patient; median time to first biopsy, 30.0 [quartiles 1–3, 22.0–38.0] d). The median number of biopsies per patient and median time to first biopsy were similar when comparing the cohort overall with those who developed AR or LB (Table 1) across the enrolling centers (Table E2) and among bilateral and single-lung recipients. The majority of biopsies (83.4%) were performed for surveillance, whereas the remainder were for cause. Among the for-cause bronchoscopies, the most common clinical indications were radiographic abnormalities, decline in pulmonary function tests, or worsening dyspnea or gas exchange.
Table 2 summarizes the findings with respect to AR and LB on every biopsy over the first post-transplant year. The majority of AR episodes were of minimal severity (grade A1) with only eight of 2,026 biopsies demonstrating grade A3 or A4 rejection. Similarly, LB was nearly always of minimal grade (B1R). Rates of minimal (grade A1) and mild (grade A2) AR were similar between for-cause and surveillance bronchoscopy. Considering all biopsies, 10.6% were deemed ungradable for AR, and 30.7% were ungradable for LB. When comparing biopsy results stratified by enrolling center (Table E2), we observed heterogeneity in the rates of specimens ungradable for AR (range, 2.5–28.0% of biopsies) or LB (range, 5.1–60.4% of biopsies). Despite the variable rates of biopsies ungradable for AR, the rates of grade A1 and A2 AR were similar among the four highest volume lung transplant centers, which contributed the overwhelming majority of the biopsies. In contrast, the rates of B1R were highly variable.
Table 2.
Results for All Biopsies Taken over the First Post-transplant Year with Respect to the Histological Findings of AR and LB
| All Biopsies (N = 2,026) | For Cause (n = 323) | Surveillance (n = 1,689) | Not Specified (n = 14) | |
|---|---|---|---|---|
| AR (A grade), n (%) | ||||
| None (A0) | 1,403 (69.2) | 204 (63.2) | 1,193 (70.6) | 6 (42.9) |
| Minimal (A1) | 287 (14.2) | 50 (15.5) | 236 (14.0) | 1 (7.1) |
| Mild (A2) | 114 (5.6) | 25 (7.7) | 87 (5.2) | 2 (14.3) |
| Moderate (A3) | 6 (0.3) | 1 (0.3) | 4 (0.2) | 1 (7.1) |
| Severe (A4) | 2 (0.1) | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| Ungradable (Ax) | 214 (10.6) | 41 (12.7) | 169 (10.0) | 4 (28.6) |
| LB (B-grade rejection)*, n (%) | ||||
| None (B0) | 1,333 (65.8) | 204 (63.4) | 1,122 (66.4) | 7 (50.0) |
| Low-grade (B1R) | 66 (3.3) | 14 (4.3) | 52 (3.1) | 0 (0.0) |
| High-grade (B2R) | 4 (0.2) | 1 (0.3) | 2 (0.1) | 1 (7.1) |
| Ungradable (Bx) | 622 (30.7) | 103 (32.0) | 513 (30.4) | 6 (42.9) |
Definition of abbreviations: AR = acute perivascular rejection; LB = lymphocytic bronchiolitis.
Presented overall and as stratified by whether the bronchoscopy was performed for surveillance purposes or for evaluation of a clinical symptom or sign (for cause).
N = 1; B grade was missing because the biopsy was performed at an outside hospital.
Over half of patients in this cohort (213/400, 53.3%) experienced at least one episode of AR during the first post-transplant year, with a median time to first occurrence of 43 (quartile 1–quartile 3, 27–100) days. These 213 patients accounted for 409 episodes of AR (maximum episodes per patient = 8). When compared with AR, LB occurred much less frequently. Only 14.8% (59/400) of patients in our cohort developed at least one episode of LB during the first post-transplant year, with a median time to first occurrence of 48 (quartile 1–quartile 3, 24–104) days. These 59 patients accounted for 70 episodes of LB (maximum episodes per patient = 3). The majority (78.0%) of subjects with LB also experienced at least one AR. Nearly two-thirds (61.4%) of the biopsies demonstrating LB demonstrated concurrent AR. Thus, although LB was relatively infrequently observed in this cohort, when it was observed it typically co-occurred with AR.
Clinical Risk Factors for AR
We next performed univariable and multivariable Cox proportional hazards modeling with random center effects to evaluate the impact of donor and recipient factors on the risk for first AR occurrence. In univariable analyses, the number and loci of HLA mismatches between the donor and recipient influenced the risk for AR. In particular, recipients with four or more total HLA mismatches had a twofold increase in AR risk (unadjusted hazard ratio [HR], 2.10; 95% confidence interval [CI], 1.34–3.31; P < 0.01). Although degree of HLA mismatch at the HLA A or B loci did not significantly influence AR risk, recipients with two (vs. zero or one) mismatches at the HLA DR locus had a significant increase in the hazards for AR (unadjusted HR, 1.33; 95% CI, 1.01–1.75; P = 0.04). When considering combinations of mismatch at the HLA class I (A and B) or class I and II (A and DR) loci, we noted that patients with four mismatches at the combined A and DR loci had an increased risk for AR (unadjusted HR, 2.33; 95% CI, 1.01–5.37; P = 0.05). Interestingly, receipt of a bilateral (vs. single) transplant was associated with a significant decrease in the hazards of AR (unadjusted HR, 0.56; 95% CI, 0.41–0.76; P < 0.01). We also observed a small but significant reduction in the hazards of AR with each 5-unit increase in donor age. None of the other donor or recipient variables evaluated were significantly associated with AR risk (Table 3).
Table 3.
Univariable Associations between Covariates of Interest and Time to First AR Event in the First Year after Lung Transplantation
| Covariate of Interest | Effect Estimate [HR (95% CI)] | P Value |
|---|---|---|
| Time-independent covariates | ||
| Age at transplant, ≥65 yr vs. <65 yr | 1.13 (0.84–1.53) | 0.41 |
| Sex, F vs. M | 0.77 (0.58–1.02) | 0.06 |
| UNOS native lung disease category | ||
| B vs. A | 0.61 (0.25–1.53) | 0.29 |
| C vs. A | 1.20 (0.76–1.89) | 0.44 |
| D vs. A | 1.23 (0.90–1.69) | 0.19 |
| Transplant type, bilateral vs. single | 0.56 (0.41–0.76) | <0.01 |
| LAS at transplant, continuous, per 10 points | 0.99 (0.90–1.08) | 0.82 |
| Induction immunosuppression, yes vs. no | 0.59 (0.30–1.18) | 0.13 |
| Maintenance immunosuppression, cyclosporine vs. tacrolimus | 1.07 (0.63–1.85) | 0.80 |
| Donor age, continuous, per 5 yr | 0.95 (0.91–0.99) | 0.03 |
| Donor race | ||
| Other vs. African American | 1.35 (0.83–2.20) | 0.22 |
| White vs. African American | 1.27 (0.86–1.87) | 0.23 |
| Donor sex, F vs. M | 0.97 (0.73–1.27) | 0.81 |
| Donor cause of death, ICH/blunt injury vs. other | 1.01 (0.76–1.33) | 0.96 |
| Ischemic time, continuous, h | 1.01 (0.96–1.05) | 0.81 |
| Total HLA mismatch, 4–6 vs. 0–3 | 2.10 (1.34–3.31) | <0.01 |
| HLA A mismatch, 2 vs. 0–1 | 1.13 (0.86–1.48) | 0.37 |
| HLA B mismatch, 2 vs. 0–1 | 1.15 (0.85–1.55) | 0.36 |
| HLA DR mismatch, 2 vs. 0–1 | 1.33 (1.01–1.75) | 0.04 |
| HLA A/B mismatch | ||
| 2 vs. 0–1 | 0.86 (0.39–1.89) | 0.71 |
| 3 vs. 0–1 | 1.27 (0.62–2.62) | 0.52 |
| 4 vs. 0–1 | 1.14 0.55–2.36) | 0.73 |
| HLA A/DR mismatch | ||
| 2 vs. 0–1 | 1.95 (0.84–4.56) | 0.12 |
| 3 vs. 0–1 | 1.82 (0.79–4.18) | 0.16 |
| 4 vs. 0–1 | 2.33 (1.01–5.37) | 0.05 |
| Recipient HLA-sensitized pretransplant, yes vs. no | 1.01 (0.76–1.35) | 0.94 |
| pTLC ratio, continuous | 0.58 (0.24–1.38) | 0.22 |
| Recipient/donor sex mismatch | ||
| F/M vs. matched | 0.74 (0.48–1.15) | 0.18 |
| M/F vs. matched | 1.12 (0.78–1.63) | 0.54 |
| PGD grade 3 within 72 h, yes vs. no | 0.74 (0.50–1.08) | 0.11 |
| Time-dependent covariates | ||
| Any bacterial infection, yes vs. no | 1.16 (0.86–1.56) | 0.35 |
| Any fungal infection, yes vs. no | 1.11 (0.75–1.63) | 0.61 |
| Any viral infection, yes vs. no | 1.10 (0.76–1.59) | 0.62 |
| Any mycobacterial infection, yes vs. no | 1.14 (0.69–1.86) | 0.61 |
| Any CMV, yes vs. no | 1.09 (0.54–2.21) | 0.81 |
| Any augmented immunosuppression, yes vs. no | 0.87 (0.61–1.24) | 0.43 |
Definition of abbreviations: AR = acute perivascular rejection; CI = confidence interval; CMV = cytomegalovirus; HR = hazard ratio; ICH = intracranial hemorrhage; LAS = lung allocation score; PGD = primary graft dysfunction; pTLC ratio = donor to recipient predicted TLC ratio; UNOS = United Network for Organ Sharing; UNOS native lung disease category A = obstructive lung disease; UNOS native lung disease category B = pulmonary vascular disease; UNOS native lung disease category C = cystic fibrosis; UNOS native lung disease category D = restrictive lung disease.
Enrolling center is included in the model as a random effect.
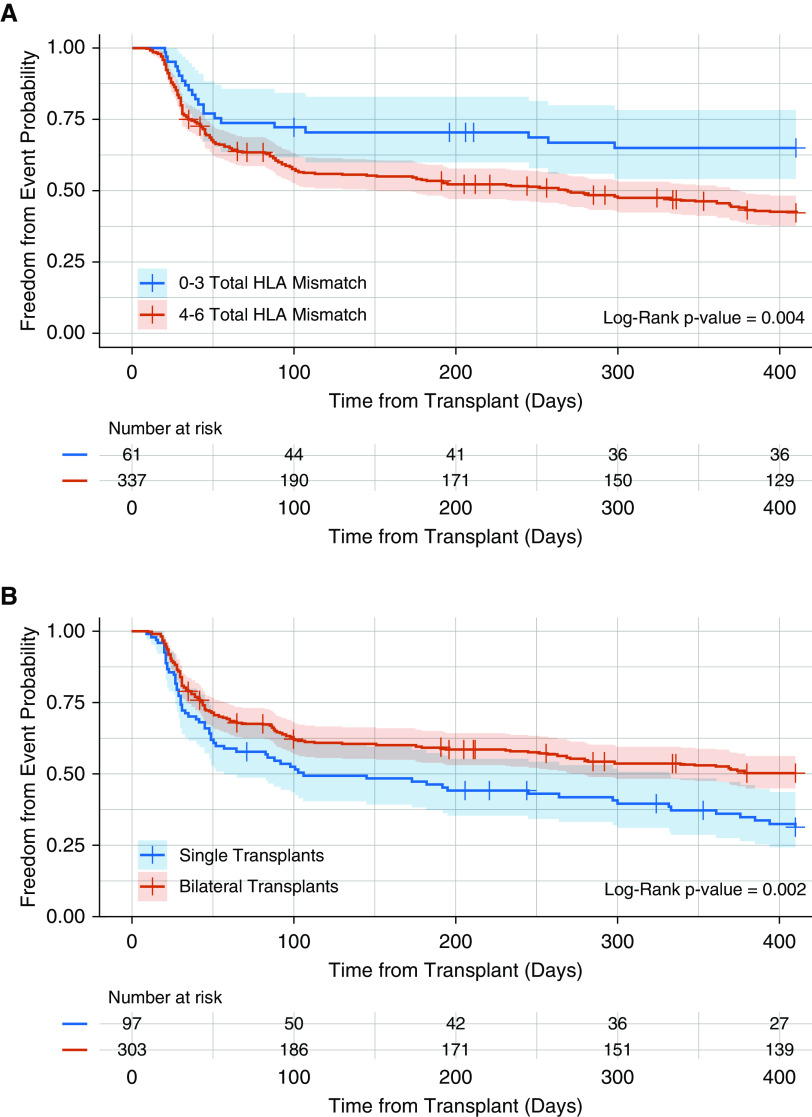
Multivariable analyses revealed that four or more total HLA mismatches continued to be associated with an increased risk for AR (adjusted HR, 2.06; 95% CI, 1.30–3.27; P < 0.01), whereas receipt of a bilateral lung transplant (adjusted HR, 0.57; 95% CI, 0.38–0.83; P < 0.01) was independently associated with reduced AR risk (Figure 1). The Kaplan-Meier plots for freedom from AR for each of these independent AR risk factors are illustrated in Figure 2. We explored whether these factors identified as influencing the time to first AR occurrence also influenced the overall frequency or severity of AR over the first post-transplant year, as reflected by the normalized AR score. The median normalized AR score over the first year after transplantation was significantly lower for patients with fewer than four versus those with four or more HLA mismatches (0.0 vs. 0.19; P < 0.01) and for bilateral lung recipients versus single lung recipients (0.00 vs. 0.29; P < 0.01). Figure 3 illustrates the observed differences in the distribution of the normalized AR scores over the first post-transplant year when stratified by total HLA mismatch or type of transplantation.
Figure 1.
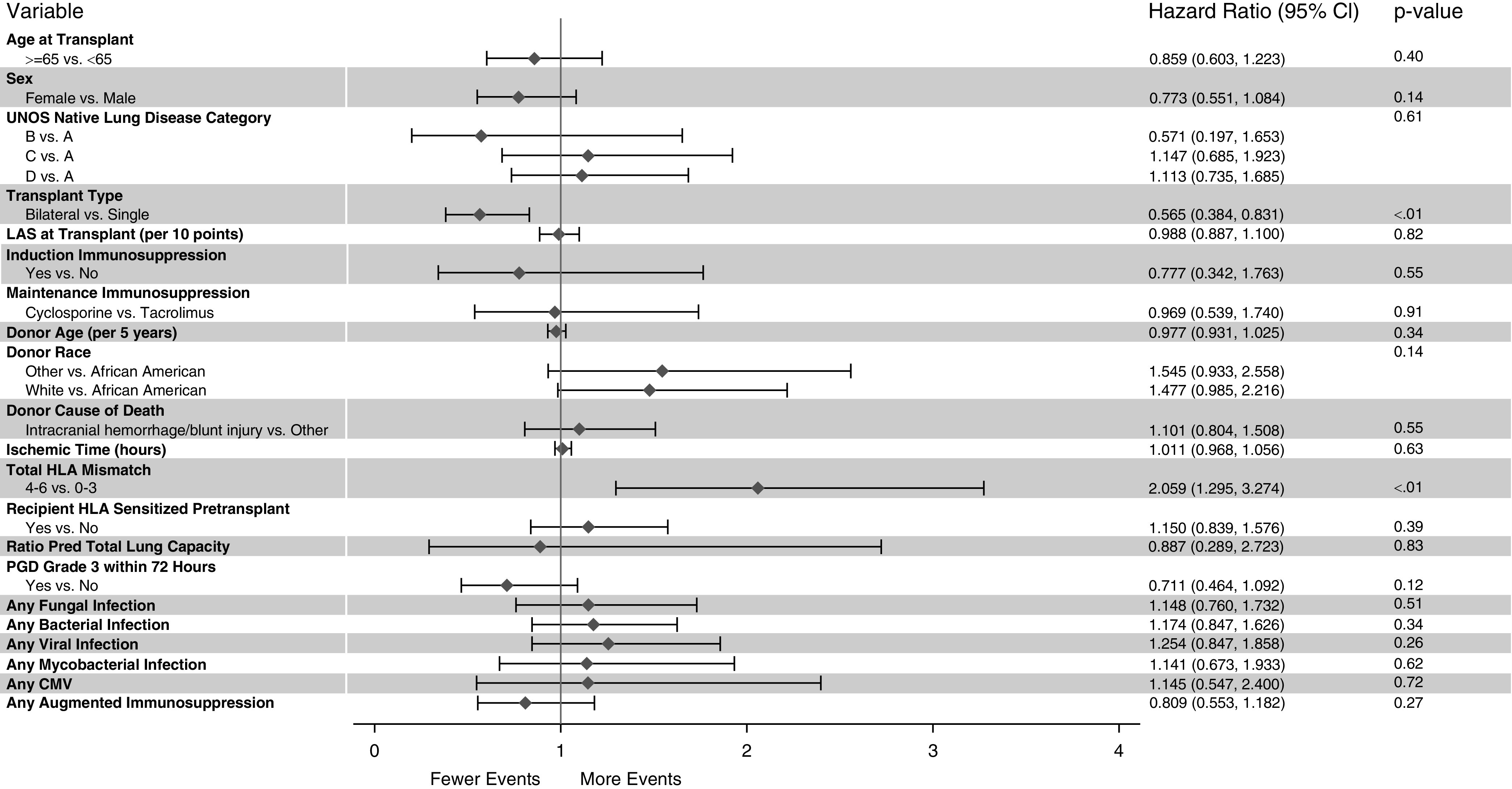
Forest plot of multivariable associations from the Cox regression model evaluating the impact of donor and recipient factors on the time to development of the first acute perivascular rejection event in the first year after lung transplantation. The enrolling center is included in the model as a random effect. Acute perivascular rejection–free survival was censored for death, retransplant, study termination, and the end of first post-transplant year. CI = confidence interval; CMV = cytomegalovirus; LAS = lung allocation score; PGD = primary graft dysfunction; UNOS = United Network for Organ Sharing.
Figure 2.
Descriptive Kaplan-Meier plots illustrating the time to development of the first acute perivascular rejection event in the first year after lung transplantation as stratified by presence or absence of identified clinical risk factor. (A) Fewer than four versus four or more HLA mismatches. (B) Single versus bilateral lung transplant recipient. Acute perivascular rejection–free survival was censored for death, retransplant, study termination, and the end of first post-transplant year.
Figure 3.
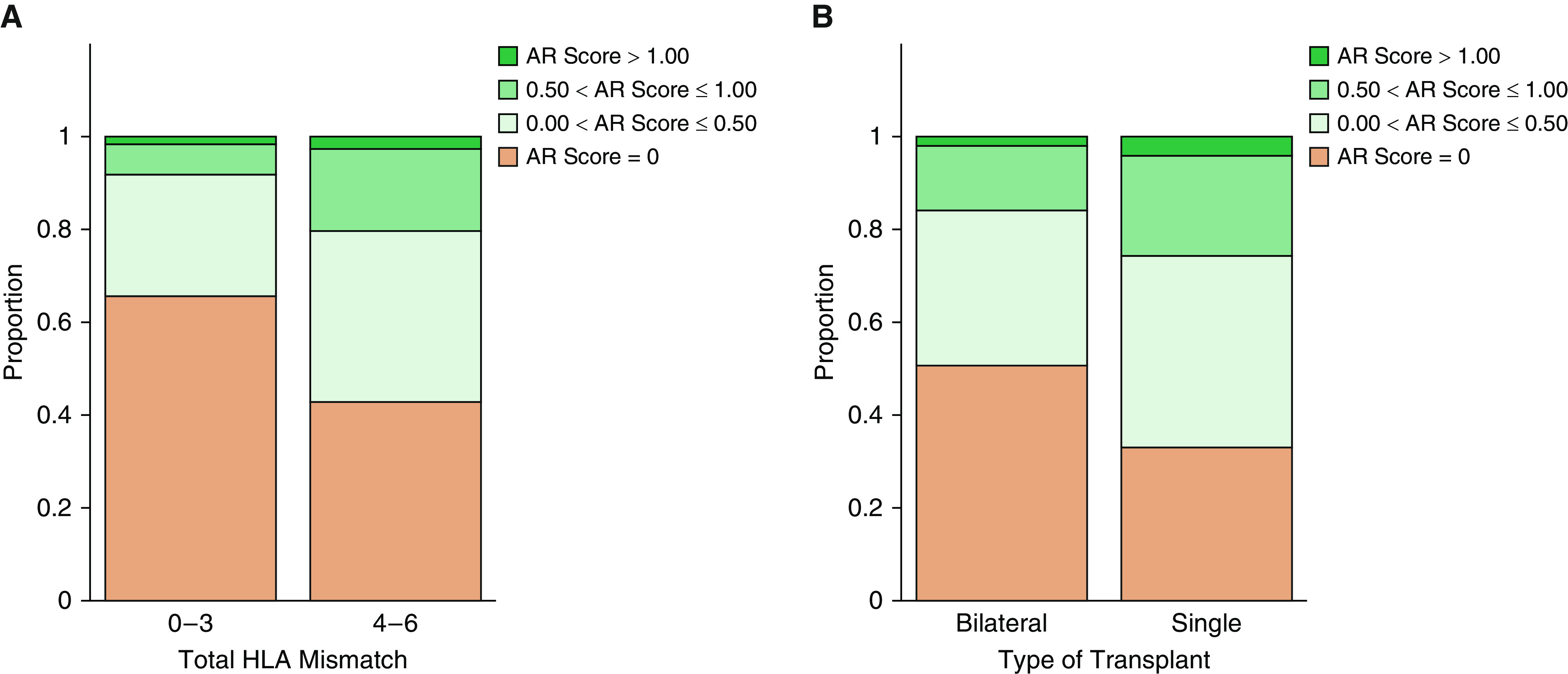
Distribution of normalized acute perivascular rejection scores over the first year after lung transplantation as stratified by the presence or absence of identified clinical risk factors. (A) Fewer than four versus four or more HLA mismatches. (B) Single versus bilateral lung transplant recipient. AR = acute perivascular rejection.
Discussion
Using a present-day, multicenter cohort of 400 lung transplant recipients who underwent frequent and consistent surveillance biopsies, we demonstrated over half of lung transplant recipients experience at least one episode of AR during the first year after transplant, with most episodes occurring within 3 months after transplantation. Most LB episodes were concurrent with AR. Multivariable analyses accounting for center effects indicated that the receipt of a bilateral, as opposed to single, lung transplant significantly reduced AR risk, whereas four or more total HLA mismatches between the donor and the recipient increased the risk for AR.
Although the cumulative incidence of AR in the first year after transplantation observed in our cohort (53%) is higher than that reported by some studies, including the ISHLT registry (1), all five of the CTOT-20 centers perform scheduled surveillance biopsies throughout the first post-transplant year. In contrast, the ISHLT registry includes data from lung transplant centers around the world with variable clinical practices in relation to the use of surveillance biopsies (1). Moreover, registry data are self-reported, retrospectively collected, and unmonitored, with a high degree of missingness. In contrast, CTOT-20 data are prospective, provide complete biopsy information on every patient, and include onsite monitoring with source data verification to ensure the accuracy of reported data.
We observed similar rates of minimal and mild AR on for-cause biopsies compared with surveillance biopsies. Specifically, approximately 20% of biopsies in this cohort were positive for minimal or mild AR, irrespective of the reason for the bronchoscopy. In contrast, a 2008 study compared a cohort of patients managed by a surveillance versus for-cause bronchoscopy schedule and concluded that no AR episode requiring treatment (defined as ≥A2) was detected by a true surveillance biopsy (8). However, other studies support the idea that surveillance biopsies frequently detect asymptomatic allograft rejection (9, 10). Consistent with our results, McWilliams and colleagues reported a similar proportion of surveillance and for-cause biopsies performed in the first post-transplant year demonstrated grade ≥A2 AR (18.7% vs. 15.7%) (9). Although our results demonstrate a high yield of biopsies performed for AR surveillance purposes, the study was not designed to determine whether detection or AR with a surveillance approach influences longer-term patient outcomes.
Despite the importance of AR among lung recipients, there are no contemporary prospective multicenter analyses of AR risk factors. A prior single-center study examined select donor and recipient risks for AR using repeated-measures modeling over extended post-transplant follow-up. Similar to our study, the authors found the highest incidence of AR in the first 2 months after lung transplantation. However, the prevalence of AR at any time after transplant in this former study was dominated by donor factors, including donor age, race, and mechanism of death, with the only associated recipient factor being the pretransplant class II HLA panel reactive antibody exceeding 10% (11). In addition to the different analytic methodology, it is notable the previous work included an older era cohort comprised predominantly of patients transplanted for chronic obstructive pulmonary disease, with very little use of induction immunosuppression. Moreover, it is not evident that the impact of transplant type or important time-dependent risks, such as infection, on AR risk were considered, thus providing several explanations for the divergent results.
Our data suggest that recipients with four or more HLA mismatches are at an elevated risk for AR. Although colinearity precluded us from simultaneously evaluating total HLA mismatch and HLA mismatch at each locus in our multivariable analyses, our univariable results suggest that mismatches at the class II HLA DR locus in particular may drive this effect. Several prior studies have associated increasing HLA mismatches with worse post-transplant survival and CLAD (12–15), although fewer have investigated the impact of HLA mismatch on AR (13, 15). Peltz and colleagues analyzed data from the Organ Procurement and Transplantation Network database to report that mismatches at the class II HLA locus, but not those at the class I locus, increased the risk for treated AR. In a separate study, Quantz and colleagues examined data from the ISHLT registry to report an association between class I, but not class II, HLA mismatches and either treated AR or hospitalization for AR. Both studies involved patients transplanted before 2004 and were limited in focus to the outcome of treated AR events. Thus, our analyses provide a significant extension of this work by confirming the degree of HLA mismatch is independently associated with any occurrence of AR in a lung transplant cohort managed according to contemporary clinical practice.
Although induction immunosuppression is commonly employed in lung transplantation, its use remains controversial given the lack of prospective data supporting its influence on post-transplant outcomes. Approximately 50% of patients in our cohort received induction immunosuppression, most commonly with basiliximab, a monoclonal antibody to the IL-2 receptor, and the use of induction was highly colinear with center. Receipt of induction immunosuppression was not associated with a significant reduction in AR risk when controlling for center effects in our analysis. This finding is in contrast to ISHLT registry data suggesting patients receiving basiliximab are significantly less likely to experience AR over the first post-transplant year compared with those not receiving induction immunosuppression (1). As noted previously, however, the incidence of AR was higher in our cohort than that reported in the ISHLT registry and suggests differences in the ascertainment of AR in these cohorts either because of differences in bronchoscopy/biopsy practices or data collection methods. Moreover, the ISHLT analyses are not adjusted for other confounders, including potential heterogeneity, in center-specific hazards for AR. Finally, as the effect estimate for induction on the outcome of AR was in the protective direction both in the univariate and multivariate analyses, it is possible we were underpowered to detect a very small effect difference between patients receiving induction therapy versus those not receiving induction therapy. This suggests if there is a benefit to induction therapy in relation to AR, the benefit may be small and require a large sample size to become evident.
A novel outcome of our analysis is the finding that bilateral, as compared with single, lung recipients are at a lower risk for AR within the first postoperative year, independent of the other covariates, such as age, native lung disease, or receipt of induction immunosuppression. Although unexpected, these findings parallel prior reports suggesting an association between transplant type and CLAD. Specifically, Hadjialiadis and colleagues demonstrated a higher rate of CLAD in single lung recipients compared with bilateral lung recipients (49.3% vs. 31.7%) (16). Furthermore, in multivariable analyses type of transplant remained a significant risk factor for CLAD. Similar observations were made by Gerbase and colleagues (17) and Neurohr and colleagues in a smaller series of patients with pulmonary fibrosis (18). A more recent ISHLT registry analysis demonstrated that bilateral lung recipients had significantly greater CLAD-free survival than single lung recipients did (19). Ours, however, is the first study to demonstrate significant differences in the rates of AR between single and bilateral lung recipients, suggesting that the difference in CLAD rates and overall survival among single and bilateral lung recipients may reflect immunological differences that could influence the timing of CLAD onset. The plausibility of this finding is strengthened by the additional finding of reduced severity and frequency of AR in bilateral lung recipients compared with single lung recipients as well as the observation that the influence of transplant type on AR risk was generally consistent across centers.
Although the mechanism underlying the association of reduced early AR in bilateral lung recipients is not immediately clear, prior studies provide biological plausibility for these clinical findings. For example, He and colleagues demonstrated in experimental models of heart or skin transplantation that recipients of smaller-sized donor grafts experienced a higher rate of acute rejection and inferior survival compared with those transplanted with larger donor tissue mass (20). T-cell exhaustion, characterized by impaired cytokine secretion and loss of proliferative capacity, represents a potential mechanism underlying the relationship between graft tissue mass, or antigen burden, and graft survival. Consistent with this idea, exposure to an increased quantity of antigens contributes to an exhausted T-cell phenotype in various infections (21, 22). Further mechanistic studies in experimental lung transplant models, including those that test the hypothesis of T-cell exhaustion, are necessary to inform the biological interpretation of these results.
Our study represents a contemporary, multicenter experience including several large volume North American lung transplant centers; however, there are some limitations. The study relied on histological diagnoses made at each enrolling center. Although there was no central adjudication, pathologists from each center used commonly agreed on interpretation approaches supported by published guidelines (5). Despite this effort, observed differences in LB rates, in particular, across centers suggests the need to further refine the ISHLT biopsy-grading approach. In addition, although we considered the approach to immunosuppression in general, we did not collect detailed information on calcineurin inhibitor trough amounts, which over time could contribute to differences in rejection rates. However, each center follows relatively similar management with respect to target calcineurin inhibitor amounts in the first post-transplant year, and differences in trough amounts are unlikely to explain the finding we observed.
In summary, in a contemporary multicenter lung transplant cohort with consistent biopsy sampling, AR is demonstrated to be more common over the first post-transplant year than previously appreciated. These data underscore the importance of AR in lung recipients compared with recipients of other solid organs and highlight the critical need to develop innovative approaches to immunosuppression and clinical management that are specifically effective in the lung transplant population. Our analysis of AR risk factors found that the degree of HLA mismatch influences the risk for AR and identified a novel association between bilateral transplant and attenuated AR risk. The latter observation, although supported by prior studies on CLAD and preclinical models, clearly warrants further replication and mechanistic investigation to provide a deeper understanding of the immunological effects of transplant operation. Present-day, multicenter lung transplant cohort studies, such as CTOT-20, with precise, validated, and complete clinical data are commensurate in guiding evidence-based approaches to lung recipient management and improving long-term patient outcomes.
Supplementary Material
Footnotes
Supported by NIH grant U01-AI113315 (to S.M.P).
Author Contributions: All authors contributed to the design of the study. J.L.T., C.W.F., L.D.S., E.N.P., T.M., W.T., M.Y.S., L.G.S., M.B., P.D.S., J.M.R., S.M.P., J.A.B., and S.S.W. contributed to the acquisition of the data. J.L.T., M.L.N., H.K., M.L.S., S.M.P., J.A.B., and S.S.W. contributed to the development of the analysis plan, and M.L.N., H.K., and M.L.S. performed the data analysis. All authors contributed to the interpretation of the data. J.L.T. drafted the manuscript. All authors critically revised the manuscript and approved the final version for submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-1915OC on May 7, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017. Focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1047–1059. doi: 10.1016/j.healun.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Khalifah AP, Hachem RR, Chakinala MM, Yusen RD, Aloush A, Patterson GA, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5:2022–2030. doi: 10.1111/j.1600-6143.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004;170:1022–1026. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 4.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Koutsokera A, Levy L, Pal P, Orchanian-Cheff A, Martinu T. Acute cellular rejection: is it still relevant? Semin Respir Crit Care Med. 2018;39:181–198. doi: 10.1055/s-0037-1617424. [DOI] [PubMed] [Google Scholar]
- 7.Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85:185–203. doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valentine VG, Gupta MR, Weill D, Lombard GA, LaPlace SG, Seoane L, et al. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant. 2009;28:14–20. doi: 10.1016/j.healun.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 9.McWilliams TJ, Williams TJ, Whitford HM, Snell GI. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant. 2008;27:1203–1209. doi: 10.1016/j.healun.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Minami M, Wada N, Nakagiri T, Funaki S, Kawamura T, et al. Results of surveillance bronchoscopy after cadaveric lung transplantation: a Japanese single-institution study. Transplant Proc. 2014;46:944–947. doi: 10.1016/j.transproceed.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 11.Mangi AA, Mason DP, Nowicki ER, Batizy LH, Murthy SC, Pidwell DJ, et al. Predictors of acute rejection after lung transplantation. Ann Thorac Surg. 2011;91:1754–1762. doi: 10.1016/j.athoracsur.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 12.Smits JM, Mertens BJ, Van Houwelingen HC, Haverich A, Persijn GG, Laufer G. Predictors of lung transplant survival in eurotransplant. Am J Transplant. 2003;3:1400–1406. doi: 10.1046/j.1600-6143.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- 13.Peltz M, Edwards LB, Jessen ME, Torres F, Meyer DM. HLA mismatches influence lung transplant recipient survival, bronchiolitis obliterans and rejection: implications for donor lung allocation. J Heart Lung Transplant. 2011;30:426–434. doi: 10.1016/j.healun.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Schulman LL, Weinberg AD, McGregor C, Galantowicz ME, Suciu-Foca NM, Itescu S. Mismatches at the HLA-DR and HLA-B loci are risk factors for acute rejection after lung transplantation. Am J Respir Crit Care Med. 1998;157:1833–1837. doi: 10.1164/ajrccm.157.6.9707007. [DOI] [PubMed] [Google Scholar]
- 15.Quantz MA, Bennett LE, Meyer DM, Novick RJ. Does human leukocyte antigen matching influence the outcome of lung transplantation? An analysis of 3,549 lung transplantations. J Heart Lung Transplant. 2000;19:473–479. doi: 10.1016/s1053-2498(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 16.Hadjiliadis D, Davis RD, Palmer SM. Is transplant operation important in determining posttransplant risk of bronchiolitis obliterans syndrome in lung transplant recipients? Chest. 2002;122:1168–1175. doi: 10.1378/chest.122.4.1168. [DOI] [PubMed] [Google Scholar]
- 17.Gerbase MW, Spiliopoulos A, Rochat T, Archinard M, Nicod LP. Health-related quality of life following single or bilateral lung transplantation: a 7-year comparison to functional outcome. Chest. 2005;128:1371–1378. doi: 10.1378/chest.128.3.1371. [DOI] [PubMed] [Google Scholar]
- 18.Neurohr C, Huppmann P, Thum D, Leuschner W, von Wulffen W, Meis T, et al. Munich Lung Transplant Group. Potential functional and survival benefit of double over single lung transplantation for selected patients with idiopathic pulmonary fibrosis. Transpl Int. 2010;23:887–896. doi: 10.1111/j.1432-2277.2010.01071.x. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni HS, Cherikh WS, Chambers DC, Garcia VC, Hachem RR, Kreisel D, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: an International Society for Heart and Lung Transplantation thoracic transplant registry analysis. J Heart Lung Transplant. 2019;38:5–16. doi: 10.1016/j.healun.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, et al. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- 21.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8+ T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur J Immunol. 2012;42:2290–2304. doi: 10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.