Abstract
Personality is associated with health, but examinations in patients with illnesses are lacking. We aimed to determine whether personality–physical health associations differed between community and cancer samples. This cross-sectional study involved 168 participants without cancer, 212 men with prostate cancer, and 55 women with breast cancer. We examined whether the Big Five personality dimensions were associated with health behaviors and multiple health indicators. Higher conscientiousness and lower neuroticism were associated with better health behaviors and health (rmax = .31), with few differences between community and cancer samples. Findings call for research on the implications of personality in patients with serious illnesses.
Keywords: health behaviors, health outcomes, oncology, personality, physical health
Across many studies of the Big Five personality traits (McCrae and Costa, 1987), higher conscientiousness and lower neuroticism have been associated with better physical health in community samples (e.g. Chapman et al., 2011; Strickhouser et al., 2017), but limited research exists in samples with cancer. For example, conscientiousness is a key determinant of better health behaviors (i.e. more exercise, healthier diet, less substance use, and safer sex behaviors; Bogg and Roberts, 2004), better perceived health, and reduced mortality (Jokela et al., 2013; Luo and Roberts, 2015). Additionally, high neuroticism is associated primarily with negative health behaviors (i.e. less exercise, poor diet, and greater substance use; De Moor et al., 2006; Hakulinen et al., 2015; Keller and Siegrist, 2015; Kuntsche et al., 2008), worse perceived health (Hoerger et al., 2016b), greater functional impairment (Chapman et al., 2007a), and multimorbidity (Chapman et al., 2007b). Given demographic aging and the increasing prevalence of chronic or serious illnesses, such as cancer, research on personality in disease-defined populations (Epstein and Street, 2007) will have implications for developing targeted and tailored interventions that can improve health outcomes and reduce healthcare cost (Chapman et al., 2011; Croyle, 2015).
Few studies have examined whether personality is associated with physical health in cancer samples. Given that living with cancer may be a “strong” situation (see Benjamin and Simpson, 2009) weakening personality–health associations (Duberstein et al., 2003; Löckenhoff et al., 2008), there is reason to hypothesize that these associations may be weaker in cancer samples. Some studies suggest that higher neuroticism, hostility, negative affectivity, and Type D personality are associated with worse health (Beisland et al., 2015; Husson et al., 2015; Paika et al., 2010; Schoormans et al., 2017; Zhang et al., 2016) and extraversion elements (i.e. optimism) are associated with better health in patients with cancer (Allison et al., 2003; Paika et al., 2010), but these associations were not compared to those from community samples. In addition, these studies only measured specific Big Five dimensions (e.g. neuroticism) or other personality variables (e.g. optimism, hostility, and Type D personality), and none examined conscientiousness. A more complete assessment of how each Big Five personality dimension explains physical health in cancer samples is warranted, as this could inform the design of targeted and tailored interventions for this population (Chapman et al., 2011).
This study examined the Big Five personality traits and physical health in community, prostate cancer, and breast cancer samples. Our research was informed by Smith’s (2006) health behavior model, which suggests that personality influences health behaviors, which in turn influence both subjective and objective health (Hampson et al., 2007; Smith, 2006). Support for this model exists in community samples (e.g. (Lodi-Smith et al., 2010; Mroczek et al., 2009), but it remains an open question whether the “strong” situation (see Benjamin and Simpson, 2009) of living with cancer could diminish the influence of personality (Duberstein et al., 2003; Löckenhoff et al., 2008). Our goals were (a) to determine whether personality–physical health associations differed between community and cancer samples, (b) to determine whether health behaviors explained these associations, and (c) to determine whether indirect associations were moderated by cancer diagnosis. We hypothesized that (a) higher consciousness and lower neuroticism would be associated with better health behaviors and outcomes in both community and cancer samples, (b) these personality–health associations would be weaker in the cancer samples, (c) health behaviors would explain these associations in both community and cancer samples, and (d) this indirect effect would be weaker in the cancer samples. To our knowledge, this is the first study using a complete measure of the Big Five to examine personality–health associations in cancer samples, with potential implications for building knowledge that may contribute toward developing personally tailored interventions.
Method
Participants and procedure
Participants were aged 40 years and above with no cancer history (n = 168) or prostate cancer (n = 212) or female breast cancer (n = 55; see Table 1). Inclusion criteria were being at least 18 years and ability to respond in English. For the cancer samples, patients were excluded if they were not in active oncological care. This study only included prostate or breast cancer diagnoses because these were the only sizeable cancer samples (see Hoerger et al., 2016a). They were recruited via the National Institutes of Health (NIH) ResearchMatch participant pool, health education websites, discussion forums/listservs, and search engines (see Hoerger et al., 2016a) to complete an online questionnaire. Procedures were approved by the local Institutional Review Board.
Table 1.
Participant demographics and variable descriptives by sample.
| Category | Community |
Prostate cancer |
Breast cancer |
|---|---|---|---|
| (n = 168) |
(n = 212) |
(n = 55) |
|
| n (%) or M (SD) | n (%) or M (SD) | n (%) or M (SD) | |
| Age (years) | |||
| 40–49 | 100 (59.5%) | 7 (3.3%) | 20 (36.4%) |
| 50–59 | 52 (31.0%) | 80 (37.7%) | 20 (36.3%) |
| 60–69 | 13 (7.7%) | 78 (36.8%) | 13 (23.7%) |
| 70+ | 3 (1.8%) | 47 (22.2%) | 2 (3.6%) |
| Gender | |||
| Female | 131 (78.0%) | 0 (0.0%) | 55 (100%) |
| Male | 37 (22.0%) | 212 (100%) | 0 (0.0%) |
| Race/ethnicity | |||
| White, non-Latino/a | 133 (79.2%) | 193 (91.0%) | 55 (100%) |
| Other, diverse | 35 (20.8%) | 19 (9.0%) | 0 (0.0%) |
| Marital status | |||
| Married | 90 (53.6%) | 179 (84.4%) | 37 (67.3%) |
| Unmarried | 78 (46.4%) | 33 (15.6%) | 18 (32.7%) |
| Geographic region | |||
| South | 37 (22.0%) | 67 (31.6%) | 16 (29.1%) |
| West | 38 (22.6%) | 39 (18.4%) | 9 (16.4%) |
| North | 31 (18.5%) | 25 (11.8%) | 5 (9.1%) |
| Midwest | 21 (12.5%) | 44 (20.8%) | 11 (20.0%) |
| International | 41 (24.4%) | 37 (17.5%) | 14 (25.5%) |
| Educational level | |||
| Some high school or less | 2 (1.2%) | 3 (1.4%) | 0 (0.0%) |
| High school graduate | 10 (6.0%) | 15 (7.1%) | 2 (3.6%) |
| Some college or associates degree | 63 (37.5%) | 47 (22.2%) | 16 (29.1%) |
| Bachelor’s degree | 44 (26.2%) | 75 (35.4%) | 21 (38.2%) |
| Masters or doctoral degree | 49 (29.2%) | 72 (34.0%) | 16 (29.1%) |
| Time since diagnosis | |||
| Less than 1 year | 76 (35.8%) | 33 (60.0%) | |
| 1–2.9 years | 68 (32.1%) | 9 (16.4%) | |
| 3–4.9 years | 29 (13.7%) | 3 (5.4%) | |
| 5 or more years | 39 (18.4%) | 10 (18.2%) | |
| Treatments | |||
| No treatment | 47 (22.2%) | 5 (9.1%) | |
| Radiation | 63 (29.7%) | 19 (34.5%) | |
| Chemotherapy | 20 (9.4%) | 28 (50.9%) | |
| Surgery | 47 (22.2%) | 21 (38.2%) | |
| Biologic/targeted therapy | 19 (9.0%) | 13 (23.6%) | |
| Unknown treatments | 19 (9.0%) | 2 (3.6%) | |
| Other treatments | 51 (24.1%) | 12 (21.8%) | |
| Cancer metastasized | 58 (27.4%) | 15 (27.3%) | |
| Personality | |||
| Conscientiousness (α = .67) | 14.70 (3.31) | 14.75 (2.93) | 14.42 (2.94) |
| Neuroticism (α = .76) | 11.48 (3.51) | 10.80 (3.46) | 11.25 (3.36) |
| Extraversion (α = .81) | 12.22 (3.85) | 11.76 (3.55) | 12.64 (4.11) |
| Openness (α = .67) | 15.70 (3.10) | 15.80 (2.68) | 15.42 (2.67) |
| Agreeableness (α = .74) | 16.45 (3.06) | 15.60 (2.55) | 17.22 (2.22) |
| Physical health | |||
| Perceived health | 3.65 (0.98) | 3.18 (0.92)* | 3.33 (0.98)* |
| Poor | 2 (1.2%) | 10 (4.7%) | 1 (1.8%) |
| Fair | 20 (11.9%) | 31 (14.6%) | 12 (21.8%) |
| Good | 48 (28.6%) | 94 (44.3%) | 15 (27.3%) |
| Very good | 63 (37.5%) | 65 (30.7%) | 22 (40.0%) |
| Excellent | 35 (20.8%) | 12 (5.7%) | 5 (9.1%) |
| Functional status (1 day in bed)a | 61 (36.3%) | 47 (22.2%) | 27 (49.1%)*** |
| Health history (comorbidities)a | 81 (48.2%) | 152 (71.7%) | 25 (45.5%) |
| Reported physical symptoms (α = .81)a | 0.67 (0.67) | 1.00 (0.79)*** | 1.30 (0.97)*** |
| Health behaviors (α = .78) | 12.99 (4.30) | 14.39 (3.96) | 13.16 (3.87) |
M (SD) = mean (standard deviation).
Patients may have received multiple treatments. Alphas are presented in parentheses for measures with multiple items. Asterisks represent personality, physical health, and health behaviors that differed from the community sample controlling for covariates using analysis of covariance (ANCOVA) or binary logistic regression as needed. Numbers and percents for functional status represent participants who spent one or more days in bed during the past month.
Variables indicate poorer health here, but were reverse coded for all remaining analyses, so all health variables represented better physical health.
p < .05, **p < .01, and ***p < .001.
Measures
Personality
The 20-item Mini-International Personality Item Pool (IPIP) scales (Donnellan et al., 2006) were used to assess personality. Participants responded to statements such as whether they “Have frequent mood swings” (neuroticism) and “Like order” (conscientiousness) using a 5-point scale (1 = very inaccurate to 5 = very accurate). The Mini-IPIP has shown evidence of internal consistency, test–retest reliability, and construct validity when compared with other Big Five measures (Donnellan et al., 2006) and is suitable for patient populations (e.g. Moran et al., 2011). Internal-consistency reliability was comparable to that of prior studies and across samples (Table 1).
Physical health
We assessed four indicators of physical health—health history, functional status, reported physical symptoms, and perceived health. To assess health history, participants reported whether they had been previously diagnosed with any of 13 health conditions, including diabetes, stroke, and heart attack, using an adapted version of the Midlife in the United States (MIDUS) Health History Checklist (e.g. Costanzo et al., 2012). Patient and physician reports of these diagnoses have shown consistency (Fortin et al., 2005). Functional status was measured with an item adapted from the Functional Status Questionnaire (FSQ) that read “During the past month, how many days did you spend the majority of your time in bed?” (Jette et al., 1986) measured from 0 to 30 days. Participants also completed the Functional Assessment of Cancer Therapy–General (FACT-G) Physical Well-Being Subscale (Cella et al., 1993) rating from 0 (not a lot) to 4 (very much), the extent to which they experienced symptoms (e.g. “I have pain” and “I have nausea”) during the past week. The final item of the FACT-G (time spent in bed) was not administered due to redundancy with the FSQ. Perceived health was measured with the Short Form Health Survey (SF-1) (Ware and Sherbourne, 1992) which reads “In general, how would you say your health is?” measured from 1 (poor) to 5 (excellent). Due to non-normality in functional status, D(435) = .35, p < .001, and health history, D(435) = .22, p < .001, responses were dichotomized for functional status (0 = one or more days in bed, 1 = zero days in bed) and health history (0 = any comorbidity, 1 = no comorbidities). Principal Component Analysis was used to create a single physical health indicator from the four indicators (supported by scree plots) with higher scores indicating better health. Each of these scales has shown evidence of reliability and validity (Cleary and Jette, 2000; DeSalvo et al., 2006; Martin et al., 2000; Weitzner et al., 1995).
Health behaviors
Participants responded to three items adapted from the Health Behavior Marker Scale (Vickers et al., 1990) regarding how often they exercised and monitored their diet and weight during the past month from 1 (never) to 7 (always). A higher summated score reflected better health behaviors. Internal-consistency reliability was acceptable (Table 1), and the validity of lengthier versions of the scale has been documented (Lodi-Smith et al., 2010; Vickers et al., 1990).
Statistical analyses
First, we examined descriptive statistics, including central tendency, dispersion, frequencies, and distribution shapes. Second, we examined demographic, personality, health behavior, and physical health differences between samples using analysis of variance (ANOVA) with Tukey’s honest significant difference (HSD) tests, analysis of covariance (ANCOVA), chi-square, or binary logistic regression. Third, we examined zero-order correlations between personality and health variables across all participants. Fourth, we examined hierarchical regression analyses that entered covariates in the first step and all five personality dimensions in the second step; separate analyses were conducted for each health-related dependent variable. Covariates included age (continuous), education (continuous), marital status (coded 0 = unmarried and coded 1 = married), gender (coded 0 = female and coded 1 = male), and race/ethnicity (coded 0 = White non-Latino and coded 1 = racially/ethnically diverse). We chose to include these covariates a priori because these variables have well-documented associations with physical health outcomes; so, their inclusion reduces the risk of confounding and provides more precise estimates of effects. These regression analyses allowed us to examine the combined effect of all five personality dimensions beyond the effects of covariates (ΔR2) and examine the unique contribution of each personality dimension (standardized beta). Linear regression was used for continuous dependent variables, whereas binary logistic regression was used for dichotomized ones. Fifth, to examine whether personality and overall physical health or health behavior associations differed between community and cancer samples, we examined interaction terms from regression models including sample, a single personality trait, the mean-centered personality trait by sample interaction term, and covariates. Sample was coded using two dummy variables (prostate cancer variable: prostate cancer = 1, breast cancer and community = 0; breast cancer variable: breast cancer = 1, prostate cancer and community = 0).
Finally, we used PROCESS for SPSS (version 2.16; Model 4) to test for indirect effects controlling for covariates. When indirect effects were significant, we conducted moderated mediation analyses (Model 7) using bias correcting bootstrapping with 10,000 resamples to determine whether health behaviors indirectly explained the pathway between personality and physical health and to determine whether sample moderated this indirect effect.
Results
Across samples, the majority were White and college-educated and varied in terms of gender, age, marital status, and geographic region (Table 1). The cancer samples ranged in time since diagnosis and varied in treatment types (Table 1). The community sample was younger (M = 49.3 years, SD = 7.7 years) than the cancer samples (prostate: M = 62.4, SD = 8.2 years; breast: M = 53.9, SD = 8.8 years; F (2432) = 124.75, p < .001) and more racially/ethnically diverse (χ2 = 21.07, p < .001). Samples varied by gender (χ2 = 317.12, p < .001) due to gender-specific cancer sites. The prostate cancer sample was more likely to be married (χ2 = 43.08, p < .001).
Analyses of between-group differences (Table 1) indicated that personality scores, health history, and health behaviors were comparable across groups. As anticipated, the cancer samples reported worse perceived health (prostate cancer: p = .04; breast cancer: p = .03) and more physical symptoms (p < .001 for both cancer samples) than the community sample. The breast cancer sample reported worse functional status than the community sample (p < .001). Thus, the cancer samples were in worse physical health than the community sample suggesting that living with cancer could represent a strong situation that may alter associations.
Personality associations with physical health and health behaviors
Personality was associated with several indicators of physical health across samples (Table 2). Consistent with hypotheses, higher conscientiousness and lower neuroticism were associated with better overall physical health. Conscientiousness was positively correlated with four measures of better physical health (r = .13–.22), and neuroticism was negatively correlated with all five measures of physical health (r = –.11 to –.31). Although not hypothesized, extraversion was positively correlated with three measures of physical health (r = .10–.13), openness was positively correlated with three measures of physical health (r = .10–.11), and agreeableness was associated with a better health history (r = .14). Findings were comparable after controlling for covariates (Table 2), except that those involving openness were no longer significant.
Table 2.
Associations among personality dimensions and indicators of physical health across all participants (N = 435).
| Personality | Physical Health |
|||||
|---|---|---|---|---|---|---|
| Better health behaviors r (β) |
Better health history r (β) |
Better functional status r (β) |
Better reported physical symptoms r (β) |
Better perceived health r (β) |
Better physical health composite r (β) |
|
| Conscientiousness | .19*** (.16**) | .08 (.05) | .13** (.09) | .16** (.13**) | .20*** (.17***) | .22*** (.18***) |
| Neuroticism | −.23*** (−.16**) | −.11* (−.17***) | −.20*** (−.14**) | −.29*** (−.28***) | −.22*** (−.20***) | −.31*** (−.29***) |
| Extraversion | .07 (.03) | .10* (.04) | −.01 (−.03) | .06 (.02) | .20*** (.15**) | .13** (.07) |
| Openness | .15** (.10*) | −.01 (−.09) | .06 (.01) | .10* (.00) | .11* (.02) | .11* (−.01) |
| Agreeableness | .02 (−.04) | .14** (.07) | .02 (.04) | .00 (−.09) | .07 (−.08) | .07 (−.05) |
| ΔR2 | .08*** | .22*** | .16*** | .10*** | .11*** | .13*** |
Findings show correlations with standardized betas in parentheses. Standardized betas control for the effects of other personality dimensions, as well as covariates that included age, education, marital status, gender, and race/ethnicity. The ΔR2 represents the combined contribution of the five personality dimensions beyond covariates. Logistic regression with the Nagelkerke approximated R2 was conducted for better health history and better functional status. Linear regression was conducted for better reported physical symptoms, better perceived health, and the better physical health composite.
p < .05, **p < .01, and ***p < .001.
Personality was associated with engagement in health behaviors across samples (Table 2). As hypothesized, higher conscientiousness and lower neuroticism were associated with better health behaviors (r = .19 and r = −.23, respectively). Openness was also significantly correlated with better health behaviors (r = .15). Controlling for covariates did not alter associations (Table 2).
We examined whether sample moderated the associations between personality and health outcomes. There were no sample by personality trait interactions on overall physical health. Only one interaction was found on health behaviors: a prostate cancer by agreeableness interaction (β = .16, p = .02), such that agreeableness was associated with better health behaviors in the prostate cancer sample (r = .17) and weak-to-worse health behaviors in the other samples (breast cancer: r = −.13, community: r = −.04). Overall, these results indicate that personality–health associations were comparable across the prostate cancer, breast cancer, and community samples.
Tests of indirect effects
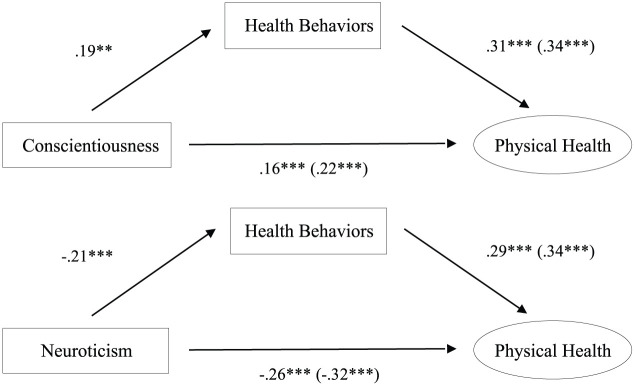
Tests of indirect effects showed that the association between personality and overall physical health was mediated by health behaviors (Figure 1). These pathways were significant for conscientiousness and neuroticism (Table 2); health behaviors and physical health were correlated (r = .34, p < .001). Health behaviors explained 26.8 percent of the total effect of conscientiousness on physical health (a × b = .02, standard error (SE) = .01, z = 3.38, p < .001) and 18.9 percent of the total effect of neuroticism on physical health (a × b = −.02, SE = <.01, z = −3.50, p < .001).
Figure 1.
Analyses of indirect effects demonstrating that the associations between conscientiousness or neuroticism and physical health are explained by engagement in health behaviors. Standardized betas controlling for covariates are presented. Parenthetical values provide coefficients for the total association, whereas non-parenthetical values provide coefficients for the direct association when including personality and health behaviors in the model simultaneously.
**p < .01 and ***p < .001.
Four moderated mediation analyses examined whether sample moderated the indirect effect of health behaviors on the conscientiousness– or neuroticism–overall physical health association. First, we examined whether the indirect effect of health behaviors on the conscientiousness–overall physical health association was moderated by having prostate cancer. Second, we examined the same model moderated by having breast cancer. In the third and fourth models, conscientiousness was replaced with neuroticism. Analyses indicated that having prostate or breast cancer did not moderate the indirect effect of conscientiousness (prostate cancer: z = 0.60, p = .55; breast cancer: z = 1.39, p = .17) or neuroticism (prostate cancer: z = 1.31, p = .19; breast cancer: z = 0.38, p = .70) on overall physical health. Thus, the role of health behaviors in explaining the association between conscientiousness or neuroticism and physical health was not significantly different across samples.
Discussion
Although it was an open question whether the strong situation of living with cancer would diminish the effects of personality on health (Benjamin and Simpson, 2009; Löckenhoff et al., 2008), we found no evidence to support the idea that associations between personality and health are significantly different across participants with prostate cancer, breast cancer, and no history of cancer. This investigation shows that higher conscientiousness and lower neuroticism are associated with better physical health and health behaviors, while extraversion, openness, and agreeableness showed few associations with health regardless of cancer diagnosis. Furthermore, according to Smith’s (2006) Health Behavior Model, personality may account for physical health by explaining variation in health behaviors, and our findings support this model regardless of cancer diagnosis. Despite using a brief measure of health behaviors that could have underestimated effects, the health behaviors we assessed explained 18–27 percent of the effect of conscientiousness and neuroticism on physical health, consistent with previous reports in community samples (Turiano et al., 2015). Our results indicate that personality has implications for health, even in strong situations, such as living with cancer (Akrami et al., 2009).
Given the rising emphasis on “personalized medicine,” or tailoring health interventions to a patient’s unique qualities, including personality dimensions (e.g. Hirsh et al., 2012), neuroticism and conscientiousness may be useful targets. Individuals higher in neuroticism may experience difficulty regulating negative emotions, which they may manage through unhealthy behaviors (e.g. poor diet and withdrawing from exercise); therefore, future studies should examine whether incorporating stress management into interventions for patients high in neuroticism could improve intervention effectiveness (Chapman et al., 2011). Individuals low in conscientiousness may experience difficulty following instructions from healthcare providers or following through on planned health behavior changes; therefore, future studies should examine whether providing more detailed medical instructions, reminders, care management, and/or home visits could improve health for patients low in conscientiousness (Bogg and Roberts, 2013). Examinations of interventions with and without personality-tailored components are warranted.
Limitations of this study should be noted. First, although our samples were comparable to others in terms of personality scores (Donnellan et al., 2006), participants were mainly White and college-educated, and a larger breast cancer sample would allow for smaller effects to be detected. Second, this study used relatively brief measures, and given the encouraging findings, future studies should assess personality, the full array of health behaviors (e.g. smoking, alcohol consumption, vaccination, etc.), and physical health (including cancer severity) in greater detail. Third, given the cross-sectional design, longitudinal studies are needed to understand the causal nature and mediational pathways of effects. Fourth, objective health indicators (e.g. number of medications) were not included, though our health outcomes did range in subjectiveness. These questions could potentially be answered through secondary analyses of patient samples in existing large-scale longitudinal datasets (i.e. Midlife in the United States, Hawaii Personality and Health Cohort, and Normative Aging Study). Our study had a number of strengths as well. First, there is a pressing public health priority for psychology studies to assess patients with significant health needs, especially patients with cancer (Croyle, 2015), and we included two cancer samples. Second, this is the only study to our knowledge to assess all five Big Five personality dimensions as they relate to health in a cancer sample.
In conclusion, personality dimensions, especially conscientiousness and neuroticism, are associated with health outcomes in community and cancer samples. Research employing longitudinal designs will be helpful for drawing more definitive causal inferences about the observed associations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Rochester Medical Center Department of Psychiatry Leonard F. Salzman Research Award, the National Institute of Mental Health (T32MH018911), and the National Institute of General Medical Sciences (U54GM104940).
ORCID iD: Catherine Rochefort  https://orcid.org/0000-0001-8289-4148
https://orcid.org/0000-0001-8289-4148
References
- Akrami N, Ekehammar B, Bergh R, et al. (2009) Prejudice: The person in the situation. Journal of Research in Personality 43(5): 890–897. [Google Scholar]
- Allison PJ, Guichard C, Fung K, et al. (2003) Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. Journal of Clinical Oncology 21(3): 543–548. [DOI] [PubMed] [Google Scholar]
- Beisland E, Beisland C, Hjelle KM, et al. (2015) Health-related quality of life, personality and choice of coping are related in renal cell carcinoma patients. Scandinavian Journal of Urology 49(4): 282–289. [DOI] [PubMed] [Google Scholar]
- Benjamin LT, Jr, Simpson JA. (2009) The power of the situation: The impact of Milgram’s obedience studies on personality and social psychology. American Psychologist 64(1): 12–19. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. (2004) Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin 130(6): 887–919. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. (2013) The case for conscientiousness: Evidence and implications for a personality trait marker of health and longevity. Annals of Behavioral Medicine 45(3): 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, et al. (1993) The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology 11(3): 570–579. [DOI] [PubMed] [Google Scholar]
- Chapman B, Duberstein P, Lyness JM. (2007. a) Personality traits, education, and health-related quality of life among older adult primary care patients. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences 62(6): 343–352. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Lyness JM, Duberstein P. (2007. b) Personality and medical illness burden among older adults in primary care. Psychosomatic Medicine 69(3): 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Roberts B, Duberstein P. (2011) Personality and longevity: Knowns, unknowns, and implications for public health and personalized medicine. Journal of Aging Research 2011: 759170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary PD, Jette AM. (2000) Reliability and validity of the Functional Status Questionnaire. Quality of Life Research 9(1): 747–753. [Google Scholar]
- Costanzo ES, Stawski RS, Ryff CD, et al. (2012) Cancer survivors’ responses to daily stressors: Implications for quality of life. Health Psychology 31(3): 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle RT. (2015) Risks and opportunities for psychology’s contribution to the war on cancer. American Psychologist 70(2): 221–224. [DOI] [PubMed] [Google Scholar]
- De Moor MH, Beem AL, Stubbe JH, et al. (2006) Regular exercise, anxiety, depression and personality: A population-based study. Preventive Medicine 42(4): 273–279. [DOI] [PubMed] [Google Scholar]
- DeSalvo KB, Bloser N, Reynolds K, et al. (2006) Mortality prediction with a single general self-rated health question. Journal of General Internal Medicine 21(3): 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan MB, Oswald FL, Baird BM, et al. (2006) The mini-IPIP scales: Tiny-yet-effective measures of the Big Five factors of personality. Psychological Assessment 18(2): 192–203. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Sörensen S, Lyness JM, et al. (2003) Personality is associated with perceived health and functional status in older primary care patients. Psychology and Aging 18(1): 25–37. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Street RL., Jr (2007) Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda, MD: National Cancer Institute. [Google Scholar]
- Fortin M, Bravo G, Hudon C, et al. (2005) Prevalence of multimorbidity among adults seen in family practice. The Annals of Family Medicine 3(3): 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen C, Hintsanen M, Munafò MR, et al. (2015) Personality and smoking: Individual-participant meta-analysis of nine cohort studies. Addiction 110(11): 1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Goldberg LR, Vogt TM, et al. (2007) Mechanisms by which childhood personality traits influence adult health status: Educational attainment and healthy behaviors. Health Psychology 26(1): 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, Kang SK, Bodenhausen GV. (2012) Personalized persuasion tailoring persuasive appeals to recipients’ personality traits. Psychological Science 23(6): 578–581. [DOI] [PubMed] [Google Scholar]
- Hoerger M, Chapman BP, Mohile SG, et al. (2016. a) Development and psychometric evaluation of the Decisional Engagement Scale (DES-10): A patient-reported psychosocial survey for quality cancer care. Psychological Assessment 28(9): 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerger M, Coletta M, Sörensen S, et al. (2016. b) Personality and perceived health in spousal caregivers of patients with lung cancer: The roles of neuroticism and extraversion. Journal of Aging Research 2016: 5659793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson O, Vissers PA, Denollet J, et al. (2015) The role of personality in the course of health-related quality of life and disease-specific health status among colorectal cancer survivors: A prospective population-based study from the PROFILES registry. Acta Oncologica 54(5): 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette AM, Davies AR, Cleary PD, et al. (1986) The Functional Status Questionnaire. Journal of General Internal Medicine 1(3): 143–149. [DOI] [PubMed] [Google Scholar]
- Jokela M, Batty GD, Nyberg ST, et al. (2013) Personality and all-cause mortality: Individual-participant meta-analysis of 3,947 deaths in 76,150 adults. American Journal of Epidemiology 178(5): 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Siegrist M. (2015) Does personality influence eating styles and food choices? Direct and indirect effects. Appetite 84: 128–138. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Von Fischer M, Gmel G. (2008) Personality factors and alcohol use: A mediator analysis of drinking motives. Personality and Individual Differences 45(8): 796–800. [Google Scholar]
- Löckenhoff CE, Sutin AR, Ferrucci L, et al. (2008) Personality traits and subjective health in the later years: The association between NEO-PI-R and SF-36 in advanced age is influenced by health status. Journal of Research in Personality 42(5): 1334–1346. [Google Scholar]
- Lodi-Smith J, Jackson J, Bogg T, et al. (2010) Mechanisms of health: Education and health-related behaviours partially mediate the relationship between conscientiousness and self-reported physical health. Psychology and Health 25(3): 305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Roberts BW. (2015) Concurrent and longitudinal relations among conscientiousness, stress, and self-perceived physical health. Journal of Research in Personality 59: 93–103. [Google Scholar]
- McCrae RR, Costa PT. (1987) Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology 52(1): 81–90. [DOI] [PubMed] [Google Scholar]
- Martin LM, Leff M, Calonge N, et al. (2000) Validation of self-reported chronic conditions and health services in a managed care population. American Journal of Preventive Medicine 18(3): 215–218. [DOI] [PubMed] [Google Scholar]
- Moran AM, Everhart DE, Davis CE, et al. (2011) Personality correlates of adherence with continuous positive airway pressure (CPAP). Sleep and Breathing 15(4): 687–694. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A, Turiano NA. (2009) Do health behaviors explain the effect of neuroticism on mortality? Longitudinal findings from the VA Normative Aging study. Journal of Research in Personality 43(4): 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paika V, Almyroudi A, Tomenson B, et al. (2010) Personality variables are associated with colorectal cancer patients’ quality of life independent of psychological distress and disease severity. Psycho-Oncology 19(3): 273–282. [DOI] [PubMed] [Google Scholar]
- Schoormans D, Husson O, Denollet J, et al. (2017) Is type D personality a risk factor for all-cause mortality? A prospective population-based study among 2625 colorectal cancer survivors from the PROFILES registry. Journal of Psychosomatic Research 96: 76–83. [DOI] [PubMed] [Google Scholar]
- Smith TW. (2006) Personality as risk and resilience in physical health. Current Directions in Psychological Science 15(5): 227–231. [Google Scholar]
- Strickhouser JE, Zell E, Krizan Z. (2017) Does personality predict health and well-being? A metasynthesis. Health Psychology 36(8): 797–810. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Chapman BP, Gruenewald TL, et al. (2015) Personality and the leading behavioral contributors of mortality. Health Psychology 34: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers RR, Conway TL, Hervig LK. (1990) Demonstration of replicable dimensions of health behaviors. Preventive Medicine 19: 377–401. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 30(6): 473–483. [PubMed] [Google Scholar]
- Weitzner MA, Meyers CA, Gelke CK, et al. (1995) The Functional Assessment of Cancer Therapy (FACT) scale: Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75(5): 1151–1161. [DOI] [PubMed] [Google Scholar]
- Zhang JK, Fang LL, Zhang DW, et al. (2016) Type D personality in gastric cancer survivors: Association with poor quality of life, overall survival, and mental health. Journal of Pain and Symptom Management 52(1): 81–91. [DOI] [PubMed] [Google Scholar]