Abstract
PURPOSE:
The median overall survival (OS) for metastatic pancreatic ductal adenocarcinoma (mPDAC) is < 1 year. Factors that contribute to quality of life during treatment are critical to quantify. One factor—time spent obtaining clinical services—is understudied. We quantified total outpatient time among patients with mPDAC receiving palliative systemic chemotherapy.
METHODS:
We conducted a retrospective analysis using four patient-level time measures calculated from the medical record of patients with mPDAC receiving 5-fluorouracil infusion, leucovorin, oxaliplatin, and irinotecan; gemcitabine/nab-paclitaxel; or gemcitabine within the University of Pennsylvania Health System between January 1, 2011 and January 15, 2019. These included the total number of health care encounter days (any day with at least one visit) and total visit time. Total visit time represented the time spent receiving care (care time) plus time spent commuting and waiting for care (noncare time). We performed descriptive statistics on these outpatient time metrics and compared the number of encounter days to OS.
RESULTS:
A total of 362 patients were identified (median age, 65 years; 52% male; 78% white; 62% received gemcitabine plus nab-paclitaxel). Median OS was 230.5 days (7.6 months), with 79% of patients deceased at the end of follow-up. On average, patients had 22 health care encounter days, accounting for 10% of their total days survived. Median visit time was 4.6 hours, of which 2.5 hours was spent commuting or waiting for care.
CONCLUSION:
On average, patients receiving palliative chemotherapy for mPDAC spend 10% of survival time on outpatient health care. More than half of this time is spent commuting and waiting for care. These findings provide an important snapshot of the patient experience during ambulatory care, and efforts to enhance efficiency of care delivery may be warranted.
INTRODUCTION
Pancreatic ductal adenocarcinoma is the fourth leading cause of cancer death,1 with a 5-year overall survival (OS) of 9%,1 despite multimodality therapies. Palliative chemotherapy is the therapeutic backbone for patients with metastatic disease, with the most commonly used regimens being 5-fluorouracil infusion, leucovorin, oxaliplatin, irinotecan (FOLFIRINOX) and gemcitabine plus nab-paclitaxel.2-4 Single-agent gemcitabine, a less-toxic but also less-effective regimen, is typically reserved for patients who cannot tolerate multiagent treatment. Currently used chemotherapy regimens have the potential to add between 6 weeks and 11 months to a patient’s expected life span.2-4
Considering this relatively limited benefit, one of the fundamental activities of an oncologist is to disclose all relevant information about the risks, benefits, and logistics of cancer treatment.5 Therefore, health care providers must be equipped to adequately prepare patients with advanced cancer for the opportunity costs—the alternative benefits forgone while receiving cancer care—associated with palliative treatment. Opportunity costs of cancer therapy are traditionally studied in cost-effectiveness analyses in which treatment benefit is reported as gains in survival or quality of life.6-8 One key variable, the amount of time spent engaging in health care, is understudied.
In our clinical experience, patients undergoing treatment of metastatic pancreatic ductal adenocarcinoma travel frequently to and from appointments and spend substantial time both receiving and waiting to receive care. This disease often requires a multidisciplinary approach: patients may have appointments with radiation oncology providers, medical oncology providers, palliative care team members, pain specialists, and others. Although time spent in the health care system would be an important subject on which to counsel patients, to our knowledge, there is no currently available literature on this subject. A better appreciation of the patient experience can also instruct initiatives to improve palliative cancer care.9 To address this information gap, we estimated patient time burden associated with ambulatory cancer care among a cohort of patients receiving palliative chemotherapy for metastatic pancreatic ductal adenocarcinoma.
METHODS
Patient Population
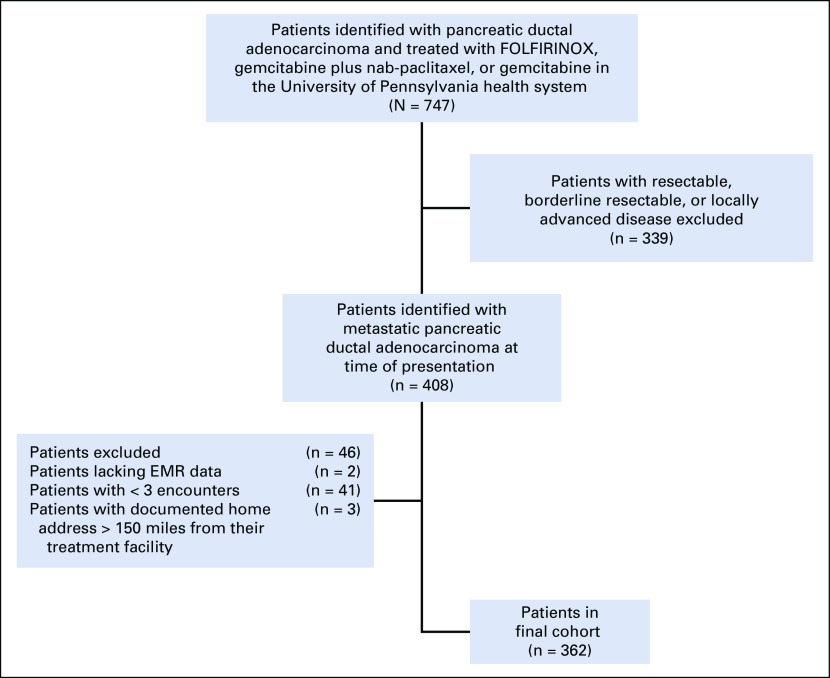
We conducted a retrospective cohort study of consecutive patients with metastatic pancreatic ductal adenocarcinoma who received systemic, palliative chemotherapy between January 1, 2011 and January 15, 2019 within the University of Pennsylvania Health System, which includes 10 university-affiliated and community ambulatory care practices. Patients were included if they received either palliative FOLFIRINOX, gemcitabine plus nab-paclitaxel, or gemcitabine monotherapy at one of the 10 practice sites. Patients who did not have metastatic disease at time of diagnosis or whose tumor histology was not consistent with adenocarcinoma were excluded. Patients who were lost to follow-up, defined as having fewer than three encounters, were also excluded (Appendix Fig A1, online only). This project was approved by the institutional review board at the University of Pennsylvania.
Data Collection Procedures
For each eligible patient, corresponding ambulatory cancer care encounters were identified within the Epic electronic medical record (EMR) system (Epic Systems Corporation, Verona, WI), including medical oncology and radiation oncology provider visits, treatment infusion visits, and any radiology imaging visit performed in the ambulatory setting. Palliative care visits were not exclusively identified, as many GI oncologists within our health system are also trained in palliative care. Visits with nononcology providers (eg, primary care or subspecialty care) were not identified, as care outside the health system could not be reliably captured. Radiation treatment delivery sessions were identified within the Aria (Varian Medical Systems, Palo Alto, CA) radiation oncology information system (ROIS) database. Using an iterative process, we evaluated potential EMR and ROIS timestamps for suitability as markers of key events in a patient’s day.
All potential timestamps were first evaluated for completeness across all relevant patient encounters. Those with > 5% missing values were excluded from analysis. In addition, individual timestamps valued between 10:00 pm (22:00:00) and 6:00 am (06:00:00) were believed to be spurious and excluded. Only outpatient health care visits were included.
Next, an iterative qualitative review of randomly sampled, patient-level data was performed to assess the order of events for logical consistency and compared the extracted data to events documented in the medical record. In addition, interviews with key cancer center personnel, including providers, chemotherapy nurses, and practice operation staff were performed to better understand workflow, Epic utilization throughout clinical care, and practice standards. These reviews and discussions led to further refinement of the queries and re-evaluation of the sample data.
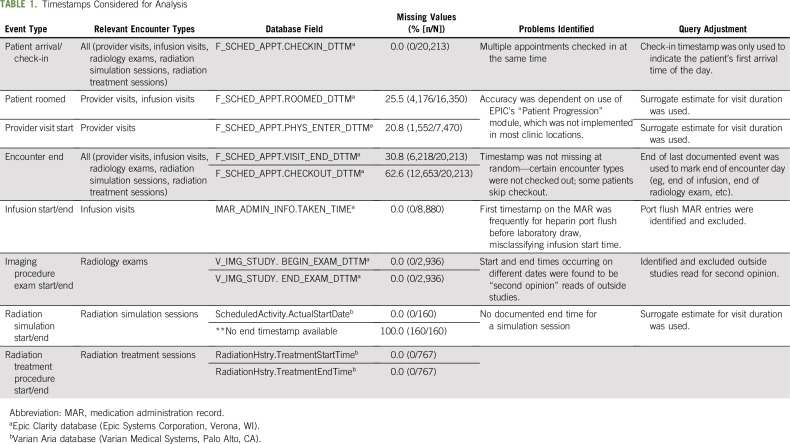
Table 1 describes the data elements considered and examples of challenges that were identified and addressed through query refinement. For example, the start and end times of the face-to-face portion of provider visits were found to be unreliable. Therefore, on the basis of expert consensus among medical and radiation oncology providers, a surrogate 30-minute length of visit time was estimated for each encounter. Similarly, for encounters related to radiation planning (simulation), a surrogate 60-minute visit time was estimated for each encounter.
TABLE 1.
Timestamps Considered for Analysis
Commute times were estimated using the Google Maps Platform (Google, Mountain View, CA). Patient’s home address was determined using the patient’s most recent mailing address documented in Epic and geocoded using ArcGIS software (Esri, Redlands, CA). Because of the large volume of patient encounters studied, it was not feasible to calculate travel time for each individual encounter day. Therefore, a standardized date and time of January 28, 2019 at 09:00 was used to estimate the time required to travel between the patient’s home address and the patient’s care facility. The travel time was doubled to create an estimate of total round-trip commute time. Patients with a documented home address > 150 miles from their cancer center were excluded from the study because of the assumption that the addresses did not reflect the patients’ living arrangements during treatment, as this would equate to a > 6-hour round-trip commute
EPIC was also used to abstract the patient factors, including age, sex, race, date of diagnosis, date of treatment initiation for metastatic cancer, type of insurance provider, location where cancer care was received, Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis, tumor location, sites of metastases, and date of death or last follow-up. Total number of laboratory/specimen collections, outpatient radiology encounters, office encounters (including nononcology providers), radiation treatment sessions, and infusion visits were also abstracted.
Calculation of Time Metrics
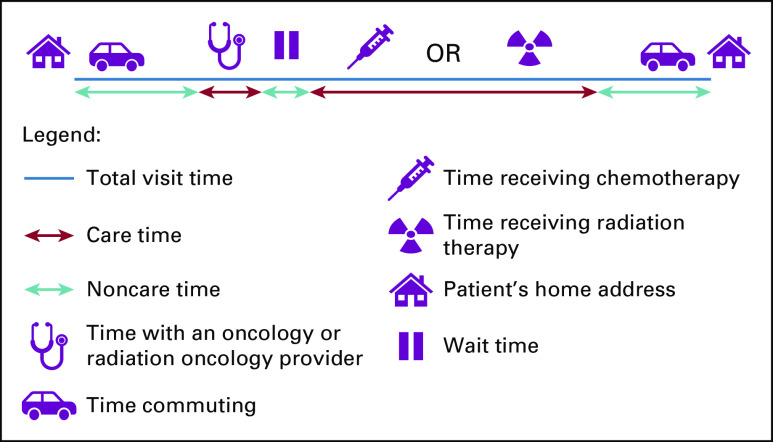
For each patient, the following time metrics were determined: number of health care encounter days, total visit time, care time, and noncare time using the EMR timestamps identified above (Fig 1). Encounter days were defined as any day with at least one health care visit. Days with only a laboratory visit were excluded because of the inability to reliably capture these visits with the timestamps available through the EMR. For each encounter day, total visit time was established using the first and last EMR-based time stamps at the facility plus the estimated round-trip commute time. Care time included estimated time with a medical or radiation oncology provider, time receiving chemotherapy within the infusion suite, time receiving radiation therapy or undergoing radiation simulation, and time undergoing imaging studies. Time waiting to receive care represented the difference between total visit time at the facility and the care time. Noncare time included round-trip commute time and time waiting to receive care
Fig 1.
Visual representation of the time metrics calculated.
Statistical Analysis
Descriptive statistics were calculated to summarize patient health system encounters and associated time metrics, including total visit time, care time, and noncare time, for all patients. Using the Kaplan-Meier method, overall survival was calculated from the start of treatment of metastatic disease to the date of death from any cause, last contact, or end of data collection (January 15, 2019). Among cohort members, the median number of calendar days with at least one outpatient visit was compared with the median overall survival to estimate the median proportion of time spent in health care relative to total survival time.
Several subgroup and sensitivity analyses were performed. To measure the impact of demographics, treatment, and practice factors, the primary analysis was repeated in subgroups defined by age (age greater or less than 65 years), chemotherapy regimen (FOLFIRINOX, gemcitabine, or gemcitabine plus nab-paclitaxel), treatment facility (community v academic), and distance from treatment facility (per quartiles). Kruskal-Wallis analysis was used to assess differences between subgroups. In addition, sensitivity analyses were conducted by varying provider visit office times (using 15- and 45-minute time lengths) to account for variation in visit length, as well as excluding patients starting chemotherapy after January 15, 2018 to ensure adequate follow-up time at the time of data extraction (January 15, 2019).
RESULTS
The final cohort consisted of 362 patients diagnosed with metastatic pancreatic ductal adenocarcinoma who received palliative chemotherapy within the University of Pennsylvania Health System. Baseline characteristics are listed in Table 2. Median age was 65 years (interquartile range [IQR], 58-72 years), median ECOG performance status was 1 (IQR, 0-1), 52% were male, 78% were white, and 50% used Medicare as their insurance payer. Liver (75%) and lymph nodes (54%) were the most common sites of metastatic disease. Most patients (62%) received gemcitabine plus nab-paclitaxel. Median duration of follow-up was 202 days (IQR, 84-385 days), and 79% of patients were deceased by the end of follow-up. The median overall survival was 230.5 days (7.6 months; IQR, 110-431 days).
TABLE 2.
Baseline Characteristics (N = 362)
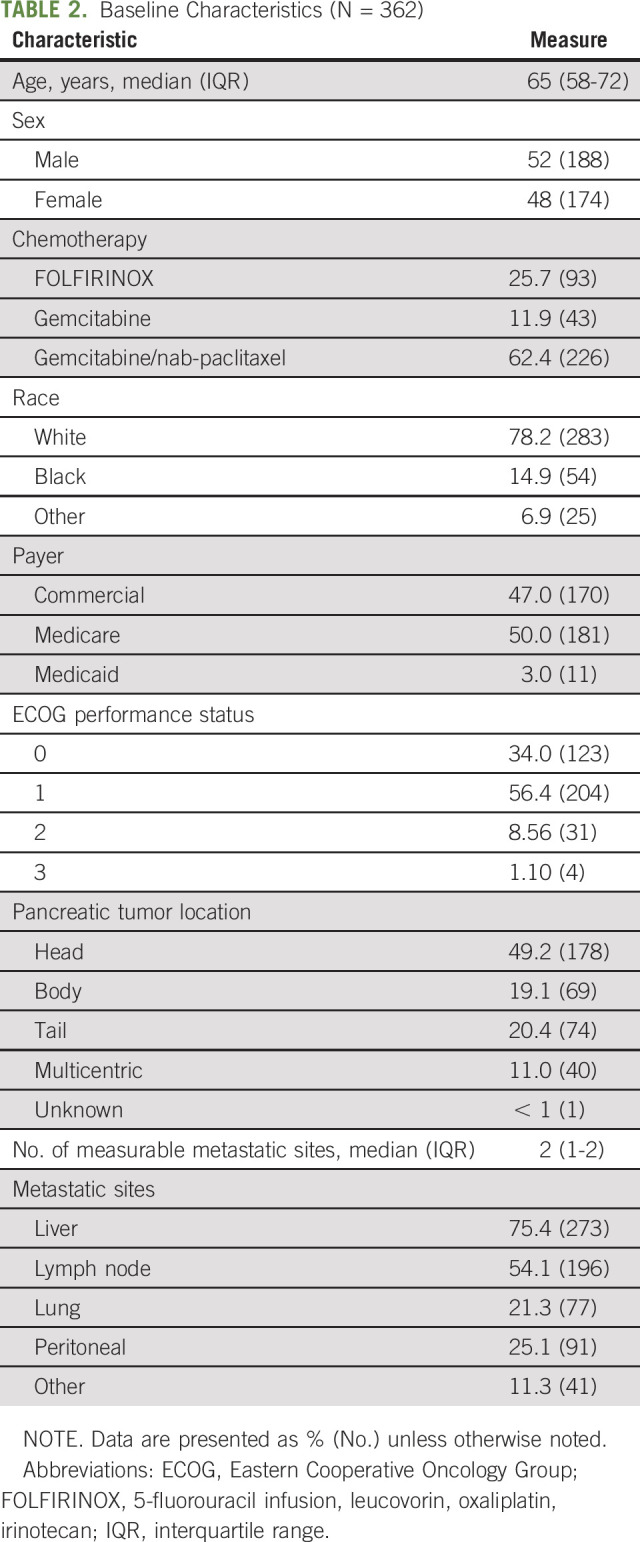
Table 3 shows summary estimates of time burden. Notably, over the course of treatment, patients had a median of 22 (IQR, 10-41) encounter days, accounting for 10% of their total days survived. When evaluating total number of discrete visits, patients had a median of 15 office visits (IQR, 7-27), 33 laboratory visits (IQR, 15-54), 17 infusion visits (IQR, 7-29), and three radiology visits (IQR, 1-7). Of the 51 patients who received radiation therapy, the median number of radiation treatment sessions was 12 (IQR, 5-25).
TABLE 3.
Survival Time Analysis
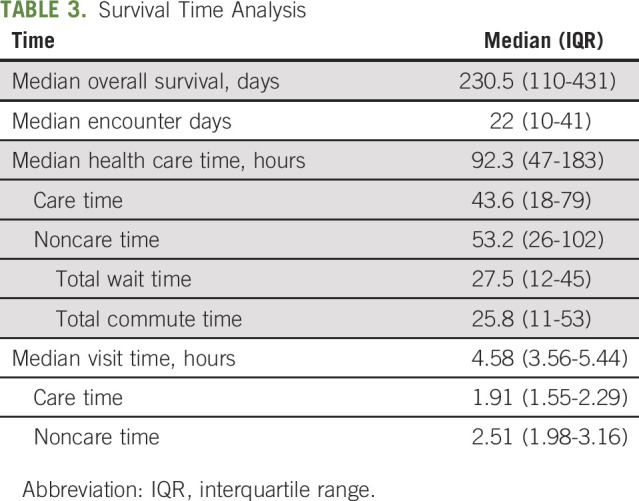
In aggregate, the encounters resulted in a median of 92.3 hours (IQR, 47-183 hours) spent engaging in outpatient health care, of which 43.6 (IQR, 18-79) hours represented “care time,” which included time spent either with a provider, receiving cancer treatment, or undergoing imaging studies. The remaining 53.2 (IQR, 26-102) hours represented “noncare time,” which included time spent commuting (25.8 hours; IQR, 11-53 hours) or waiting to receive care (27.5 hours; IQR, 12-45 hours).
Per encounter, median visit time was 4.6 hours (IQR, 3.56-5.44 hours), of which > 50% of time was accounted for by noncare time, including wait and commute time.
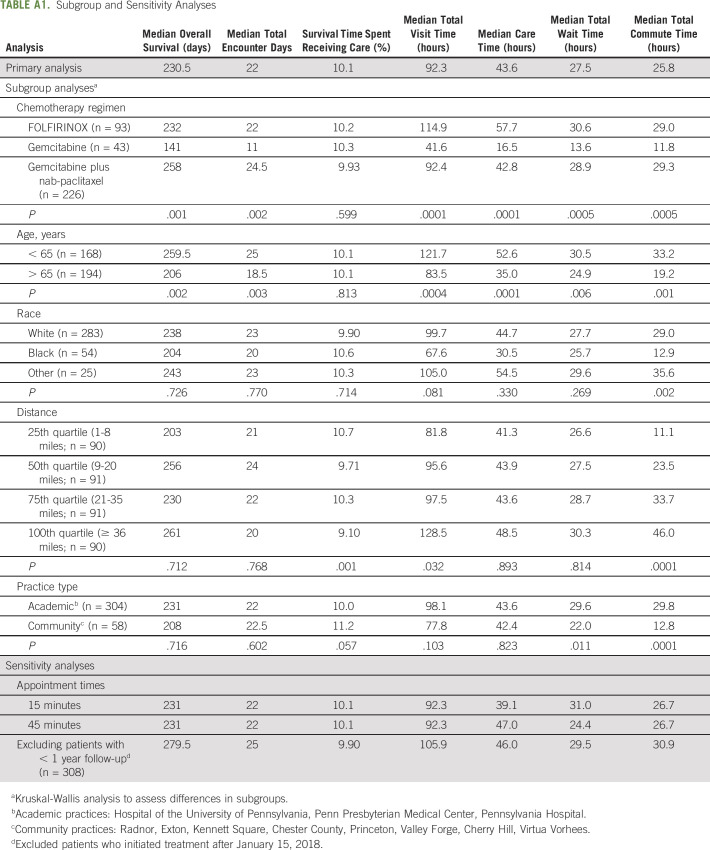
Subgroup analyses evaluating the impact of clinical, demographic, and practice factors on the time metrics measured are listed in Appendix Table A1 (online only). Importantly, the proportion of encounter days relative to survival time was similar across all subgroups (approximately 10%). Differences in total health care time were noted by treatment type, age, race, and practice type. This was in part due to differences in cumulative time spent in the infusion suite between treatment regimens (gemcitabine, 9 hours; FOLFIRINOX, 46.3 hours; gemcitabine plus nab-paclitaxel, 32.6 hours), commute time between black and white patients (12.9 v 29.0 hours), and shorter commute time (12.8 v 29.8 hours) and wait times (22.0 v 29.6 hours) noted at the community practices. In sensitivity analyses, results were similar regardless of a 15-, 30-, or 45-minute provider visit length. Median overall survival was longer (279.5 days v 230.5 days) after excluding patients who initiated treatment after January 15, 2018; all other time metrics were similar (Appendix Table A1).
DISCUSSION
Patients who are adequately prepared for potential toxicities associated with cancer treatment are more satisfied with their care10 and report improved psychological outcomes.11 Therefore, understanding the patient experience, including the total outpatient time receiving care, is essential to guide informed consent and identify deficiencies in cancer care delivery. This knowledge is particularly important for patients with metastatic pancreatic cancer receiving palliative chemotherapy, as the expected overall survival is a mere 6-11 months.2-4 We observe that 10% of a patient’s days survived involve a health care encounter, and of those days, more than half of the time is spent either commuting to or from care or waiting for care. These findings provide a critical snapshot of the patient experience during ambulatory care and highlight the need for enhanced efficiency in care delivery.
Although oncologists routinely discuss the risks and benefits of chemotherapy, including safety and efficacy of treatment, information on time costs to patients with advanced disease, such as time spent traveling to, waiting for, and receiving care, is limited.12,13 Cheng and colleagues14 have quantified the burden of routine outpatient care among patients with early-stage breast cancer, reporting an average of 44 encounter days over an 18-month period, with an average encounter length of 3.6 hours. Presley and colleagues15 have demonstrated extensive health care utilization among Medicare beneficiaries with early-stage lung cancer, showing that patients spent 1 in every 3 days interacting with the health system. Notably, these studies were limited to patients with nonmetastatic cancer, whereas it has been suggested that patients with more advanced disease have a higher burden of appointments, longer clinic sessions, and higher total commute times.14,16,17 Indeed, results from a recent survey regarding patient flow within infusion centers of National Comprehensive Cancer Network member institutions showed a high degree of variation in patient wait times based on type and stage of malignancy, treatment modality, and health system protocol.18 Collectively, the results of these studies highlight the importance of understanding the patient experience, particularly patients with advanced disease, to improve quality, efficiency, and expectations of care.
To specifically address the paucity of data on the time cost of receiving palliative cancer treatment and overcome the limitations of prior studies, we designed a study of patients with metastatic pancreatic ductal adenocarcinoma from multiple different health care settings (community and academic) and across multiple years of care (2011-2019). We used a patient-centric approach by quantifying the allocation of patient treatment time during routine outpatient care, including commute time, wait time, and time spent in testing and treatment. To capture a real-world experience, we used real-time metrics, such as patient-specific clinic check-in time and chemotherapy infusion and radiation therapy start times. Subgroup analyses identified notable differences in health care time by age, race, chemotherapy type, and treatment facility but showed nearly identical proportions of survival time on outpatient health care across these groups. Sensitivity analyses demonstrated that the results were robust to multiple assumptions.
Our work has several limitations. First, this is a retrospective, single-institution study, potentially limiting its generalizability. Nevertheless, we included a large array of practice types that are affiliated with our institution, including a large, urban academic center and multiple smaller community-based practices. Second, because of lack of reliable hospital and emergency room electronic records before 2016, we limited our assessment of time allocation to ambulatory care. Eliminating capture of emergency room visits and hospital admissions likely underestimates true patient care time. Third, because of the large volume of encounter days, we were not able to calculate daily travel times and instead extrapolated commute time on the basis of a standardized date and time. Therefore, potential variability in commute time experienced throughout the day was not captured in our study. Similarly, our commute time analysis did not account for travel by public transportation or walking or the effect of home community (rural, suburban, urban) on travel time. Finally, the retrospective design of this study meant we were limited to the available time metrics captured in the EMR. Because the EMR timestamp data could not accurately capture the true length of a provider visit or a radiation planning session, an estimated 30 minutes for provider visits and 60 minutes for radiation planning sessions was used. Therefore, our calculated care time could represent an over- or underestimate of the true patient experience. Also, this limited our ability to capture actual wait time data for these encounters and may have led to an underestimation of true patient wait times. Similarly, visits with other health care providers (primary care, palliative care, other medical or surgical specialties) were not captured in our analysis, which would likely increase the total care time for patients in our cohort.
Our work represents a critical first step in improving our understanding of the treatment burden imposed on patients with advanced cancer during routine care. Prospective cohort studies to more accurately capture the patient experience, including time spent receiving inpatient and emergency care and time spent recovering from treatment, are important next steps to quantify the total treatment burden. Likewise, qualitative studies describing patient expectations and experiences during treatment are needed to fully understand the patient perspective. Ultimately, these data could result in initiatives to streamline patient care, such as optimized patient scheduling practices, comanagement with community practices, increased availability of home care, and utilization of telemedicine.9,19,20 Our results demonstrate that the total time patients spend interacting with the health care system is substantial and signals the need to create innovative care models to reduce treatment burden and streamline care delivery.
Appendix
Fig A1.
Flowchart of the study cohort. EMR, electronic medical record; FOLFIRINOX, 5-fluorouracil infusion, leucovorin, oxaliplatin, irinotecan.
TABLE A1.
Subgroup and Sensitivity Analyses
PRIOR PRESENTATION
Presented at the 35th Annual Meeting of the International Society for Pharmacoepidemiology, Philadelphia, PA, August 24-29, 2019.
SUPPORT
Supported by National Institutes of Health Grant No. K23CA187185 (R.M.) and a Conquer Cancer Foundation Career Development award (K.A.R.).
AUTHOR CONTRIBUTIONS
Conception and design: Erin M. Bange, Peter E. Gabriel, Bethany I. Mooney, Kim A. Reiss, Ronac Mamtani
Provision of study material or patients: Kim A. Reiss
Collection and assembly of data: Erin M. Bange, Abigail Doucette, Peter E. Gabriel, Florence Porterfield, James J. Harrigan, Robin Wang, Andrez P. Wojcieszynski
Data analysis and interpretation: Erin M. Bange, Peter E. Gabriel, Robin Wang, Andrez P. Wojcieszynski, Ben Boursi, Kim A. Reiss, Ronac Mamtani
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Opportunity Costs of Receiving Palliative Chemotherapy for Metastatic Pancreatic Ductal Adenocarcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter E. Gabriel
Research Funding: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
Andrzej Wojcieszynski
Consulting or Advisory Role: Gerson Lehrman Group
Research Funding: Philips Healthcare
Bethany I. Mooney
Honoraria: Merck
Kim Reiss
Research Funding: Eli Lilly (Inst), Clovis Oncology, Bristol-Myers Squibb (Inst), Tesaro (Inst)
Ronac Mamtani
Honoraria: Flatiron Health
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics/Astellas
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Cancer Institute: Cancer Stat Facts: Pancreatic cancer. https://seer.cancer.gov/statfacts/html/pancreas.html.
- 2.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storm C, Casillas J, Grunwald H, et al. Informed consent for chemotherapy: ASCO member resources. J Oncol Pract. 2008;4:289–295. doi: 10.1200/JOP.0866002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabhan C, Feinberg BA. Value-based calculators in cancer: Current state and challenges. J Oncol Pract. 2017;13:499–506. doi: 10.1200/JOP.2017.022947. [DOI] [PubMed] [Google Scholar]
- 7.Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: Revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925–2934. doi: 10.1200/JCO.2016.68.2518. [DOI] [PubMed] [Google Scholar]
- 8.Carlson RW, Jonasch E. NCCN evidence blocks. J Natl Compr Cancer Netw. 2016;14:616–619. doi: 10.6004/jnccn.2016.0177. [DOI] [PubMed] [Google Scholar]
- 9.Santibáñez P, Chow VS, French J, et al. Reducing patient wait times and improving resource utilization at British Columbia Cancer Agency’s ambulatory care unit through simulation. Health Care Manage Sci. 2009;12:392–407. doi: 10.1007/s10729-009-9103-1. [DOI] [PubMed] [Google Scholar]
- 10. Waller A, Forshaw K, Bryant J, et al: Interventions for preparing patients for chemotherapy and radiotherapy: A systematic review. Support Care Cancer 22:2297-2308, 2014. [DOI] [PubMed]
- 11.Duckworth KE, Morrell R, Russell GB, et al. Goals and adverse effects: Rate of concordance between patients and providers. J Oncol Pract. 2019;15:e798–e806. doi: 10.1200/JOP.19.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faller H, Koch U, Brähler E, et al. Satisfaction with information and unmet information needs in men and women with cancer. J Cancer Surviv. 2016;10:62–70. doi: 10.1007/s11764-015-0451-1. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1186/s12904-018-0346-9. Wang T, Molassiotis A, Chung BPM, et al: Unmet care needs of advanced cancer patients and their informal caregivers: A systematic review. BMC Palliat Care 17:96, 2018. [DOI] [PMC free article] [PubMed]
- 14. Cheng AC, Levy MA: Data driven approach to burden of treatment measurement: A study of patients with breast cancer. AMIA Annu Symp Proc 2016:1756–1763, 2017. [PMC free article] [PubMed] [Google Scholar]
- 15.Presley CJ, Soulos PR, Tinetti M, et al. Treatment burden of Medicare beneficiaries with stage I non-small-cell lung cancer. J Oncol Pract. 2017;13:e98–e107. doi: 10.1200/JOP.2016.014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng AC, Levy MA: Measures of treatment workload for patients with breast cancer. JCO Clin Cancer Inform . [DOI] [PMC free article] [PubMed]
- 17. Cheng AC, Levy MA: Determining burden of commuting for treatment using online mapping services - A study of breast cancer patients. AMIA Annu Symp Proc 2017:555–564, 2018. [PMC free article] [PubMed] [Google Scholar]
- 18.Sugalski JM, Kubal T, Mulkerin DL, et al. National Comprehensive Cancer Network Infusion Efficiency Workgroup study: Optimizing patient flow in infusion centers. J Oncol Pract. 2019;15:e458–e466. doi: 10.1200/JOP.18.00563. [DOI] [PubMed] [Google Scholar]
- 19.Handley NR, Bekelman JE. The oncology hospital at home. J Clin Oncol. 2019;37:448–452. doi: 10.1200/JCO.18.01167. [DOI] [PubMed] [Google Scholar]
- 20.Doolittle GC, Spaulding AO. Providing access to oncology care for rural patients via telemedicine. J Oncol Pract. 2006;2:228–230. doi: 10.1200/jop.2006.2.5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]