Abstract
PURPOSE:
Low health literacy (HL) and language negatively affect cancer screening and prevention behaviors; less is known about how they affect the patient’s experience during cancer treatment. This study explores associations among HL, spoken language, and dimensions of cancer-related needs within 6 months of receiving a breast cancer diagnosis.
METHODS:
Women speaking English, Spanish, or Haitian Creole, enrolled in a patient navigation study at diagnosis, completed a survey in their primary spoken language at baseline and 6 months to characterize their cancer-related needs. HL was measured using the Brief Health Literacy Screening Tool. Outcomes included the Cancer Needs Distress Inventory (CaNDI; n = 38 items) and the Communication and Attitudinal Self-Efficacy scale (CASE-Cancer) for cancer (n = 12 items). Linear regressions measured the impact of HL and language on total CaNDI and CASE-Cancer scale for cancer scores and subscales, adjusted for demographics.
RESULTS:
At baseline, 262 women participated and 228 (87%) followed up at 6 months. Of these, 38% had adequate HL, 33% had marginal HL, and 29% had inadequate HL. Women with inadequate or marginal HL had higher median baseline CaNDI scores (P = .02) and lower self-efficacy scores (P = .008), relative to those with adequate HL. Haitian-Creole speakers had significantly lower CANDI scores at baseline (P = .03). Adjusting for demographics, differences in CaNDI scores at baseline remained significant for those with lower HL and Haitian-Creole speakers. At 6 months, differences in self-efficacy persisted for Haitian-Creole speakers.
CONCLUSION:
Findings suggest that interventions oriented to mitigating HL and language barriers might reduce distress at the time of diagnosis and improve self-efficacy over the course of treatment.
INTRODUCTION
Cancer remains a leading cause of morbidity and mortality. Advances in cancer biology, the development of targeted therapies, and the introduction of personalized medicine to clinical practice have improved physiologic outcomes and survival.1,2 However, these advances add complexity to treatment choices, tasking patients with assimilating genetic, lifestyle, and pharmacologic information to choose therapies while managing potentially debilitating disease and treatment effects in the short and long term.3 Patients with cancer may be vulnerable to poor outcomes during treatment if they lack the necessary skills to meet high informational demands and manage psychosocial stressors. Two such skills are health literacy (HL) and English proficiency, because these independently affect a patient’s ability to understand, integrate, and communicate about medical information.4
Literature suggests that patients with breast cancer have unmet informational needs over the course of treatment in the context of health care interactions5,6 and printed materials,7,8 and needs are higher among those with low HL and non-English speakers.5,9,10 One proposed mechanism for these disparities is that HL is a predictor of successful learning and of ability to cope with psychosocial challenges11-13: Those with adequate HL are better able to recognize relevant health problems and process related information. As such, evidence indicates that lower HL is associated with disengagement in health care and psychosocial distress, including anxiety, fear, depression, disempowerment, feelings of being overwhelmed, and having decision regret.14-20 Likewise, non-English speakers are less likely to understand treatment effects and the reasons for cancer tests, are less likely to discuss advantages and disadvantages of treatment with a doctor,6 have poorer symptom management, and higher anxiety and symptom-related distress.21,22 Thus, HL and spoken language are crucial elements in effectively managing one’s health care, supporting positive patient-centered outcomes. Given the high demand for these skills during cancer diagnosis and treatment, identifying patients at risk for poor outcomes and providing appropriate information and psychosocial support could have an important impact on a variety of outcomes.23,24
Although research has documented that low HL and limited English proficiency negatively affect understanding about cancer screening and prevention behaviors,25-30 less is known about how HL and spoken language independently affect patients during cancer treatment. Thus, we used data from a single-center, randomized, controlled trial to examine the associations among language, HL, cancer-related needs and self-efficacy among a sample of patients with newly diagnosed breast cancer. We hypothesized that marginal or inadequate HL would be associated with more cancer-related needs at both baseline and 6 months relative to adequate HL, but this would differ across three language groups, after adjusting for relevant demographics. We also posited that marginal or inadequate HL and non-English language would be associated with lower mean self-efficacy scores relative to adequate HL and English speakers. Building a better understanding of the relationships among these factors in the immediate aftermath of a breast cancer diagnosis and during treatment may inform the development of targeted psychosocial interventions to empower patients as partners in their health care and identify services that are responsive to patients’ needs.
METHODS
This is a secondary analysis of data collected during a randomized, comparative effectiveness trial. Women speaking English, Spanish, or Haitian Creole who had received a diagnosis of breast cancer within the prior 30 days and had yet to receive their first breast cancer treatment were enrolled in a patient navigation intervention study at a single, urban, safety-net hospital.31 Women diagnosed with cancer within the prior 5 years, cognitive impairment, or those who sought to transfer their care to another institution were excluded. Women were surveyed in their primary spoken language at enrollment and again 6 months later. Surveys included in this secondary analysis were used to characterize the impact of HL and language on cancer-related needs and self-efficacy at baseline and 6 months.
Measures
Data sources for this study included research assistant–administered surveys lasting 30 minutes and electronic medical record abstraction. All demographic variables were collected at the time of enrollment via the electronic medical record and included age, race, ethnicity, cancer stage, and type of insurance. Race and ethnicity were recorded as white (non-Hispanic), black, Hispanic, or other. Language was captured from the medical record and verified in person; all participants spoke English, Spanish, or Haitian Creole. Insurance was classified as either private or public, with public including Medicare, Medicaid, and other government-funded insurances. Employment was elicited via self-report and categorized as employed, unemployed, unable to work, and nonworking/unknown. The nonworking group included those who identified as retired, a student, a homemaker, and those whose employment status was unknown. The main independent variable of interest, HL, was measured using the Brief Health Literacy Screening Tool (BRIEF) at enrollment.32,33 This four-item HL screening tool includes three items developed by Chew et al34 and a fourth item that assesses how well people understand verbal health communication. BRIEF scores < 12 denote inadequate HL, scores from 13 to 16 suggest marginal HL and from 17 to 20 indicate adequate HL.35
Cancer-related needs, the primary outcome, were measured using an adapted version of the Cancer Needs Distress Inventory (CaNDI) instrument collected at enrollment and 6 months. The CaNDI is a 39-item, validated survey measuring anxiety, depression, and emotional, social, health care, practical, and physical needs.36 The version used in this study removed one question about suicidality that was not included in any validated subscale. The main outcome was the average total CaNDI score, which minimizes the impact of occasional missing data on individual items.36 The subscales of anxiety (two items; score range: 2 to 10), depression (four items; score range: 4 to 20), health care distress (four items; score range: 4 to 20), and practical distress (six items; score range: 6 to 30) were examined as secondary outcomes. Each item was scored from one (not a problem) to five (very severe problem), with a total possible range of 38 to 190. Scores are reported as the average across items overall and for each subscale.
To measure patient self-efficacy, the Communication and Attitudinal Self-Efficacy scale for cancer (CASE-cancer) was collected at the time of enrollment and again 6 months after diagnosis. It is a validated, 12-item, self-reported measure covering three domains37—seeking and obtaining information, understanding and participating in care, and maintaining a positive attitude—each assessed via four items rated on a four-point scale. The theoretical range of the CASE-cancer score is 12 to 48, with higher scores capturing higher self-efficacy. We report the total summed score overall and for each subscale.
Although HL and both outcome measures were validated in English and Spanish, all three measures required translation into Haitian Creole. Translation was conducted by a bilingual staff member with independent back-translation to English by a second bilingual staff member. The back-translation was compared with the original and consensus was obtained to ensure questions’ meaning were appropriately reflected in the translated version.
Data Analysis
Descriptive data were compiled and examined for differences by HL and language. The outcomes of cancer-related needs and self-efficacy and their subscales were examined by both literacy and language separately using Kruskal-Wallis tests to explore the independent associations between these constructs. before performing the multivariate analyses, we examined variables for collinearity and removed race from subsequent models, due to high collinearity with language (Fisher exact test, P < .0001). Because there were no statistically significant differences in the primary clinical outcomes by study arm in the parent study, all data are pooled.
Initial and final multivariate analyses.
Multivariate linear regression models that incorporated an interaction term between the two categorical variables (literacy × language) tested potential differential effects among language and literacy on cancer-related needs and self-efficacy, adjusting for age, type of insurance, disease stage, employment, and language. Models were created separately for baseline and 6-month outcomes. In 6-month multivariate models, baseline outcome score was included as a covariate. When we examined the primary adjusted model with the total cancer-related needs score at baseline with and without the interaction term, the interaction term model added 0.5% to the total explanatory power. Given that these models became much more complex, and the interaction added only marginally to the explanatory power, our final models do not include an interaction between HL and language.
After final models were constructed, we tested the normality assumption by generating residual plots. Data were not normally distributed, so outcomes were log-transformed and models rerun. These models resulted in findings consistent with nontransformed variables. Models are presented without log transformation to facilitate interpretation.
RESULTS
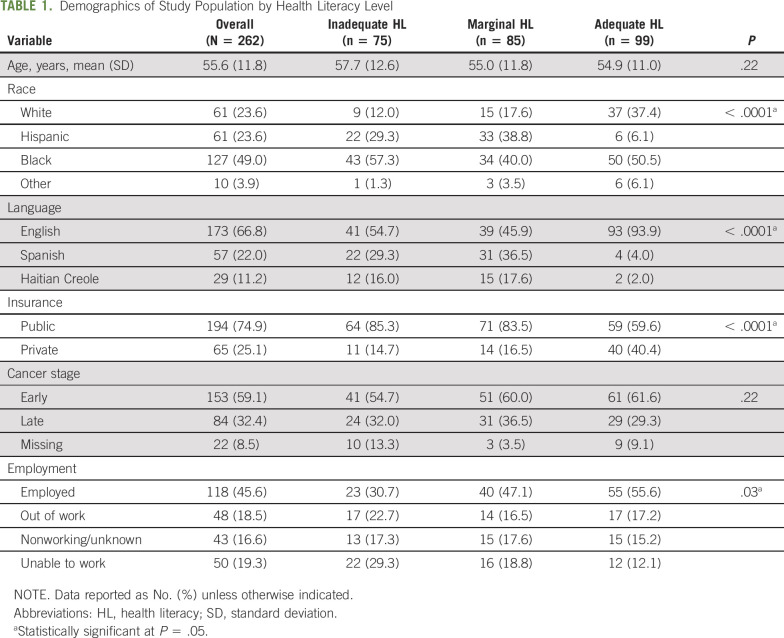
A total of 262 individuals were eligible for analysis and completed surveys at enrollment; 228 (87%) were retained at 6 months. The median number of days from enrollment to baseline survey completion was 3 (first quartile, 0 days, third quartile, 8 days). An analysis of those retained versus those not retained indicated that women with missing cancer stage data were less likely to be retained at 6 months. Demographics are described in Table 1, displayed for the entire sample and by HL level. Participants with adequate HL were more likely to be white (37% v 18% with marginal HL and 12% with inadequate HL). When examining the distribution of HL within language groups, the Haitian-Creole group (n = 29) had 41% inadequate, 52% marginal, and 7% adequate HL. Similarly, the Spanish-speaking group (n = 57), had 39% inadequate, 54% marginal, and 7% adequate. These groups varied significantly from the distribution among English-speaking participants (n = 173), among which 23% were classified each as having marginal and inadequate HL, and 54% had adequate HL (P < .001 for the between-language difference). These differences are reflected in the HL distribution in the sample overall, where 94% of the adequate HL group spoke English, relative to 46% marginal HL and 55% in the inadequate HL group. Although most (75%) of the sample had public insurance, a higher proportion of those in the adequate HL group had private insurance (40% v 17% marginal HL and 15% inadequate HL). Finally, the majority of participants with adequate HL were employed (56%), in contrast to the marginal and inadequate HL groups (47% and 32%, respectively). There were no significant differences across literacy groups by age or in the stage of diagnosis (early v late).
TABLE 1.
Demographics of Study Population by Health Literacy Level
Bivariate Associations
Figure 1 illustrates the variation in cancer-related needs across literacy and language groups. Bars represent median scores and lines represent the interquartile range. There were significant differences in the median cancer-related needs score at baseline across literacy groups, with those with adequate HL having lower total cancer-related needs scores. This trend was also seen for the depression, health care needs, and practical needs subscales, whereas anxiety was not significantly different by literacy group. By 6 months postdiagnosis, these differences dissipated such that those with adequate HL still had lower median practical needs but all other associations were no longer significant (Fig 1B). Language-related differences were also significant only at baseline (Fig 1C). Those speaking Haitian Creole had lower cancer-related needs scores relative to English and Spanish speakers. Health care needs were lowest among English speakers, whereas Spanish speakers had the highest median scores on the practical needs subscale.
Fig 1.

Differences in cancer-related needs scores (CaNDIScores) by literacy and language through 6 months after diagnosis. (A) Cancer-related needs by health literacy level at baseline. (B) Cancer-related needs by health literacy level at 6 months. (C) Cancer-related needs by language at baseline. (D) Cancer-related needs by language at 6 months. (*)Difference statistically significant at P < .05.
For the outcome of self-efficacy using the CASE-cancer scale, self-efficacy scores were significantly higher among those with adequate HL at baseline, as were the information-seeking and understanding patient care subscales relative to those with marginal or inadequate HL (Fig 2). There was no difference in the subscale for maintaining a positive attitude. Unlike cancer-related needs, where differences did not persist at 6 months, the total self-efficacy score and two subscales that were significant at baseline remained significant at 6 months across HL groups. Examining scores by language, English speakers had higher self-efficacy related to the information-seeking subscale both at baseline and 6 months relative to the non-English–speaking groups. No other significant differences were observed across language groups.
Fig 2.
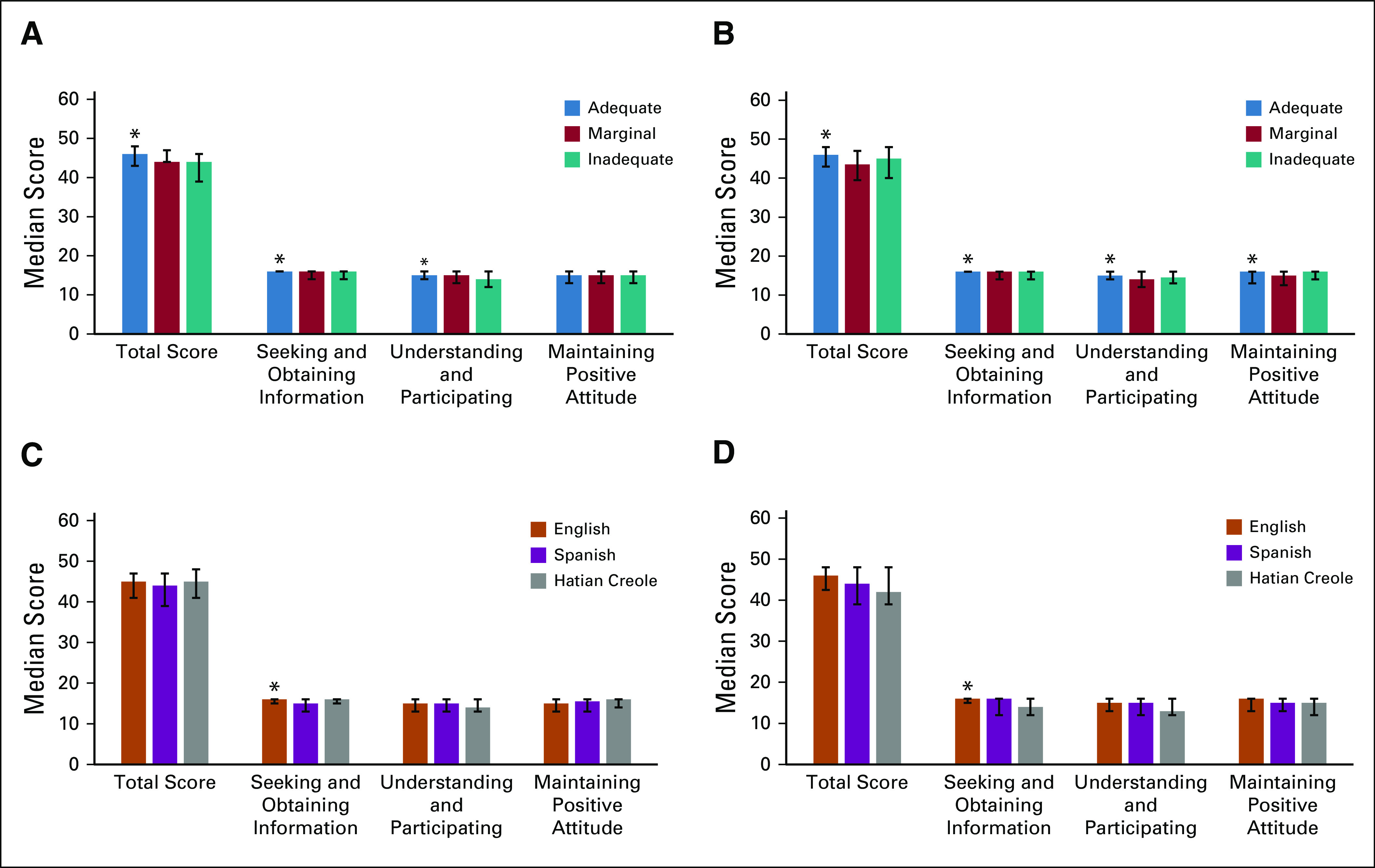
Differences self-efficacy by literacy and language through 6-months after diagnosis. (A) Self-efficacy by health literacy level at baseline. (B) Self-efficacy by health literacy level at 6 months. (C) Self-efficacy by language at baseline. (D) Self-efficacy by language at 6 months. (*)Difference statistically significant at P < .05.
Multivariate Models
Multivariable linear regressions (Table 2) demonstrated that having inadequate and marginal HL was associated with higher average cancer-related needs scores at baseline compared with those with adequate HL. Haitian-Creole language was associated with significantly lower cancer-related needs scores, as was older age. Older age, having public insurance, being nonworking, and baseline CaNDI score remained significant predictors of cancer-related needs at 6 months, after adjusting for other factors. For self-efficacy, on average, having inadequate HL was associated with lower self-efficacy at baseline, as was being unable to work. Older age and baseline self-efficacy were associated with higher self-efficacy at 6 months; speaking Haitian Creole was associated with lower self-efficacy after adjusting for other factors. The large difference in R2 values in 6-month models were driven by the baseline score adjustment.
TABLE 2.
Multivariable Linear Regression Models for Total CaNDI and Total CASE-Cancer Scores
DISCUSSION
HL and language independently contributed to differences in cancer-related needs and self-efficacy in the immediate aftermath of a breast cancer diagnosis. Lower HL was associated with higher cancer-related needs scores at diagnosis, but differences dissipated by 6 months. Those speaking Haitian Creole had lower cancer-related needs scores at baseline and lower self-efficacy at 6 months. Finally, self-efficacy scores were significantly lower among those with inadequate HL at baseline.
Consistent with other data that suggest patients with breast cancer who have low HL have greater unmet information needs relative to those with higher HL,5,10 we have shown that those with low HL report more cancer-related needs at the time of a diagnosis. Although this study could not ascertain the informational needs of women and how these might relate to cancer-related needs, we posit that meeting informational needs could be a mechanism through which cancer-related needs and self-efficacy outcomes are improved. We base this on observed differences in self-efficacy related to information seeking. Other researchers have found that there are few informational materials that address the needs of patients with breast cancer who have low HL.7,8 Although some published, video-based informational resources and decision aids do exist, they are not in widespread use.38,39 Use of simple text and illustrations boost informed decision-making and understanding among those with lower HL without compromising attitudes or outcomes among those with higher HL.40 Clear, simple information sources in multiple languages may help women navigate this time when self-efficacy is lower, especially because we observed lower self-efficacy among Haitian-Creole speakers over time, which was driven by lower scores in seeking and obtaining information. Lower self-efficacy is associated with poorer symptom management,41 which, in turn, is associated with functional health status, treatment interruptions or delays, and disease status.41,42 There are tested interventions that health care providers can use to improve women’s knowledge and self-efficacy to manage symptoms during cancer treatment.43-45 However, few of these trainings are tested in non-English speakers, a group for whom our findings suggest this capacity building could have greatest benefit, especially if cultural and linguistic differences are attended to in tandem.46 Medical oncology teams may benefit from evaluating their capacity to provide support to patients throughout treatment, with special attention to how linguistic services can best support non-English speakers.
We also observed differences in cancer-related needs by language. Those who spoke Haitian Creole had lower cancer-related needs scores at baseline relative to English speakers and lower self-efficacy at 6 months in adjusted models. The mechanisms driving these associations are unknown, and findings should be interpreted with caution because surveys were not validated in Haitian Creole. However, lower cancer-related needs scores could be influenced by unmet communication needs related to having limited English proficiency, cultural perceptions of cancer, or other social factors. Other research has described that women of Haitian decent have more fatalistic and spiritually driven beliefs in the causes and outcomes of cancer.47,48 These certainly may influence dimensions of cancer needs, emotional response to a diagnosis, and treatment choice, but the interplay of these factors is not yet clear.49,50 Alternatively, these women may have greater social support, which can mitigate distress associated with a cancer diagnosis.51 We did obtain Berkman-Syme social network scores52 for a subset of patients and observed higher reported social support among non-English participants (data not shown), suggesting a potential relationship that requires additional exploration to understand the causal links among social support, culturally based beliefs, and perception of cancer-related needs.
This exploratory analysis demonstrated differences in cancer-related needs and self-efficacy over the first 6 months after a breast cancer diagnosis. However, this analysis does not discern the clinical meaning of these measures. The cancer-related needs measures, in particular, have little data on what is considered a meaningful change or difference in scores.36 Thus, although we saw small differences on the average scale, how changes relate to patient experience is not discernable. These data represent these psychosocial outcomes alone, and they have not been linked with clinical data, including treatment adherence, completion, experienced adverse effects, or other health outcomes. The multiple contributors to clinical outcomes are important: other studies have begun to establish associations between self-efficacy and treatment choice, demonstrating disparities by literacy and language.50,53-56 Although not powered to do so in this study, better characterizing drivers of self-efficacy, and its influence on cancer-related needs and clinical outcomes is warranted. How psychosocial measures relate to patient outcomes in both the short and long term should be an area of investigation. Timing in the course of cancer care is particularly salient, because many support resources are provided to patients in the early months after diagnosis, and later distress may influence treatment adherence once such supports are no longer in place; however, the role of these factors is not yet well characterized in the literature.
As a secondary analysis, this study has limitations. Cancer-related needs scores may have been influenced by all women in this study being assigned to a patient navigator as part of the parent trial. Although all women had navigators at the time of diagnosis, they were not language concordant with patients. Language-concordant navigators have been associated with women achieving timelier diagnostic resolution among the screening population57 and may be a solution to overcome differences in needs and self-efficacy observed here. Furthermore, the HL, cancer-related needs, and self-efficacy measures were not validated in Haitian Creole, which limits conclusions that can be drawn about this group’s experiences. Validation studies of patient-reported measures in multiple languages are needed to strengthen the quality of evidence of patient experiences during cancer care and must go beyond language to characterize the unique contributions of cultural beliefs and values in addition to language.
Exploring these concepts in a more representative segment of oncology patients without such access to one-on-one support is essential. This study was conducted in a single, safety-net institution, sampling only 262 women with recently diagnosed breast cancer. The small sample size may have limited our ability to detect potentially meaningful differences in these outcomes. These findings also represent point-in-time associations, and causality cannot be established. However, findings do point to the importance of understanding associations between cancer care and experiences, with particular attention to the distinct roles of patient HL and language. Language is one facet of culture and offers a window into how people perceive health and illness.46 This particular analysis is limited by data collected in the parent trial; thus, factors that may contribute to these understandings, including acculturation, country of birth, years of schooling, and related cancer beliefs, are unmeasured. Additional research should enhance our understanding of the interconnectedness of language, culture, beliefs, and values as they relate to cancer care and the tailoring of services to match patients’ desired health practices.
In conclusion, the contributions of language and HL to patient well-being during cancer treatment is an important area for quality improvement. Health systems and cancer centers are building programs such as patient navigation and/or social work services that seek to address patient needs. Understanding how patient needs may differ according to various characteristics and over time may assist health systems in determining appropriate services and supports.49 Hiring multilingual personnel in medical oncology settings, like patient navigators, and ensuring cancer communications are available in accessible, culturally appropriate formats and multiple languages is foundational to achieving a health literate organization and may improve cancer outcomes.46,58 Characterizing needs across the cancer continuum may also help programs make decisions about effectively deploying limited personnel and material resources. Finally, attending to the experiences of patients during treatment is essential as a means of reducing disparities. In this article, we focused on HL and language as potential contributors to disparities in psychosocial outcomes. Engaging patients who speak other languages and/or have low HL and recognizing their background, cultural beliefs, and values may empower them to participate fully in their cancer care and reduce disparities in adherence and outcomes.
PRIOR PRESENTATION
Preliminary results were presented at the Health Literacy Annual Research Conference, Bethesda, MD, October 11, 2018.
SUPPORT
Supported by Patient-Centered Outcomes Research Institute Award No. AD-1304-6272 (T.A.B.) and the American Cancer Society Award No. RSG-13-368-01-CPPB (T.A.B.). C.M.G. was funded in part by the National Cancer Institute Award No. 1K07CA221899.
AUTHOR CONTRIBUTIONS
Conception and design: Christine M. Gunn, Tracy A. Battaglia
Financial support: Tracy A. Battaglia
Administrative support: Sharon Bak
Provision of study material or patients: Sharon Bak, Tracy A. Battaglia
Collection and assembly of data: Christine M. Gunn, Sharon Bak, Jennifer Pamphile, Tracy A. Battaglia
Data analysis and interpretation: Christine M. Gunn, Michael K. Paasche-Orlow, Sharon Bak, Na Wang, Kerrie Nelson, Samantha Morton, Tracy A. Battaglia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Health Literacy, Language, and Cancer-Related Needs in the First 6 Months after a Breast Cancer Diagnosis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samantha Morton
Consulting or Advisory Role: Steward Health Care System (Inst), Steward Medicaid Care Network (Inst)
Tracy A. Battaglia
Research Funding: American Cancer Society, Patient-Centered Outcomes Research Institute
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tsimberidou A-M. Initiative for molecular profiling and advanced cancer therapy and challenges in the implementation of precision medicine. Curr Probl Cancer. 2017;41:176–181. doi: 10.1016/j.currproblcancer.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Shore ND. Advances in the understanding of cancer immunotherapy. BJU Int. 2015;116:321–329. doi: 10.1111/bju.12692. [DOI] [PubMed] [Google Scholar]
- 3.Engqvist Boman L, Sandelin K, Wengström Y, et al. Patients’ learning and understanding during their breast cancer trajectory. Patient Educ Couns. 2017;100:795–804. doi: 10.1016/j.pec.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Nutbeam D. The evolving concept of health literacy. Soc Sci Med. 2008;67:2072–2078. doi: 10.1016/j.socscimed.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 5.Matsuyama RK, Kuhn LA, Molisani A, et al. Cancer patients’ information needs the first nine months after diagnosis. Patient Educ Couns. 2013;90:96–102. doi: 10.1016/j.pec.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Hyatt A, Lipson-Smith R, Schofield P, et al. Communication challenges experienced by migrants with cancer: A comparison of migrant and English-speaking Australian-born cancer patients. Health Expect. 2017;20:886–895. doi: 10.1111/hex.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch NP, Lang B, Angelov S, et al. Breast reconstruction post mastectomy- let’s Google it. Accessibility, readability and quality of online information. Breast. 2017;32:126–129. doi: 10.1016/j.breast.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Tran BNN, Singh M, Singhal D, et al. Readability, complexity, and suitability of online resources for mastectomy and lumpectomy. J Surg Res. 2017;212:214–221. doi: 10.1016/j.jss.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Halbach SM, Ernstmann N, Kowalski C, et al. Unmet information needs and limited health literacy in newly diagnosed breast cancer patients over the course of cancer treatment. Patient Educ Couns. 2016;99:1511–1518. doi: 10.1016/j.pec.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt A, Ernstmann N, Wesselmann S, et al. After initial treatment for primary breast cancer: Information needs, health literacy, and the role of health care workers. Support Care Cancer. 2016;24:563–571. doi: 10.1007/s00520-015-2814-6. [DOI] [PubMed] [Google Scholar]
- 11.Husson O, Mols F, Fransen MP, et al. Low subjective health literacy is associated with adverse health behaviors and worse health-related quality of life among colorectal cancer survivors: Results from the profiles registry. Psychooncology. 2015;24:478–486. doi: 10.1002/pon.3678. [DOI] [PubMed] [Google Scholar]
- 12.Adams RJ. Improving health outcomes with better patient understanding and education. Risk Manag Healthc Policy. 2010;3:61–72. doi: 10.2147/RMHP.S7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.2165/11313790-000000000-00000. Dixon A, Hibbard J, Tusler M: How do people with different levels of activation self-manage their chronic conditions? Patient 2:257-268, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Halbach SM, Enders A, Kowalski C, et al. Health literacy and fear of cancer progression in elderly women newly diagnosed with breast cancer--a longitudinal analysis. Patient Educ Couns. 2016;99:855–862. doi: 10.1016/j.pec.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Koay K, Schofield P, Jefford M. Importance of health literacy in oncology. Asia Pac J Clin Oncol. 2012;8:14–23. doi: 10.1111/j.1743-7563.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1007/s00520-013-1780-0. Koay K, Schofield P, Gough K, et al: Suboptimal health literacy in patients with lung cancer or head and neck cancer. Supportive Care Cancer 21:2237-2245, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Smith SK, Trevena L, Nutbeam D, et al. Information needs and preferences of low and high literacy consumers for decisions about colorectal cancer screening: Utilizing a linguistic model. Health Expect. 2008;11:123–136. doi: 10.1111/j.1369-7625.2008.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Wagner C, Semmler C, Good A, et al. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Educ Couns. 2009;75:352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Kugbey N, Meyer-Weitz A, Oppong Asante K. Access to health information, health literacy and health-related quality of life among women living with breast cancer: Depression and anxiety as mediators. Patient Educ Couns. 2019;102:1357–1363. doi: 10.1016/j.pec.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Livaudais JC, Franco R, Fei K, et al. Breast cancer treatment decision-making: Are we asking too much of patients? J Gen Intern Med. 2013;28:630–636. doi: 10.1007/s11606-012-2274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva MD, Genoff M, Zaballa A, et al. Interpreting at the end of life: A systematic review of the impact of interpreters on the delivery of palliative care services to cancer patients with limited English proficiency. J Pain Symptom Manage. 2016;51:569–580. doi: 10.1016/j.jpainsymman.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi JK, Swartz MD, Reyes-Gibby CC. English proficiency, symptoms, and quality of life in Vietnamese- and Chinese-American breast cancer survivors. J Pain Symptom Manage. 2011;42:83–92. doi: 10.1016/j.jpainsymman.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Smits R, Bryant J, Sanson-Fisher R, et al. Tailored and integrated web-based tools for improving psychosocial outcomes of cancer patients: The DoTTI development framework. J Med Internet Res. 2014;16:e76. doi: 10.2196/jmir.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. doi: 10.1007/s00520-018-4370-3. Mora-Pinzon MC, Chrischilles EA, Greenlee RT, et al: Variation in coordination of care reported by breast cancer patients according to health literacy. Supportive Care Cancer 27:857-865, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson MD, Hoffman-Goetz L. Cancer information comprehension by English-as-a-second-language immigrant women. J Health Commun. 2011;16:17–33. doi: 10.1080/10810730.2010.529496. [DOI] [PubMed] [Google Scholar]
- 26.King-Marshall EC, Mueller N, Dailey A, et al. “It is just another test they want to do”: Patient and caregiver understanding of the colonoscopy procedure. Patient Educ Couns. 2016;99:651–658. doi: 10.1016/j.pec.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Bennett IM, Chen J, Soroui JS, et al. The contribution of health literacy to disparities in self-rated health status and preventive health behaviors in older adults. Ann Fam Med. 2009;7:204–211. doi: 10.1370/afm.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores BE, Acton GJ. Older Hispanic women, health literacy, and cervical cancer screening. Clin Nurs Res. 2013;22:402–415. doi: 10.1177/1054773813489309. [DOI] [PubMed] [Google Scholar]
- 29.Sentell T, Braun KL, Davis J, et al. Colorectal cancer screening: low health literacy and limited English proficiency among Asians and Whites in California. J Health Commun. 2013;18(suppl 1):242–255. doi: 10.1080/10810730.2013.825669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldach BR, Katz ML. Health literacy and cancer screening: A systematic review. Patient Educ Couns. 2014;94:149–157. doi: 10.1016/j.pec.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko N, Bak S, Gunn C, et al. An innovation in patient navigation: Background, methods, and measures. J Oncol Navig Surviv. 2019;10:218–226. [Google Scholar]
- 32. doi: 10.1080/10810730.2012.712615. Haun J, Luther S, Dodd V, et al: Measurement variation across health literacy assessments: Implications for assessment selection in research and practice. J Health Commun 17:141-159, 2012 (suppl 3). [DOI] [PubMed] [Google Scholar]
- 33.Haun J, Noland Dodd V, Graham-Pole J, et al. Testing a health literacy screening tool: Implications for utilization of a BRIEF health literacy indicator. Fed Pract. 2009;26:24–31. [Google Scholar]
- 34.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 35.Sarkar U, Schillinger D, López A, et al. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J Gen Intern Med. 2011;26:265–271. doi: 10.1007/s11606-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowery AE, Greenberg MA, Foster SL, et al. Validation of a needs-based biopsychosocial distress instrument for cancer patients. Psychooncology. 2012;21:1099–1106. doi: 10.1002/pon.2008. [DOI] [PubMed] [Google Scholar]
- 37.Wolf MS, Chang C-H, Davis T, et al. Development and validation of the communication and attitudinal self-efficacy scale for cancer (CASE-cancer) Patient Educ Couns. 2005;57:333–341. doi: 10.1016/j.pec.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Hart TL, Blacker S, Panjwani A, et al. Development of multimedia informational tools for breast cancer patients with low levels of health literacy. Patient Educ Couns. 2015;98:370–377. doi: 10.1016/j.pec.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Jibaja-Weiss ML, Volk RJ, Granchi TS, et al. Entertainment education for breast cancer surgery decisions: A randomized trial among patients with low health literacy. Patient Educ Couns. 2011;84:41–48. doi: 10.1016/j.pec.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Meppelink CS, Smit EG, Buurman BM, et al. Should we be afraid of simple messages? The effects of text difficulty and illustrations in people with low or high health literacy. Health Commun. 2015;30:1181–1189. doi: 10.1080/10410236.2015.1037425. [DOI] [PubMed] [Google Scholar]
- 41.White LL, Cohen MZ, Berger AM, et al. Perceived self-efficacy: A concept analysis for symptom management in patients with cancer. Clin J Oncol Nurs. 2017;21:E272–E279. doi: 10.1188/17.CJON.E272-E279. [DOI] [PubMed] [Google Scholar]
- 42. doi: 10.1007/s00520-015-2698-5. Wyatt G, Sikorskii A, Tesnjak I, et al: Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Supportive Care Cancer 23:3183-3191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adam R, Bond C, Murchie P. Educational interventions for cancer pain. A systematic review of systematic reviews with nested narrative review of randomized controlled trials. Patient Educ Couns. 2015;98:269–282. doi: 10.1016/j.pec.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Gilbertson-White S. Developing an ehealth intervention for cancer symptom management (S725) J Pain Symptom Manage. 2018;55:669–670. [Google Scholar]
- 45.Goldberg J, Hinchey J, Feder S, et al. Developing and evaluating a self-management intervention for women with breast cancer. West J Nurs Res. 2016;38:1243–1263. doi: 10.1177/0193945916650675. [DOI] [PubMed] [Google Scholar]
- 46.Andrulis DP, Brach C. Integrating literacy, culture, and language to improve health care quality for diverse populations. Am J Health Behav. 2007;31(suppl 1):S122–S133. doi: 10.5555/ajhb.2007.31.supp.S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David M. Communication, cultural models of breast cancer beliefs and screening mammography: An assessment of attitudes among Haitian immigrant women in Eastern Massachusetts. Boston, MA: Boston Medical Center; 2001. [Google Scholar]
- 48.Consedine NS, Magai C, Spiller R, et al. Breast cancer knowledge and beliefs in subpopulations of African American and Caribbean women. Am J Health Behav. 2004;28:260–271. doi: 10.5993/ajhb.28.3.7. [DOI] [PubMed] [Google Scholar]
- 49.Meade CD, Menard J, Thervil C, et al. Addressing cancer disparities through community engagement: Improving breast health among Haitian women. Oncol Nurs Forum. 2009;36:716–722. doi: 10.1188/09.ONF.716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Sereika SM, Bender CM, et al. Beliefs in chemotherapy and knowledge of cancer and treatment among African American women with newly diagnosed breast cancer. Oncol Nurs Forum. 2016;43:180–189. doi: 10.1188/16.ONF.180-189. [DOI] [PubMed] [Google Scholar]
- 51.Andreu Y, Galdón MJ, Durá E, et al. A longitudinal study of psychosocial distress in breast cancer: Prevalence and risk factors. Psychol Health. 2012;27:72–87. doi: 10.1080/08870446.2010.542814. [DOI] [PubMed] [Google Scholar]
- 52.Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 53.DeWalt DA, Boone RS, Pignone MP. Literacy and its relationship with self-efficacy, trust, and participation in medical decision making. Am J Health Behav. 2007;31(suppl 1):S27–S35. doi: 10.5555/ajhb.2007.31.supp.S27. [DOI] [PubMed] [Google Scholar]
- 54. doi: 10.1007/s00520-016-3389-6. Farias AJ, Ornelas IJ, Hohl SD, et al: Exploring the role of physician communication about adjuvant endocrine therapy among breast cancer patients on active treatment: a qualitative analysis. Supportive Care Cancer 25:75-83, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keim-Malpass J, Doede A, Camacho F, et al. Impact of patient health literacy on surgical treatment of breast cancer. Breast J. 2018;24:633–636. doi: 10.1111/tbj.13011. [DOI] [PubMed] [Google Scholar]
- 56.Keim-Malpass J, Doede A, Showalter SL. Does patient health literacy impact adherence to adjuvant endocrine therapy in breast cancer patients? Patient Prefer Adherence. 2018;13:47–51. doi: 10.2147/PPA.S190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charlot M, Santana MC, Chen CA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121:1477–1483. doi: 10.1002/cncr.29221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brach C, Keller D, Hernandez L, et al: Ten attributes of health literate health care organizations. Discussion paper. NAM Perspectives Washington, DC, National Academy of Medicine, 2012. [Google Scholar]