Abstract
Background
The shelterin complex is composed of six proteins that protect and regulate telomere length, including protection of telomeres 1 (POT1). Germline POT1 mutations are associated with an autosomal dominant familial cancer syndrome presenting with diverse malignancies including glioma, angiosarcoma, colorectal cancer, and melanoma. Although somatic POT1 mutations promote telomere elongation and genome instability in chronic lymphocytic leukemia, the contribution of POT1 mutations to development of other sporadic cancers is largely unexplored.
Methods
We performed logistic regression, adjusted for tumor mutational burden, to identify associations between POT1 mutation frequency and tumor type in 62,368 tumors undergoing next-generation sequencing.
Results
A total of 1,834 tumors harbored a non-benign mutation of POT1 (2.94%), of which 128 harbored a mutation previously reported to confer familial cancer risk in the setting of germline POT1-deficiency. Angiosarcoma was 11 times more likely than other tumors to harbor a POT1 mutation (P=1.4×10−20), and 65% of POT1-mutated angiosarcoma had >1 mutations in POT1. Malignant gliomas were 1.7 times less likely to harbor a POT1 mutation (P=1.2×10−3) than other tumor types. Colorectal cancer was 1.2 times less likely to harbor a POT1 mutation (P=0.012), while melanoma showed no differences in POT1 mutation frequency versus other tumors (P=0.67).
Conclusions
These results confirm a role for shelterin dysfunction in angiosarcoma development, but suggest that gliomas arising in the context of germline POT1 deficiency activate a telomere lengthening mechanism that is uncommon in gliomagenesis.
Keywords: POT1 mutation, telomere, hereditary cancer syndrome families, TERT, ATRX
INTRODUCTION
Eukaryotic telomeres, located at the ends of linear chromosomes, function to protect against the loss of genomic content during repeated rounds of cellular replication.1 The shelterin complex is a protein complex that binds and protects human telomere ends and regulates telomere length.2,3 Protection of telomeres 1 (POT1) is one of six proteins in the shelterin complex and one of the core components that directly bind to telomeres. POT1 loss causes telomere uncapping and leads to unprotected telomere ends, aberrant telomere lengthening, and chromosomal fusions.4,5 As such, telomere dysregulation caused by POT1 deficiency is associated with tumorigenesis.6
POT1 is frequently mutated in aggressive forms of chronic lymphocytic leukemia (CLL).7–11 Because POT1 mutations arise early in CLL pathogenesis, they are believed to function as drivers in disease progression.7–11 Furthermore, germline POT1 mutations have been shown to underlie a number of hereditary familial cancer syndromes involving CLL,11 glioma,12 melanoma13–18 and colorectal cancer.19 Furthermore, a deleterious germline missense variant in POT1 (p. R117C) was identified in three Li-Fraumeni-like (LFL) families with angiosarcomas,20 and additional POT1 variants were subsequently identified in sporadic angiosarcoma patients.21
Tumor-based next-generation sequencing (NGS) panels are increasingly common in clinical practice, and disease-associated variants identified from tumor sequencing can provide clinical insights.22–24 The frequency and spectrum of POT1 mutations in solid tumors, especially those that are associated with POT1 germline deficiency, have not been rigorously evaluated. In the current study, we obtained NGS data from 62,368 tumors and reviewed POT1 mutation frequency in pan-cancer analyses.
METHODS
Next Generation Sequencing
This study involved collection of existing data and publicly available diagnostic specimens, and the information gathering process precludes direct and indirect identification of subjects, which therefore exempts it from requiring institutional review board approval under HHS regulations at 45 CFR 46.101(b). POT1 sequencing data were obtained for 62,368 tumors from Caris Life Sciences. Each tumor was reviewed by board certified pathologists and has been classified into one of 172 tumor categories based on World Health Organization (WHO) classification guidelines.
NGS was performed on genomic DNA isolated from formalin-fixed paraffin-embedded (FFPE) tumor samples using the NextSeq platform (Illumina, Inc., San Diego, CA). Tumor specimens were collected via manual microdissection and harvesting targeted tumor tissue. Matched normal tissue was not sequenced. A custom-designed SureSelect XT assay was used to enrich 592 whole-gene targets, including POT1 (Agilent Technologies, Santa Clara, CA). All variants were detected with >99% confidence based on allele frequency and amplicon coverage with an average sequencing depth across all amplicons >500x and an average depth across POT1 amplicons >750x. DNA sequencing results are validated per Clinical Laboratory Improvement Amendments (CLIA) and Internal Organization for Standardization (ISO) requirements. All analyses were completed in the same testing environment with the same sequencing and analytic pipeline, and in compliance with CLIA and ISO regulations.
Genetic variants were categorized according to the American College of Medical Genetics and Genomics (ACMG) standards (Richards et al., 2015, Tables 3–5) as: “pathogenic”, “presumed pathogenic”, “variant of unknown significance”, “unclassified”, “presumed benign”, or “benign”, according to ACMG guidelines.25 “Presumed benign” and “benign” mutations were excluded from POT1 mutation frequency analyses. The remaining categories are collectively referred to in this study as “non-benign”. A flowchart of variant classification is included in Supplementary Figure 1. A sensitivity analysis was also performed limiting to only the variants classified as “pathogenic” and “presumed pathogenic”.
Tumor Mutation Burden
Tumor mutation burden (TMB) was measured by counting all non-synonymous single-nucleotide variants found per tumor (592 genes). TMB was calculated in accordance with the Friends of Cancer Research TMB harmonization project.26 Nonsynonymous, nonsense, in-frame deletion and frameshift variants were added after filtering out presumed germline variants determined from a cosmopolitan pooled reference database including: the Genome Aggregation Database (release 2.1), the Single Nucleotide Polymorphism Database human build 137, and the Caris in-house benign database. Per tumor sample, a total of 1.4 Mb was sequenced.27 Although comparison of tumors to a reference database rather than to matched germline specimen may inflate estimates of TMB, the similar treatment of all sequenced samples in these analyses and the comparison of these samples to a common reference database suggests that modest inflations of TMB are unlikely to create substantial biases in downstream analyses across samples.
Statistical Analysis
Data were analyzed using multivariate logistic regression in SPSS v25.0 (SPSS Inc., Chicago, IL, USA). POT1 mutation status (mutated/non-mutated) was used as the outcome of interest and tumor classification was used as the predictor of interest, adjusting for TMB to prevent passenger mutations in highly-mutated tumor types (e.g., melanoma) from confounding associations. TMBs of tumors were compared among different POT1 mutation categories using the non-parametric Mann-Whitney U-test.
RESULTS
Each of 62,368 tumors was classified into one of 172 tumor categories, with 83 tumor categories including more than 50 cases (Supplementary Table 1). A total of 1,834 tumors harbored a non-benign mutation of POT1 (2.94%), with all mutations having variant allele frequencies ≥7% (mean 34.8%, median 33%). Of these, 128 tumors harbored a mutation previously reported to confer familial cancer risk in the setting of germline POT1-deficiency (Figure 1, Supplementary Table 2). Logistic regression analyses were conducted in tumor types previously associated with germline POT1 deficiency, including: 2,200 malignant gliomas, 1,735 melanomas, 8,904 colorectal cancers, and 86 angiosarcomas. The database is almost exclusively comprised of solid tumors, so hematologic malignancies (N=306), including CLL (N=10), were not assessed.
Figure 1:
Summary of reported POT1 mutations in POT1-associated hereditary familial cancer syndromes.
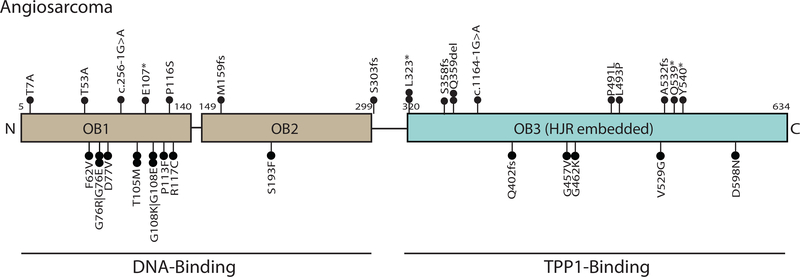
After adjustment for TMB, angiosarcoma was 11 times more likely than all other tumors combined to harbor a POT1 mutation (N=20/86, 23%; P=1.4×10−20) (Table 1). Deleterious mutations were found on both the DNA oligonucleotide/oligosarccharide and the TPP-1-binding domains of POT1 (Figure 2, Supplementary Table 3). Of these 20 POT1-mutated angiosarcoma, 12 tumors harbored two POT1 mutations and 1 harbored three POT1 mutations. No angiosaroma harbored >1 frameshift or nonsense mutation (Supplementary Table 4). However, the average variant allele frequency difference between POT1 mutations within the same tumor was 13.5%, suggesting a mutational process wherein a “second hit” in POT1 arises later during clonal evolution of the tumor. We did not observe any evidence that second hits in POT1 were more or less likely to affect the same POT1 protein domain (i.e. DNA-binding versus TPP1-binding) as the more clonal mutation.
Table 1:
Association of POT1 mutation frequency with tumor type among cancers known to be associated with germline POT1mutations.
| Tumor Type | N | Average Tumor Mutation Burden (mutations/Megabase) | Percent with POT1 mutationa | Odds Ratio (95% Confidence Interval)b | P-value |
|---|---|---|---|---|---|
| Angiosarcoma | 86 | 8.91 | 23.26% | 10.99 (6.62–18.18) | 1.40 × 10−20 |
| Malignant gliomac | 2200 | 8.03 | 1.73% | 0.58 (0.41–0.80) | 1.2×10−3 |
| Colorectal cancer | 8904 | 10.48 | 2.59% | 0.83 (0.72–0.96) | 0.012 |
| Melanoma | 1735 | 21.36 | 3.69% | 0.95 (0.73–1.23) | 0.67 |
The following are included in the calculation of percent with POT1 mutation: “mutated, pathogenic”, “mutated, presumed pathogenic”, “mutated, variant of unknown significance”, and “unclassified mutation”. 20 angiosarcomas harbored a POT1mutation, of which 12 harbored a second POT1 mutation and 1 harbored three POT1 mutations. 38 gliomas harbored a POT1 mutation, of which 3 also harbored a second POT1 mutation. 231 colorectal cancers harbored a POT1 mutation, of which 14 also harbored a second POT1 mutation. 64 melanomas harbored a POT1 mutation, of which 5 harbored a second POT1 mutation.
Association is adjusted for tumor mutation burden.
Includes glioblastoma, anaplastic astrocytoma, diffuse astrocytoma, anaplastic oligodendroglioma, and oligodendroglioma.
Figure 2:
Summary of POT1 mutations in angiosarcoma. Deleterious mutations were found on both the DNA oligonucleotide/oligosarccharide and the TPP-1-binding domains of POT1. Variants are listed with complete HGVS nomenclature in Supplementary Table 3.
Glioblastoma and other malignant gliomas were 1.7 times less likely than all other tumors combined to harbor a POT1 mutation (N=38/2200, 1.73%; P=0.0012). Of the 38 POT1-mutated gliomas, only 3 harbored a second POT1 mutation. Colorectal cancer was 1.2 times less likely to harbor a POT1 mutation than all other tumors combined (N=231/8904, 2.59%; P=0.012), with 14/231 harboring a second POT1 mutation. Melanoma showed no difference from other tumor types in POT1 mutation frequency (N=64/1735, 3.69%; P=0.67), and 5/64 POT1-mutated melanomas harbored a second POT1 mutation. Sensitivity analyses limiting to only those variants classified as “pathogenic” or “presumed pathogenic” were largely consistent with results from our primary analyses, with POT1 associations being strengthened in the angiosarcoma group, attenuated in the glioma group, and remaining approximately the same in the colorectal cancer and melanoma groups (Supplementary Table 5). All POT1 variants classified as “pathogenic” or “presumed pathogenic” across all tumor histologies are listed in Supplementary Table 6.
Among glioma histologies, glioblastoma was 1.56 times less likely than other tumors to harbor a POT1 mutation (N=30/1602, 1.87%; P=0.019) after adjustment for TMB. Neither anaplastic astrocytoma nor diffuse astrocytoma displayed significant differences in the frequency of POT1 mutation (N=6/230, 2.61%; P=0.82 and N=1/125, 0.8%; P=0.23). There were no POT1 mutations identified in oligodendrogliomas or anaplastic oligodendrogliomas (N=0/156) (Table 2).
Table 2:
Association of POT1 mutation frequency with tumor type among glioblastoma and other malignant gliomas.
| Tumor Type | N | Percent with POT1 mutationa | Odds Ratio (95% Confidence Interval)b | P-value |
|---|---|---|---|---|
| Glioblastoma, WHO grade IV | 1602 | 1.87% | 0.64 (0.44–0.93) | 0.019 |
| Anaplastic Astrocytoma | 230 | 2.61% | 0.91 (0.40–2.07) | 0.822 |
| Diffuse Astrocytoma | 125 | 0.8% | 0.30 (0.04–2.14) | 0.229 |
| Oligodendroglioma and anaplastic oligodendroglioma | 156 | 0% | N/A | N/A |
The following groups are included in the calculation of Percent with POT1 mutation: “mutated, pathogenic”, “mutated, presumed pathogenic”, “mutated, variant of unknown significance”, and “unclassified mutation”.
Association is adjusted for tumor mutation burden.
Overall median TMB for 62,368 tumors was 7 mutations/Mb. The median TMB for tumors harboring a POT1 mutation characterized as “non-benign” was 11 mutations/Mb, significantly higher than the median TMB for tumors lacking a POT1 mutation (P<0.0001) and the median for tumors harboring a POT1 mutation characterized as “benign or presumed benign” (median=8.5; P<0.0001) (Figure 3). Because TMB was higher in tumors with non-benign POT1 mutations than those with benign POT1 mutations, the observed association is unlikely to be attributable to more-highly mutated tumors simply harboring more mutations in any queried gene.
Figure 3:
Box plots of average TMB for different categories of POT1 mutation status.
In addition to affecting genomic instability, POT1 mutations have been shown to augment telomere length,22 helping cells escape replicative senescence. Although TERT promoter mutation data were unavailable, ATRX mutations that lead to the alternative lengthening of telomeres (ALT) phenotype were available for analysis. Across all 62,368 tumors, logistic regression revealed that ATRX-mutated tumors were 1.7 times less likely to have a POT1 mutation after adjusting for TMB (P=0.001). However, this association was not observed when analyses were limited to angiosarcoma (P=0.999), glioma (P=0.398), colorectal cancer (P=0.398), or melanoma (P=0.369).
DISCUSSION
Our study is the first to report the prevalence of POT1 mutations in a diverse set of solid tumors, revealing that angiosarcomas are 11 times more likely than other tumor types to harbor a POT1 mutation, often harboring multiple POT1 mutations. This indicates that shelterin complex dysfunction may be a key contributor to angiosarcoma development. Pathogenic mutations were detected in both the oligonucleotide/oligosarccharide and the TPP-1-binding domains of POT1, previously associated with abnormally long telomeres and telomere fragility,10,15,28–29 genomic instability, and tumorigenesis.4,30–32 Further studies are required to reveal which of the reported POT1 mutations are particularly damaging and which POT1-dependent molecular mechanisms are critically involved. Notably, five deleterious POT1 variants have been reported in sporadic angiosarcoma patients and angiosarcoma patients in the context of Li-Fraumeni-Like Syndrome (LFLS).20–21 These POT1 mutations were distributed across all conserved domains. Our analyses of angiosarcoma tumor genomes revealed a high frequency of POT1 mutations distributed in a similar pattern, complementing other emerging studies that have observed a high frequency of POT1 mutations in angiosarcoma.33 Because the database lacks family history data, the cases profiled in the current study may contain patients meeting criteria for LFLS.
We previously identified three germline POT1 variants in association with familial glioma.12 While all affected individuals in these families were found to have oligodendroglioma or mixed oligoastrocytoma, a subsequent molecular analysis revealed that at least one proband had a molecular astrocytoma according to revised 2016 WHO criteria (i.e. IDH-mutated, 1p/19q intact).31 Here we have identified POT1 mutations in the tumor genome of astrocytic-lineage gliomas (e.g. glioblastoma, anaplastic astrocytoma) but not oligodendroglioma. The frequency of POT1 mutations was lower in glioma, particularly high-grade malignant glioblastoma, relative to other tumor types, indicating that POT1 mutations are likely not a major contributor to gliomagenesis in non-familial cases. In our previous observations of 20 familial glioma patients, those harboring a germline mutation in the shelterin complex genes POT1 or TERF2 were the only patients not to have a somatic TERT promoter or ATRX mutation.31 As the vast majority of gliomas maintain telomere length by reactivating TERT expression through hotspot TERT promoter mutations34 or by activating ALT through loss of ATRX,35 it is possible that, in rare instances, POT1 mutations activate a non-canonical mechanism of ALT via shelterin dysfunction. As pathways of telomere maintenance are associated with clinical features, including age at diagnosis36 and glioma patient survival,35 gliomas caused by shelterin dysfunction may also harbor unique clinical presentations and therapeutic vulnerabilities.
The frequency of POT1 mutations appear lineage specific and colorectal cancer was another cancer type that was less likely to harbor a POT1 mutation. Three putatively pathogenic germline POT1 variants have been identified in colorectal cancer patients.19 One of these variants, D617Efs was also identified in familial glioma.12,19 A shared risk allele could confer pleiotropic risk of both colorectal cancer and glioma through a telomere-dependent mechanism. This has precedent, as specific polymorphisms in the telomerase component TERC have been associated with longer telomere length and increased risk of both colorectal cancer and high-grade glioma.37–38
Although CDKN2A is the primary high-penetrance melanoma predisposition gene,39–40 germline POT1 variants have been reported in melanoma-prone families by several groups.13–18 More than a dozen pathogenic germline mutations of POT1 have been identified in CDKN2A-wild-type melanoma kindreds, distributed across the DNA-binding and TPP1-binding domains. Melanoma has among the highest TMB of any cancer due to its association with UV-induced DNA damage.41 In our dataset, melanoma had a TMB of 21.36 mutations/Mb, substantially higher than the average TMB across all tumors (10.12 mutations/Mb). Associations between POT1 mutation frequency and tumor histology can be confounded by TMB due to the presence of passenger mutations that do not have biological or phenotypic consequences.42 However, our analyses adjusted for TMB to reduce the possibility of this confounder and observed no differences in POT1 mutation frequency in melanoma versus other tumor types.
A limitation to our study was that the NGS data were processed in the setting of tumor-only sequencing, which precludes the ability to definitively differentiate rare germline variants from somatically-acquired mutations.23 As a result, a portion of the mutations identified in our data may indeed be rare or previously-unreported germline variants. It is worth mentioning that many identified POT1 mutations – including in angiosarcoma – were detected in substantially less than 50% of sequencing reads, suggesting a somatic rather than a germline origin and potentially a role for POT1 in disease progression as opposed to simply tumor initiation. Although the inability to definitively distinguish germline from somatic variants does not impact our interpretation that POT1 dysfunction is a hallmark of angiosarcoma development and an uncommon driver of tumorigenesis in sporadic glioma, melanoma and colorectal cancer, it does limit our ability to directly translate findings to familial cancer syndromes or risk counseling. NGS using paired tumor-normal tissue would help to separate germline from somatic mutations and should be a focus of future research efforts, especially in angiosarcoma. Additionally, our POT1 sequencing data included only the coding region of the gene. This approach may miss functionally-relevant mutations in non-coding regions. However, with the exception of splice-site mutations, pathogenic non-coding variants are typically activating in nature (e.g. TERT promoter mutations), whereas relevant POT1 mutations appear to be loss-of-function or hypomorphic variants.
In summary, we evaluate POT1 mutation status in a pan-cancer analysis and report the prevalence of POT1 mutations across tumor types, including those previously associated with germline POT1 mutations. The high prevalence of POT1 mutations in angiosarcomas suggests that loss of POT1 function is a major driver of angiosarcoma development and that angiosarcoma sequencing studies should prioritize POT1 analyses. The observation that glioblastoma and anaplastic astrocytoma, and to a lesser extent colorectal cancers, were less likely to harbor POT1 mutations than other tumor types suggests that when these tumors arise in the context of germline POT1 deficiency they are utilizing non-canonical pathways of telomere maintenance. This is underscored by our observation that POT1-mutated tumors had higher TMB, fewer ATRX mutations, and our previous report that glioma patients with germline POT1 mutations lacked a hallmark telomere-maintenance mutation in either ATRX or the TERT promoter.31 Our results provide insight into the various roles that POT1 may play across a diverse group of tumors.
Supplementary Material
Supplementary Table 5: Association of POT1 mutation frequency with tumor type among cancers known to be associated with germline POT1 mutations, limiting analyses to only “pathogenic” and “presumed pathogenic” variants.
Supplementary Table 4: POT1 mutation data for angiosarcomas harboring >1 POT1 mutation.
Supplementary Table 6: List of POT1 mutations classified as “pathogenic” or “presumed pathogenic” in data analyses.
Supplementary Table 3: Complete HGVS nomenclature of POT1 variants appearing in Figure 2.
Supplementary Table 2: POT1 variants previously reported in hereditary familial cancer syndromes and the frequency at which they were detected among 62,368 tumors undergoing clinical sequencing.
Supplementary Table 1: List of the 172 tumor categories that contain more than 50 samples, with number of cases and number of POT1 mutations noted for each category.
Acknowledgments
FUNDING: This work was supported by a Distinguished Scientist Award from The Sontag Foundation (KMW) and by R01CA217105 from The National Cancer Institute (MLB, MNB).
Footnotes
CONFLICT OF INTEREST: JX is an employee of Caris Life Sciences, Inc. ABH holds stock in, and is a paid advisory board member for, Caris Life Sciences, Inc. No other authors have identified potential conflicts of interest relevant to the manuscript.
REFERENCES
- 1.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Ann. Rev. Genet. 2000; 34, 331–358. [DOI] [PubMed] [Google Scholar]
- 2.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell 1999; 97(4), 503–514. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Podell ER, Zaug AJ, Yang Y. Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 2007; 445(7127), 506–510. [DOI] [PubMed] [Google Scholar]
- 4.Gu P, Wang Y, Bisht KK, Wu L, Kukova L, Smith EM, Xiao Y, Bailey SM, Lei M, Nandakumar J, Chang S. Pot1 OB-fold mutations unleash telomere instability to initiate tumorigenesis. Oncogene 2017; 36, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016; 8(1), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh KM, Wiencke JK, Lachance DH, Wiemels JL, Molinaro AM, Eckel-Passow JE, Jenkins RB, Wrensch MR. Telomere maintenance and the etiology of adult glioma. Neuro. Oncol. 2015; 17(11), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S Cancer chromosomes goes to POT1. Nat. Genet. 2013; 45, 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herling CD, Klaumünzer M, Rocha CK, Altmüller J, Thiele H, Bahlo J, Kluth S, Crispatzu G, Herling M, Schiller J, Engelke A, Tausch E, Döhner H, Fischer K, Goede V, Nümberg P, Reinhardt HC, Stilgenbauer S, Hallek M, Kreuzer KA. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy. Blood 2016; 128, 396–404. [DOI] [PubMed] [Google Scholar]
- 9.Ojha J, Dyagil I, Finch SC, Reiss RF, de Smith AJ, Gonseth S, Zhou M, Hansen HM, Sherborne AL, Nakamura J, Bracci PM, Gudzenko N, Hatch M, Babkina N, Little MP, Chumak VV, Walsh KM, Bazyka D, Wiemels JL, Zablotska LB. Genomic characterization of chronic lymphocytic leukemia (CLL) in radiation-exposed Chornobyl cleanup workers. Environmental Health 2018; 17(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay AJ, Quesada V, Foronda M, Conde L, Martínez-Trillos A, Villamor N, Radríguez D, Kwarciak A, Garabaya C, Gallardo M, López-Guerra M, López-Guillermo A, Puente XS, Blasco MA, Campo E, López-Otín C. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat. Genet. 2013; 45, 526–530. [DOI] [PubMed] [Google Scholar]
- 11.Speedy HE, Kinnersley B, Chubb D, Broderick P, Law PJ, Litchfield K, layne S, Dyer MJS, Dearden C, Follows GA, Catovsky D, Houlston RS. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016; 128(19), 2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bainbridge M, Armstrong GN, Gramatges MM, Bertuch AA, Jhangianoi SN, Doddapaneni H, Lewis L, Tombrello J, Tsavachidis S, Liu Y, Jalali A, Plon SE, Lau CC, Parsons DW, Claus EB, Barnholtz-Sloan J, Il’yasova D, Schildkraut J, Ali-Osman F, Sadetzki S, Johansen C, Houlston RS, Jenkins RB, Lachance D, Olson SH, Bernstein JL, Merrell RT, Wrensch MR, Walsh KM, Davis FG, Lai R, Shete S, Aldape K, Amos CI, Thompson PA, Muzny DM, Gibbs RA, Melin RS, Bondy ML, Gliogene Consortium. Germline mutations in Shelterin complex genes are associated with familial glioma. J. Natl. Cancer Inst. 2015; 107(1), dju384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrini C, Maturo MG, Martorelli C, Suppa M, Antoni A, Kostaki D, Verna L, Landi MT, Peris K, Fargnoli MC. Characterization of melanoma susceptibility genes in high-risk patients from Central Italy. Melanoma Res. 2017; 27(3), 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potrony M, Puig-Butille JA, Ribera-Sola M, Iyer V, Robles-Espinoza CD, Aguilera P, Carrera C, Malyehy J, Badenas C, Landi MT, Adams DJ, Puig S. POT1 germline mutations but not TERT promoter mutations are implicated in melanoma susceptibility in large cohort of Spanish melanoma families. Brit. J. of Dermatol. 2019; 181, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, Pooley KA, Pritchard AL, Tiffen JC, Petljak M, Palmer JM, Symmons J, Johansson P, Stark MS, Gartside MG, Snowden H, Montgomery GW, Martin NG, Liu JZ, Choi J, Makowski M, Brown KM, Dunning AM, Keane TM, López-Otín C, Gruis NA, Hayward NK, Bishop DT, Newton-Bishop JA, Adams DJ. POT1 loss-of-function variants predispose to familial melanoma. Nat. Genet. 2014; 46(5), 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Yang XR, Ballow B, Rotunno M, Calista D, Fargnoli MC, Ghiorzo P, Bressac-de Paillerets B, Nagore E, Avril MF, Caporaso NE, McMaster ML, Cullen M, Wang Z, Zhang X, NCL DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, French Familial Melanoma Study Group, Bruno W, Pastorino L, Queirolo P, Banuls-Roca J, Garcia-Casado Z, Vaysse A, Mohamdi H, Riazalhosseini Y, Foglio M, Jouenne F, Hua X, Hyland PL, Yin J, Vallabhaneni H, Chai W, Minghetti P, Pellegrini C, Ravichandran S, Eggermont A, Lathrop M, Peris K, Scarra GB, Landi G, Savage SA, Sampson JN, He J, Yeager M, Goldin LR, Demenais F, Chanock SJ, Tucker MA, Goldstein AM, Liu Y, Landi MT. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat. Genet. 2014; 46(5), 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson TL, Hattangady N, Lerario AM, Williams C, Koeppe E, Quinonez S, Osborne J, Cha KB, Else T. A new POT1 germline mutation – expanding the spectrum of POT1-associated cancers. Fam. Cancer 2017; 16(4), 561–566. [DOI] [PubMed] [Google Scholar]
- 18.Wong K, Robles-Espinoza CD, Rodriguez D, Rudat S, Puig S, Potrony M, Wong CC, Hewinson J, Aguilera P, Puig-Butille JA, Bressac-de Pailerets B, Zattara H, vander Weyden L, Fletcher CDM, Brenn T, Arends MJ, Quesada V, Newton-Bishop JA, López-Otín C, Bishop DT, Harms PW, Johnson TM, Durham AB, Lombard DB, Adams DJ. Association of the POT1 germline missense variant p.I78T with familial melanoma. JAMA Dermatology 2019; 155(5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chubb D, Broderick P, Dobbins SE, Frampton M, Kinnersley B, Penegar S, Price A, Ma YP, Sherborne AL, Palles C, Timofeeva MN, Bishop DT, Dunlop MG, Tomlinson I, Houlston RS. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat. Commun. 2016; 7, 11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvete O, Martinez P, Garcia-Pavia P, Benitez-Buelga C, Paumard-Hernández B, Fernandez V, Dominguez F, Salas C, Romero-Laorden N, Garcia-Donas J, Carrillo J, Perona R, Triviño JC, Andrés R, Cano JM, Rivera B, Alonso-Pulpon L, Setien F, Esteller M, Rodriguez-Perales S, Bougeard G, Frebourg T, Urioste M, Blasco MA, Benítez J. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat. Commun. 2015; 6, 8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvete O, Garcia-Pavia P, Domínguez F, Bougeard G, Kunze K, Braeuninger A, Teule A, Lasa A, Ramón Y CajaI T, Llort G, Fernández V, Lázaro C, Urioste M, Benitez J. The wide spectrum of POT1 gene variants correlates with multiple cancer types. Eur. J. Hum. Genet. 2017; 25, 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond VM, Gray SW, Roychowdhury S, Joffe S, Chinnaiyan AM, Parsons DW, Plon SE, Clinical Sequencing Exploratory Research Consortium Tumor Working Group. Germline findings in tumor-only sequencing: points to consider for clinicians and laboratories. J. Natl. Cancer Inst. 2016; 108(4), djv351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trottier AM, de Andrade Silva MC, Li Z, Godley LA. Somatic mutation panels: time to clear their names. Cancer Genet. 2019; 235–236, 84–92. [DOI] [PubMed] [Google Scholar]
- 24.Shen T, Pajaro-Van de Stadt SH, Yeat NC, Lin JCH. Clinical applications of next generation sequencing in cancer from panels, to exomes, to genomes. Frontiers in Genetics 2015; 6, doi:10.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015; 17(5), 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friends of Cancer Research: Tumor mutation burden. https://www.focr.org/tmb.
- 27.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber D, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens P, Frampton GM. Analysis of 100,000 human genomes reveals the landscape of tumor mutational burden. Genome Med. 2017; 9(34): DOI 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 2001; 292(5519), 1171–1175. [DOI] [PubMed] [Google Scholar]
- 29.Hockemeyer D, Sfeir AJ, Shay JW, Wright WE, de Lange T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 2005; 24(14), 2667–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazzerini-Denchi E, Sfeir A. Stop pulling my strings – what telomeres taught us about the DNA damage response. Nature 2016; 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs DI, Fukumura K, Bainbridge MN, Armstrong GN, Tsavachidis S, Gu X, Doddapaneni HV, Hu J, Jayaseelan JC, Muzny DM, Huse JT, Bondy ML. Elucidating the molecular pathogenesis of glioma: integrated germline and somatic profiling of a familial glioma case series. Neuro. Oncol. 2018, 20(12), 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinzaru AM, Hom RA, Beal A, Phillips AF, Ni E, Cardozo T, Nair N, Choi J, Wuttke DS, Sfeir A, Denchi EL. Telomere replication stress induced by POT1 inactivation accelerates tumorigenesis. Cell Rep. 2016, 15, 2170–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Painter CA, Jain E, Tomson B, Dunphy M, Stoddard RE, Thomas BS, Damon AL, Shah S, Kim D, Zañudo JGT, Hornick JL, Chen YL, Merriam P, Raut CP, Demetri GD, Van Tine BA, Lander ES, Golub TR, Wagle N. 2019. The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. bioRxiv doi: 10.1101/741744. [DOI] [PubMed] [Google Scholar]
- 34.Mancini A, Xavier-Magalhães A, Woods WS, Nguyen KT, Amen AM, Hayes JL, Fellmann C, Gapinske M, McKinney AM, Hong C, Jones LE, Walsh KM, Bell RJA, Doudna JA, Costa BM, Song JS, Perez-Pinera P, Costello JF. Disruption of the ß1L isoform of GABP reverses glioblastoma replicative immortality in a TERT promoter mutation-dependent manner. Cancer cell 2018; 34(3), 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H, Sicotte H, Kollmeyer TM, McCoy LS, Sarkar G, Perry A, Giannini C, Tihan T, Berger MS, Wiemels JL, Bracci PM, Eckel-Passow JE, Lachance DH, Clarke J, Taylor JW, Luks T, Wiencke JK, Jenkins RB, Wrensch MR. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2016; 133(6), 1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh KM, Rice T, Decker PA, Kosel ML, Kollmeyer T, Hansen HM, Zheng S, McCoy LS, Bracci PM, Anderson E, Hsuang G, Wiemels JL, Pico AR, Smirnov I, Molinaro AM, Tihan T, Berger MS, Chang SM, Prados MD, Lachance DH, Sicotte H, Eckel-Passow JE, Wiencke JK, Jenkins RB, Wrensch MR. Genetic variants in telomerase-related genes are associated with an older age at diagnosis in glioma patients: evidence for distinct pathways of gliomagenesis. Neuro. Oncol. 2013; 15(8), 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones AM, Beggs AD, Carvajal-Carmona L, Farrington S, Tenesa A, Walker M, Howarth K, Ballereau S, Hodgson SV, Zauber A, Bertagnolli M, Midgley R, Campbell H, Kerr D, Dunlop MG, Tomlinson IP. TERC polymorphisms are associated both with susceptibility to colorectal cancer and with longer telomeres. Gut 2012; 61(2), 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh KM, Codd V, Smirnov IV, Rice T, Decker PA, Hansen HM, Kollmeyer T, Kosel ML, Molinaro AM, McCoy LS, Bracci PM, Cabridga BS, Pekmezci M, Zheng S, Wiemels JL, Pico AR, Tihan T, Berger MS, Chang SM, Prados MD, Lachance DH, O’Neill BP, Sicotte H, Eckel-Passow JE, ENGAGE Consortium Telomere Group, van der Harst P, Wienche JK, Samani NJ, Jenkins RB, Wrensch MR. Variants near TERT and TERC influencing telomere length are associated with high-grade glioma risk. Nat. Genet. 2014. 46(7), 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potrony M, Badenas C, Aguilera P, Puig-Butille JA, Carrera C, Malvehy J, Puig S. Update in genetic susceptibility in melanoma. Ann. Transl. Med. 2015; 3, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, MacKie RM, Magnusson V, Mann GJ, Bishop JN, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E, Lund Melanoma Study Group, Melanoma Genetics Consortium (GenoMEL). Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J. Med. Genet. 2007; 44, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martincorena I, Campbell PJ. Somatic mutation in cancer and normal cells. Science 2015; 349, 1483–1489. [DOI] [PubMed] [Google Scholar]
- 42.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature 2009; 458(7239), 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 5: Association of POT1 mutation frequency with tumor type among cancers known to be associated with germline POT1 mutations, limiting analyses to only “pathogenic” and “presumed pathogenic” variants.
Supplementary Table 4: POT1 mutation data for angiosarcomas harboring >1 POT1 mutation.
Supplementary Table 6: List of POT1 mutations classified as “pathogenic” or “presumed pathogenic” in data analyses.
Supplementary Table 3: Complete HGVS nomenclature of POT1 variants appearing in Figure 2.
Supplementary Table 2: POT1 variants previously reported in hereditary familial cancer syndromes and the frequency at which they were detected among 62,368 tumors undergoing clinical sequencing.
Supplementary Table 1: List of the 172 tumor categories that contain more than 50 samples, with number of cases and number of POT1 mutations noted for each category.