Abstract
There is limited research examining the impact of visual impairment (VI) on older adults while considering the complexities of aging, leaving gaps in our understanding of how health consequences of VI might be averted. We created a framework integrating concepts from disability, geriatrics, and ophthalmology that conceptualizes how VI challenges successful aging. Here, VI influences multiple functional domains, and increases the risk of negative health outcomes. This model acknowledges that common causes, such as risk factors that affect eyes and other systems simultaneously, may also drive the relationship between VI and health outcomes. Finally, the model highlights how the impact of VI on aging outcomes can be addressed at multiple intervention points.
Keywords: Functional performance, Health outcomes, Vision impairment
Visual impairment (VI) is common in older adults, with the prevalence increasing with age (Congdon et al., 2004). The number of people with moderate/severe VI worldwide is estimated to increase from 217 million in 2015 to 588 million by 2050(Bourne et al., 2017). VI has widespread functional consequences with implications for families, health care systems, and society. We hypothesize that these functional implications of VI increase the risk of negative health outcomes. Therefore, there is a need to develop and implement programs which minimize downstream effects of VI among older adults.
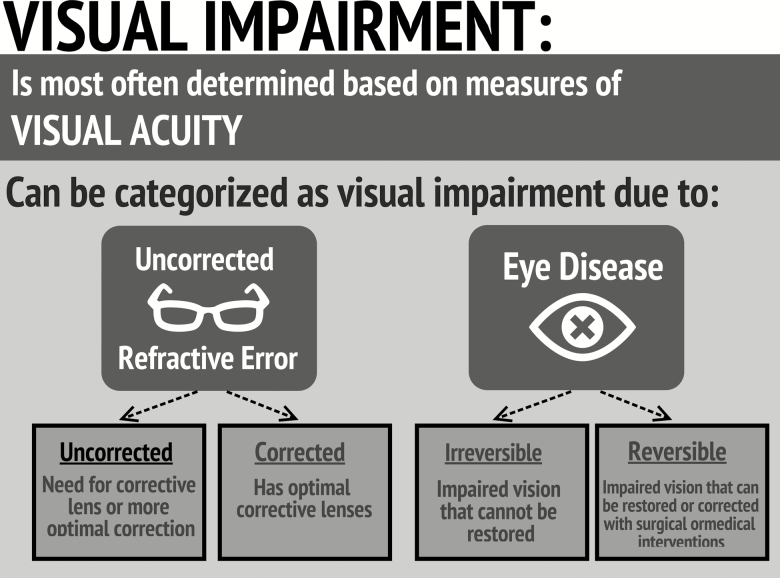
Although multiple measures can be used to assess vision, visual acuity is often the only measure used; therefore, VI is typically defined by visual acuity alone, which can be subdivided into (a) refractive error (correctable with glasses), or (b) impairment due to eye disease (often not correctable or requires surgical/medical interventions for vision improvement; Figure 1). In the United States, uncorrected refractive error accounts for approximately 79% of VI (Vitale, Cotch, & Sperduto, 2006), and cataract is the most common correctable eye disease, accounting for approximately 50% of the VI from eye disease (Congdon et al., 2004).
Figure 1.
Defining visual impairment.
The fact that two correctable conditions account for such a large portion of the societal burden of VI highlights the opportunity to improve care. One reason that this magnitude of preventable burden persists is an underappreciation—by both patients and the medical community—of the downstream consequences of VI (NASEM, 2016), likely because VI is not a direct cause of death and has been accepted as a “normal” aspect of aging. Here, we present a framework that refutes the idea that VI is a “normal” part of aging. The links in our framework are evidence based and summarizes the full scope of VI within the context of aging. It also provides a road map for understanding and improving outcomes related to VI and helps identify opportunities for future research. Further, it gives investigators who are studying geriatric outcomes more clarity about the potential role of VI.
Conceptual Framework
Gerontological research aims to understand the discrepancy between chronological and biological age. Successful aging may be more challenging for visually impaired older adults as they must manage medications, compensate for physical limitations, and cope with psychological stressors while lacking ideal vision. Thus, tailored interventions are required for visually impaired adults to age successfully.
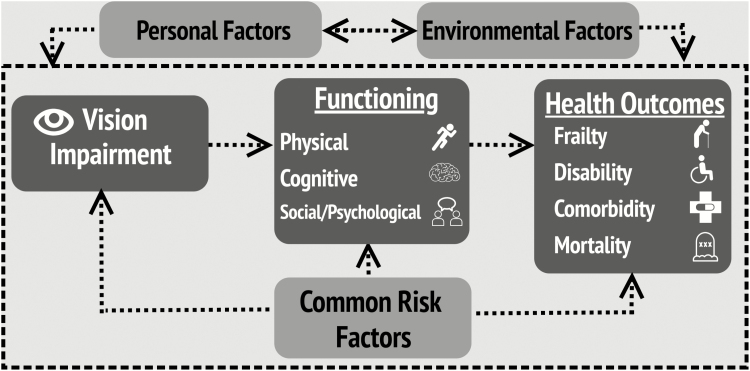
Figure 2 illustrates our model for how VI challenges successful aging. It is based on prior biopsychosocial, social, and medical models of disability (Nagi, 1976; Verbrugge & Jette, 1994; WHO, 2002), and incorporates concepts from the geriatric physical resilience model (Whitson et al., 2016) where an individual’s ability to resist decline from a stressor—in this case, VI—is affected by internal and external factors. Integrating these models allows us to capture the complexity of VI in late life.
Figure 2.
Conceptual framework: the impact of visual impairment on the health of older adults.
Unlike other disability models which begin with eye disease, this model starts with VI, as the most substantial impacts on function, health, and quality of life result in those who progress from eye disease to VI. This model is applicable to all forms of VI, and does not distinguish by VI type, although we hypothesize the relationships between the factors included here may differ by compensation for type, duration, and perception of VI.
VI may affect physical, cognitive, and psychological and social functioning, all important contributors to successful aging. This framework highlights (a) direct effects of VI on functioning—where, for example, VI affects an older adult’s ability to navigate stairs—as the impact of VI on functioning has important implications on daily life, (b) indirect or mediated effects—where VIs may cause an individual to reduce physical activity, which in turn leads to negative health outcomes, and (c) moderating effects—where an individual with VI may be less able to accommodate declines in other systems.
Health outcomes are changes to health status resulting from functional decline, including mortality, disability, comorbidity, and frailty. These outcomes help identify those who cannot live independently, consume a disproportionate share of health care resources, and are vulnerable to adverse events and escalating care needs (Fried, Ferrucci, Darer, Williamson, & Anderson, 2004).
Common risk factors that affect eye health and overall health may simultaneously drive relationships between VI, functioning, and health outcomes. For example, hypertension or smoking may serve as “common causes” that increase one’s risk for VI and functional decline. This model includes environmental factors external to the individual (i.e., features of the built/home environment), and personal factors unique to the individual (i.e., personality traits or socioeconomic status) influencing the impact of VI on health and aging. We represent a dynamic process, where the impact of VI on health may differ over time and health consequences may have a bidirectional or reverse causal relationship with VI. For example, reduced physical functioning could lead to increased risk of certain eye diseases, such as age-related macular degeneration (Knudtson, Klein, & Klein, 2006a). However, the main application of this framework will be to capture the longitudinal, downstream consequences of VI.
Later, we provide a scoping review of the literature examining the impact of VI on functioning and health in older adults, and outline gaps in our understanding of the relationship between VI and aging outcomes.
Multiple Domains of Functioning
Physical Functioning
VI is an important contributor to mobility disability—particularly walking difficulty—among older adults. Older adults with VIs report more mobility difficulty and have slower walking speeds than their nonvisually impaired counterparts (Swenor, Muñoz, & West, 2013a, 2013b). In addition, reduced contrast sensitivity and stereoacuity are independent risk factors for incident mobility disability (Deshpande, Metter, Guralnik, Bandinelli, & Ferrucci, 2014; Swenor et al., 2015). Further, research using accelerometers has demonstrated physical activity restrictions in people with VI (Van Landingham, Willis, Vitale, & Ramulu, 2012; Willis, Jefferys, Vitale, & Ramulu, 2012). These findings have been replicated for specific diseases, with lower levels of physical activity associated with glaucoma (Ramulu et al., 2012) and macular degeneration (Rovner & Casten, 2002). Given the substantial health implications of poor physical activity, persons with eye diseases and VI may be at greater risk of comorbid disease. It is important to note that the relationships between VI and physical functioning include longitudinal analyses, suggesting a temporal relationship in which VI precedes physical decline, and produces physical dysfunction beyond “normal aging.”
Cognitive Function
A consistent relationship between vision loss and cognitive function among older adults has been reported. Age-related macular degeneration, diabetic retinopathy, glaucoma, and cataract are associated with worse cognitive test scores and cognitive impairment (Baker et al., 2009; Clemons, Rankin, & McBee, 2006; Ding et al., 2010; Jefferis et al., 2013; Lindekleiv et al., 2013; Tamura et al., 2006; Wong et al., 2002). Cross-sectional associations between visual acuity and cognitive function among older adults have also been reported (Ong et al., 2013; Reyes‐Ortiz et al., 2005). Longitudinal analyses indicate that impaired vision predicts cognitive decline (Swenor et al., 2018; Zheng et al., 2018) and risk of dementia (Rogers & Langa, 2010).
Psychological and Social Function
It is well established that VI affects psychosocial functioning, substantially affecting quality of life, disability, and overall health. In older adults with VI, estimates of major depressive disorder range from 5% to 7%, whereas estimates of subthreshold depression range from 21% to 30% (van der Aa, Comijs, Penninx, van Rens, & van Nispen, 2015; Evans, Fletcher, & Wormald, 2007; Heesterbeek, van der Aa, van Rens, Twisk, & van Nispen, 2017). Older adults with low vision are two times more likely to develop depression than those with normal vision, whereas blindness is associated with a 5-fold higher likelihood of developing depression (Noran, Izzuna, Bulgiba, Mimiwati, & Ayu, 2009). Research suggests that depression in older adults with VI is attenuated by social support (Reinhardt, 1996). It has also been suggested that interventions should focus on maintaining and enhancing physical and social functioning, as activity restriction may negatively affect psychological health (Bookwala & Lawson, 2011).
A high prevalence of anxiety has also been reported among older adults with VI, ranging from 7% with a formal diagnosis of anxiety to 9.5% to 16.5% with subthreshold anxiety (van der Aa et al., 2015; Heesterbeek et al., 2017; Kempen, Ballemans, Ranchor, van Rens, & Zijlstra, 2012; Smalbrugge, Pot, Jongenelis, Beekman, & Eefsting, 2005). Anxiety has also been associated with self-reported cataracts (Eramudugolla, Wood, & Anstey, 2013) and glaucoma (Chan et al., 2015).
The social consequences of VI in older adults may significantly affect overall health. Approximately 54% of older adults with VIs report feeling lonely, and lonely individuals have more difficulty adapting to vision loss and receive less social support than those not lonely (Verstraten, Brinkmann, Stevens, & Schouten, 2005). Among nursing home residents, worse VI is associated with lower social engagement (Resnick, Fries, & Verbrugge, 1997).
Negative Health Outcomes
VI is consistently associated with declines in disability-related measures. Older adults with VI frequently report difficulties with activities of daily living and instrumental activities of daily living, as well as mobility disability (Cacciatore et al., 2004; Guralnik & LaCroix, 1992; Hochberg et al., 2012). Visually impaired older adults also have a greater number of comorbid conditions (hypertension, heart disease, stroke, arthritis, diabetes, and cancer) compared to non-impaired peers (Crews, Chou, Sekar, & Saaddine, 2017).
Multiple population-based studies have examined the relationship between VI and mortality reporting that visual acuity ≤20/40 is associated with a 20%–90% increased risk of death (AREDS, 2004; Borger et al., 2003; Knudtson, Klein, & Klein, 2006b; Wang, Mitchell, Simpson, Cumming, & Smith, 2001). Associations between specific eye diseases and mortality have also been reported. Diabetic retinopathy has been found to be a marker for poorer survival (Knudtson et al., 2006b). There is, however, conflicting evidence on glaucoma and mortality, with some studies suggesting that glaucoma does not affect life expectancy (McCarty, Nanjan, & Taylor, 2001; Taylor, McCarty, & Nanjan, 2000), but other studies indicating associations between glaucoma and age-specific mortality (Hiller et al., 1999; Rogot, Goldberg, & Goldstein, 1966). Cataract, on the other hand, has been associated with an increased risk of death (Kulmala et al., 2008; Taylor et al., 2000), but the impact of cataract surgery on survival remains unclear (Fong et al., 2014; Tseng et al., 2018; Tseng, Yu, Lum, & Coleman, 2016).
Limited research has examined the relationship between VI and frailty. Studies indicate that older visually impaired adults have lower grip strength, walk slower, and perform less physical activity—all components of the frailty phenotype (Fried et al., 2001). In addition, worse visual acuity and contrast sensitivity have been associated with worse frailty scores, and VI has been cited as a predictor of frailty (Ng, Feng, Nyunt, Larbi, & Yap, 2014).
Common Risk Factors
This conceptual model largely focuses on the downstream consequences of VI. However, there are likely common risk factors driving the relationship between VI and age-related outcomes. We hypothesize that for many older adults, both downstream consequences of VI and common causes simultaneously affect health outcomes. For example, age-related macular degeneration leads to VI among older adults and is associated with elevated inflammatory markers (Johnson, Leitner, Staples, & Anderson, 2001). Dysregulated inflammation could directly damage the macula and other organ systems, causing negative health outcomes. Further research is needed to determine to what extent these health outcomes occur as downstream consequences of VI, or as a result of common biological mechanisms.
Environmental and Personal Factors
Prior work indicates how environmental and personal factors may affect the functioning of individuals with VI (Bochsler, Legge, Gage, & Kallie, 2013; Legge et al., 2013). Features of the environment, ranging from landscape, signage, and lighting affect functioning of persons with VI (Owsley & Sloane, 1987). There is also evidence of interactions between personality traits and functioning and health of persons with VI (Crudden, O’Mally, & Antonelli, 2016; Wahl, Heyl, & Schilling, 2012). For example, a recent study found that greater self-efficacy was associated with higher levels of physical activity among individuals with VIs (Haegele, Kirk, & Zhu, 2018). However, research formally testing the effect of environmental or personal interventions for persons with VI is lacking.
Service Implications
This framework represents a dynamic process and highlights the need to better understand these relationships within the heterogeneous milieu of aging. It is also intended to help identify potential points of intervention along the pathway, including referral to low vision rehabilitation, where patients learn skills to maintain or improve their functioning, increase social interaction, and connect with mental health counseling. Of note, interventions intended to serve the broader population of older adults may need to be tailored for those with VI. For example, over the past decade, there has been a focus on increasing physical activity among older adults, but these interventions must be adapted to accommodate the mobility challenges for those with VI.
The presented conceptual framework outlines the various opportunities for interventions focused at enhancing the health of older adults with VI: (a) preventing the transition from VI to functional impairment, an important point of intervention that likely includes low vision rehabilitation services; (b) reducing the negative health outcomes for those with VI, which may include a spectrum of interventions, and requires research to determine interventions that improve the “long-term” or life course perspective on the impact of VI; (c) examining how the environment can facilitate function for persons with VI, and highlights the potential to examine how standards for building homes, hospitals, businesses, street signs, and roads may affect the functioning of older adults with VI; and (d) developing tailored approaches to care for those with VIs within the context of aging, including those who have concurrent comorbidity, frailty, and other disabilities, which may include addressing imbalances in access to care for those with VI (Spencer, Frick, Gower, Kempen, & Wolff, 2009). These efforts all must consider the specific needs of older adults with VI across ophthalmic, rehabilitation, and geriatric care settings. In addition, interventions aimed at preventing and correcting VI when applicable, such as increasing cataract surgery rates (Walker, Anstey, Hennessy, Lord, & von Sanden, 2006) and reducing the prevalence of uncorrected refractive error (Owsley, et al., 2007), may be an opportunity to enhance long-term health and aging outcomes for many older adults.
Conclusion
Advancing research at the intersection of ophthalmology and geriatrics can improve the health and quality of life of older adults with VIs. These efforts will allow for better coordination of care for those aging with VIs, as well as help identify a subset of older adults at risk of negative aging trajectories, and develop tailored and cost-effective interventions for them. Understanding the relationship between VI and these age-related health outcomes, and making progression of VI to these outcomes less likely has financial and resource implications for government, policy makers, and health care designers/providers. This broad relevance stems not only from the rising prevalence of VI, but also from the societal implications of the poor health outcomes associated with VI among older adults.
Funding
This work was supported by the National Institutes of Health (grant numbers K01AG052640 and P30AG021334 to B. K. Swenor, R01AG043438, U13AG054139, UL1TR002553, and P30AG028716 to H. E. Whitson).
Conflict of Interest
None reported.
References
- van der Aa H. P., Comijs H. C., Penninx B. W., van Rens G. H., & van Nispen R. M (2015). Major depressive and anxiety disorders in visually impaired older adults. Investigative Ophthalmology and Visual Science, 56, 849–854. doi:10.1167/iovs.14-15848 [DOI] [PubMed] [Google Scholar]
- AREDS (2004). Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Archives of Ophthalmology, 122, 716. doi:10.1001/archopht.122.5.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. L., Wang J. J., Rogers S., Klein R., Kuller L. H., Larsen E. K., & Wong T. Y (2009). Early age-related macular degeneration, cognitive function, and dementia: The Cardiovascular Health Study. Archives of Ophthalmology, 127, 667–673. doi:10.1001/archophthalmol.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochsler T. M., Legge G. E., Gage R., & Kallie C. S (2013). Recognition of ramps and steps by people with low vision. Investigative Ophthalmology and Visual Science, 54, 288–294. doi:10.1167/iovs.12-10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookwala J. & Lawson B (2011). Poor vision, functioning, and depressive symptoms: A test of the activity restriction model. Gerontologist, 51, 798–808. doi:10.1093/geront/gnr051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger P. H., van Leeuwen R., Hulsman C. A., Wolfs R. C., van der Kuip D. A., Hofman A., & de Jong P. T (2003). Is there a direct association between age-related eye diseases and mortality? The Rotterdam Study. Ophthalmology, 110, 1292–1296. doi:10.1016/S0161-6420(03)00450-0 [DOI] [PubMed] [Google Scholar]
- Bourne R. R., Flaxman S. R., Braithwaite T., Cicinelli M. V., Das A., Jonas J. B., . . . Limburg H (2017). Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Global Health, 5, e888–e897. doi:10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- Cacciatore F., Abete P., Maggi S., Luchetti G., Calabrese C., Viati L., . . . Rengo F (2004). Disability and 6-year mortality in elderly population. Role of visual impairment. Aging Clinical and Experimental Research, 16, 382–388. doi:10.1007/bf03324568 [DOI] [PubMed] [Google Scholar]
- Chan E. W., Chiang P. P., Liao J., Rees G., Wong T. Y., Lam J. S., . . . Lamoureux E (2015). Glaucoma and associated visual acuity and field loss significantly affect glaucoma-specific psychosocial functioning. Ophthalmology, 122, 494–501. doi:10.1016/j.ophtha.2014.09.030 [DOI] [PubMed] [Google Scholar]
- Clemons T., Rankin M., & McBee W (2006). Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Archives of Ophthalmology (Chicago, Ill.: 1960), 124, 537–543. doi:10.1001/archopht.124.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N., O’Colmain B., Klaver C., Klein R., Muñoz B., Friedman D. S., . . . Mitchell P (2004). Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology (Chicago, Ill.: 1960), 122, 477–485. doi:10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- Crews J. E., Chou C. F., Sekar S., & Saaddine J. B (2017). The prevalence of chronic conditions and poor health among people with and without vision impairment, aged ≥65 years, 2010-2014. American Journal of Ophthalmology, 182, 18–30. doi:10.1016/j.ajo.2017.06.038 [DOI] [PubMed] [Google Scholar]
- Crudden A., O’Mally J., & Antonelli K (2016). Transportation self-efficacy and social problem-solving of persons who are blind or visually impaired. Journal of Social Work in Disability and Rehabilitation, 15, 52–61. doi:10.1080/1536710X.2016.1124254 [DOI] [PubMed] [Google Scholar]
- Deshpande N., Metter J. E., Guralnik J., Bandinelli S., & Ferrucci L (2014). Sensorimotor and psychosocial determinants of 3-year incident mobility disability in middle-aged and older adults. Age and Ageing, 43, 64–69. doi:10.1093/ageing/aft135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Strachan M. W., Reynolds R. M., Frier B. M., Deary I. J., Gerald F. F. R., . . . Swa K (2010). Diabetic retinopathy and cognitive decline in older people with type 2 diabetes. Diabetes, 59, 2883–2889. doi:10.2337/db10-0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramudugolla R., Wood J., & Anstey K. J (2013). Co-morbidity of depression and anxiety in common age-related eye diseases: A population-based study of 662 adults. Frontiers in Aging Neuroscience, 5, 56. doi:10.3389/fnagi.2013.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., Fletcher A. E., & Wormald R. P (2007). Depression and anxiety in visually impaired older people. Ophthalmology, 114, 283–288. doi:10.1016/j.ophtha.2006.10.006 [DOI] [PubMed] [Google Scholar]
- Fong C. S., Mitchell P., Rochtchina E., de Loryn T., Tan A. G., & Wang J. J (2014). Visual impairment corrected via cataract surgery and 5-year survival in a prospective cohort. American Journal of Ophthalmology, 157, 163–170.e1. doi:10.1016/j.ajo.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Ferrucci L., Darer J., Williamson J. D., & Anderson G (2004). Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 59, 255–263. doi:10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., . . . Burke G (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56, M146–M157. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- Guralnik J &, LaCroix A. Z. (1992). Assessing physical function in older populations. In Wallace R. B. & Woolson R. F. (Eds.), The epidemiologic study of the elderly. pp. 159–181. N ew York: Oxford University Press. [Google Scholar]
- Haegele J. A., Kirk T. N., & Zhu X (2018). Self-efficacy and physical activity among adults with visual impairments. Disability and Health Journal, 11, 324–329. doi:10.1016/j.dhjo.2017.10.012 [DOI] [PubMed] [Google Scholar]
- Heesterbeek T. J., van der Aa H. P. A., van Rens G. H. M. B., Twisk J. W. R., & van Nispen R. M. A (2017). The incidence and predictors of depressive and anxiety symptoms in older adults with vision impairment: A longitudinal prospective cohort study. Ophthalmic and Physiological Optics, 37, 385–398. doi:10.1111/opo.12388 [DOI] [PubMed] [Google Scholar]
- Hiller R., Podgor M. J., Sperduto R. D., Wilson P. W., Chew E. Y., & D’Agostino R. B (1999). High intraocular pressure and survival: The Framingham Studies. American Journal of Ophthalmology, 128, 440–445. doi:10.1016/s0002-9394(99)00187-7 [DOI] [PubMed] [Google Scholar]
- Hochberg C., Maul E., Chan E. S., Van Landingham S., Ferrucci L., Friedman D. S., & Ramulu P. Y (2012). Association of vision loss in glaucoma and age-related macular degeneration with IADL disability. Investigative Ophthalmology and Visual Science, 53, 3201–3206. doi:10.1167/iovs.12-9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis J. M., Taylor J. P., Collerton J., Jagger C., Kingston A., Davies K., . . . Clarke M. P (2013). The association between diagnosed glaucoma and cataract and cognitive performance in very old people: Cross-sectional findings from the newcastle 85+ study. Ophthalmic Epidemiology, 20, 82–88. doi:10.3109/09286586.2012.757626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Leitner W. P., Staples M. K., & Anderson D. H (2001). Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Experimental Eye Research, 73, 887–896. doi:10.1006/exer.2001.1094 [DOI] [PubMed] [Google Scholar]
- Kempen G. I., Ballemans J., Ranchor A. V., van Rens G. H., & Zijlstra G. A (2012). The impact of low vision on activities of daily living, symptoms of depression, feelings of anxiety and social support in community-living older adults seeking vision rehabilitation services. Quality of Life Research, 21, 1405–1411. doi:10.1007/s11136-011-0061-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson M. D., Klein R., & Klein B. E. (2006a). Physical activity and the 15-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. British Journal of Ophthalmology, 90, 1461–1463. doi:10.1136/bjo.2006.103796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson M. D., Klein B. E., & Klein R. (2006b). Age-related eye disease, visual impairment, and survival: The Beaver Dam Eye Study. Archives of Ophthalmology, 124, 243–249. doi:10.1001/archopht.124.2.243 [DOI] [PubMed] [Google Scholar]
- Kulmala J., Era P., Törmäkangas T., Pärssinen O., Rantanen T., & Heikkinen E (2008). Visual acuity and mortality in older people and factors on the pathway. Ophthalmic Epidemiology, 15, 128–134. doi:10.1080/09286580701840388 [DOI] [PubMed] [Google Scholar]
- Legge G. E., Beckmann P. J., Tjan B. S., Havey G., Kramer K., Rolkosky D., . . . Rangarajan A (2013). Indoor navigation by people with visual impairment using a digital sign system. PLoS One, 8, e76783. doi:10.1371/journal.pone.0076783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindekleiv H., Erke M., Bertelsen G., Peto T., Arntzen K., Schirmer H., . . . Njølstad I (2013). Cognitive function, drusen, and age-related macular degeneration: A cross-sectional study. Eye, 27, 1281–1287. doi:10.1038/eye.2013.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty C. A., Nanjan M. B., & Taylor H. R (2001). Vision impairment predicts 5 year mortality. British Journal of Ophthalmology, 85, 322–326. doi:10.1136/bjo.85.3.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi S. Z. (1976). An epidemiology of disability among adults in the United States. Milbank Memorial Fund Quarterly. Health and Society, 54, 439–467. doi:10.2307/3349677 [PubMed] [Google Scholar]
- NASEM (2016). Making eye health a population health imperative: vision for tomorrow, National Academies of Sciences, Engineering, Medicine. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Ng T. P., Feng L., Nyunt M. S., Larbi A., & Yap K. B (2014). Frailty in older persons: Multisystem risk factors and the Frailty Risk Index (FRI). Journal of the American Medical Directors Association, 15, 635–642. doi:10.1016/j.jamda.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Noran N. H., Izzuna M. G., Bulgiba A. M., Mimiwati Z., & Ayu S. M (2009). Severity of visual impairment and depression among elderly Malaysians. Asia-Pacific Journal of Public Health, 21, 43–50. doi:10.1177/1010539508327353 [DOI] [PubMed] [Google Scholar]
- Ong S-Y., Ikram M. K., Haaland B. A., Cheng C-Y., Saw S-M., Wong T. Y., & Cheung C. Y (2013). Myopia and cognitive dysfunction: The Singapore Malay eye study. Investigative Ophthalmology and Visual Science, 54, 799–803. doi:10.1167/iovs.12-10460 [DOI] [PubMed] [Google Scholar]
- Owsley C., McGwin G., Scilley K., Meek G. C., Seker D., & Dyer A (2007). Effect of refractive error correction on health-related quality of life and depression in older nursing home residents. Archives of Ophthalmology, 125, 1471–1477. doi:10.1001/archopht.125.11.1471 [DOI] [PubMed] [Google Scholar]
- Owsley C. & Sloane M. E (1987). Contrast sensitivity, acuity, and the perception of “real-world” targets. British Journal of Ophthalmology, 71, 791–796. doi:10.1136/bjo.71.10.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu P. Y., Maul E., Hochberg C., Chan E. S., Ferrucci L., & Friedman D. S (2012). Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology, 119, 1159–1166. doi:10.1016/j.ophtha.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt J. P. (1996). The importance of friendship and family support in adaptation to chronic vision impairment. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 51, P268–P278. doi:10.1093/geronb/51b.5.p268 [DOI] [PubMed] [Google Scholar]
- Resnick H. E., Fries B. E., & Verbrugge L. M (1997). Windows to their world: The effect of sensory impairments on social engagement and activity time in nursing home residents. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 52, S135–S144. doi:10.1093/geronb/52b.3.s135 [DOI] [PubMed] [Google Scholar]
- Reyes-Ortiz C. A., Kuo Y. F., DiNuzzo A. R., Ray L. A., Raji M. A., & Markides K. S (2005). Near vision impairment predicts cognitive decline: Data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. Journal of the American Geriatrics Society, 53, 681–686. doi:10.1111/j.1532-5415.2005.53219.x [DOI] [PubMed] [Google Scholar]
- Rogers M. A., & Langa K. M (2010). Untreated poor vision: a contributing factor to late-life dementia. American Journal of Epidemiology, 171, 728–735. doi:10.1093/aje/kwp453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogot E., Goldberg I. D., & Goldstein H (1966). Survivorship and causes of death among the blind. Journal of Chronic Diseases, 19, 179–197. doi:10.1016/0021-9681(66)90048-8 [DOI] [PubMed] [Google Scholar]
- Rovner B. W. & Casten R. J (2002). Activity loss and depression in age-related macular degeneration. American Journal of Geriatric Psychiatry, 10, 305–310. doi:10.1097/00019442-200205000-00010 [PubMed] [Google Scholar]
- Smalbrugge M., Pot A. M., Jongenelis K., Beekman A. T., & Eefsting J. A (2005). Prevalence and correlates of anxiety among nursing home patients. Journal of Affective Disorders, 88, 145–153. doi:10.1016/j.jad.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Spencer C., Frick K., Gower E. W., Kempen J. H., & Wolff J. L (2009). Disparities in access to medical care for individuals with vision impairment. Ophthalmic Epidemiology, 16, 281–288. doi:10.1080/09286580902999439 [PubMed] [Google Scholar]
- Swenor B. K., Muñoz B., & West S. K. (2013a). Does visual impairment affect mobility over time? The Salisbury Eye Evaluation Study. Investigative Ophthalmology and Visual Science, 54, 7683–7690. doi:10.1167/iovs.13-12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor B. K., Muñoz B., & West S. K. (2013b). A longitudinal study of the association between visual impairment and mobility performance in older adults: The Salisbury Eye Evaluation Study. American Journal of Epidemiology, 179, 313–322. doi:10.1093/aje/kwt257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor B. K., Simonsick E. M., Ferrucci L., Newman A. B., Rubin S., & Wilson V (2015). Visual impairment and incident mobility limitations: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society, 63, 46–54. doi:10.1111/jgs.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenor B. K., Wang J., Varadaraj V., Rosano C., Yaffe K., Albert M., & Simonsick E. M (2018). Vision impairment and cognitive outcomes in older adults: The Health ABC Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. Advance online publication. doi:10.1093/gerona/gly244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H., Kawakami H., Kanamoto T., Kato T., Yokoyama T., Sasaki K., . . . Mishima H. K (2006). High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. Journal of the Neurological Sciences, 246, 79–83. doi:10.1016/j.jns.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Taylor H. R., McCarty C. A., & Nanjan M. B (2000). Vision impairment predicts five-year mortality. Transactions of the American Ophthalmological Society, 98, 91. [PMC free article] [PubMed] [Google Scholar]
- Tseng V. L., Chlebowski R. T., Yu F., Cauley J. A., Li W., Thomas F., . . . Coleman A. L. J. J. o (2018). Association of cataract surgery with mortality in older women: Findings from the Women’s Health Initiative. JAMA Ophthalmology, 136, 3–10. doi:10.1001/jamaophthalmol.2018.3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng V. L., Yu F., Lum F., & Coleman A. L (2016). Cataract surgery and mortality in the United States medicare population. Ophthalmology, 123, 1019–1026. doi:10.1016/j.ophtha.2015.12.033 [DOI] [PubMed] [Google Scholar]
- Van Landingham S. W., Willis J. R., Vitale S., & Ramulu P. Y (2012). Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology, 119, 2486–2492. doi:10.1016/j.ophtha.2012.06.034 [DOI] [PubMed] [Google Scholar]
- Verbrugge L. M. & Jette A. M (1994). The disablement process. Social Science and Medicine, 38, 1–14. doi:10.1016/0277-9536(94)90294-1 [DOI] [PubMed] [Google Scholar]
- Verstraten P. F. J., Brinkmann W. L. J. H., Stevens N. L., & Schouten J. S. A. G (2005). Loneliness, adaptation to vision impairment, social support and depression among visually impaired elderly. International Congress Series, 1282, 317–321. doi:10.1016/j.ics.2005.04.017 [Google Scholar]
- Vitale S., Cotch M. F., & Sperduto R. D (2006). Prevalence of visual impairment in the United States. JAMA, 295, 2158–2163. doi:10.1001/jama.295.18.2158 [DOI] [PubMed] [Google Scholar]
- Wahl H. W., Heyl V., & Schilling O (2012). Robustness of personality and affect relations under chronic conditions: The case of age-related vision and hearing impairment. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 67, 687–696. doi:10.1093/geronb/gbs002 [DOI] [PubMed] [Google Scholar]
- Walker J. G., Anstey K. J., Hennessy M. P., Lord S. R., & von Sanden C (2006). The impact of cataract surgery on visual functioning, vision-related disability and psychological distress: A randomized controlled trial. Clinical and Experimental Ophthalmology, 34, 734–742. doi:10.1111/j.1442-9071.2006.01340.x [DOI] [PubMed] [Google Scholar]
- Wang J. J., Mitchell P., Simpson J. M., Cumming R. G., & Smith W (2001). Visual impairment, age-related cataract, and mortality. Archives of Ophthalmology, 119, 1186–1190. doi:10.1001/archopht.119.8.1186 [DOI] [PubMed] [Google Scholar]
- Whitson H. E., Duan-Porter W., Schmader K. E., Morey M. C., Cohen H. J., & Colón-Emeric C. S (2016). Physical resilience in older adults: Systematic review and development of an emerging construct. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 71, 489–495. doi:10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2002). Towards a common language for functioning, disability and health: ICF. Geneva, Switzerland: World Health Organization. Retrieved from https://www.who.int/classifications/icf/icfbeginnersguide.pdf. Accessed January 29, 2019. [Google Scholar]
- Willis J. R., Jefferys J. L., Vitale S., & Ramulu P. Y (2012). Visual impairment, uncorrected refractive error, and accelerometer-defined physical activity in the United States. Archives of Ophthalmology, 130, 329–335. doi:10.1001/archopthalmol.2011.1773 [DOI] [PubMed] [Google Scholar]
- Wong T. Y., Klein R., Nieto F. J., Moraes S. A., Mosley T. H., Couper D. J., . . . Sharrett A. R (2002). Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. American Journal of Ophthalmology, 134, 828–835. doi:10.1016/s0002-9394(02)01672-0 [DOI] [PubMed] [Google Scholar]
- Zheng D. D., Swenor B. K., Christ S. L., West S. K., Lam B. L., & Lee D. J (2018). Longitudinal associations between visual impairment and cognitive functioning: The Salisbury Eye Evaluation study. JAMA Ophthalmology, 136, 989–995. doi:10.1001/jamaophthalmol.2018.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]