Abstract
Background and Objectives
Stroke is a chronic, complex condition that disproportionally affects older adults. Health systems are evaluating innovative transitional care (TC) models to improve outcomes in these patients. The Comprehensive Post-Acute Stroke Services (COMPASS) Study, a large cluster-randomized pragmatic trial, tested a TC model for patients with stroke or transient ischemic attack discharged home from the hospital. The implementation of COMPASS-TC in complex real-world settings was evaluated to identify successes and challenges with integration into the clinical workflow.
Research Design and Methods
We conducted a concurrent process evaluation of COMPASS-TC implementation during the first year of the trial. Qualitative data were collected from 4 sources across 19 intervention hospitals. We analyzed transcripts from 43 conference calls with hospital clinicians, individual and group interviews with leaders and clinicians from 9 hospitals, and 2 interviews with the COMPASS-TC Director of Implementation using iterative thematic analysis. Themes were compared to the domains of the RE-AIM framework.
Results
Organizational, individual, and community factors related to Reach, Adoption, and Implementation were identified. Organizational readiness was an additional key factor to successful implementation, in that hospitals that were not “organizationally ready” had more difficulty addressing implementation challenges.
Discussion and Implications
Multifaceted TC models are challenging to implement. Facilitators of implementation were organizational commitment and capacity, prioritizing implementation of innovative delivery models to provide comprehensive care, being able to address challenges quickly, implementing systems for tracking patients throughout the intervention, providing clinicians with autonomy and support to address challenges, and adequately resourcing the intervention.
Clinical Trial Registration
Keywords: Continuum of care, Care coordination, Person-centered care, Qualitative research methods, Quality of care
An estimated 800,000 people suffer a stroke in the United States annually (Benjamin et al., 2019). Stroke is a chronic and complex condition that disproportionally affects older adults with the risk for stroke doubling every 10 years after age 55. Approximately 75% of all strokes occur in adults aged 65 and older (Yousufuddin & Young, 2019).
Over the past decade, health systems have become better prepared for managing complex stroke patients presenting to the emergency department (Guzik & Bushnell, 2017; Higashida et al., 2013; Li et al., 2020; Miller et al., 2019). Clear and functional links between emergency medical services (EMS) and acute care, especially in stroke-certified hospitals, have improved acute stroke interventions and reduced, but not eliminated, poststroke disability (Lahr, van der Zee, Luijckx, & Buskens, 2020). However, stroke is the leading cause of major, long-term disability, requiring ongoing medical care and rehabilitation after discharge from acute care to optimize functional independence and community reintegration (Benjamin et al., 2019). Clear and functional links to poststroke follow-up care and community-based services are needed but often absent as patients transition home and continue recovery (Bettger et al., 2019; Lutz, Young, Cox, Martz, & Creasy, 2011). The missing links between hospital and community-based service providers illustrate the fragmented approach to health care. Hospital-based clinicians often do not understand the postdischarge experiences of patients. When patients are discharged back to the community, they are often left to their own means to identify what resources and services they may need and what is available in their area to further their recovery (Lutz et al., 2017). They often do not know the warning signs of stroke, cannot manage their medications, and do not monitor their blood pressure (Nicol & Thrift, 2005). Patients may not seek stroke-specific follow-up care because they have not been told to do so, do not have the cognitive ability (Fens et al., 2013), or because they cannot afford additional therapies.
To be truly comprehensive, stroke care should cover the continuum from prehospital recognition of symptoms to postdischarge follow-up and linkages to relevant community-based resources and primary care (Winstein et al., 2016). Additionally, efforts toward value-based care and bundled payments in the United States require health systems to evaluate innovative delivery models to provide comprehensive care and improve outcomes across the continuum for patients with complex medical needs (Bettger et al., 2015).
The Comprehensive Post-Acute Stroke Service–Transitional Care (COMPASS-TC) is an innovative delivery model designed to bridge this gap from hospital to return-to-community. The COMPASS-TC model was created and implemented in a pragmatic clinical trial to address these gaps in postacute transitional care (TC) for patients with mild-to-moderate stroke and transient ischemic attack (TIA) discharged directly home from the hospital (Duncan et al., 2017). Community stakeholders, including patients, caregivers, hospital administrators, and clinicians, and other community health and human service providers developed COMPASS-TC and were engaged throughout implementation (Gesell et al., 2017).
Implementing comprehensive interventions like COMPASS-TC that cross the care continuum from hospital to home can be difficult to integrate into existing complex hospital system structures and processes, especially without allocating sufficient resources (Gesell et al., 2019). This study provides valuable information about the challenges and solutions from real-world hospital settings to help facilitate implementation of this type of TC.
Overview of COMPASS-TC
COMPASS-TC was implemented in two phases in 40 North Carolina (NC) hospital units as part of the first large pragmatic clinical trial funded by Patient-Centered Outcomes Research Institute (PCORI; Duncan et al., 2017). In Phase 1, hospitals were randomized to either the 12-month intervention group or usual care group. In Phase 2, intervention hospitals were encouraged to sustain COMPASS-TC and usual care hospitals implemented the 12-month intervention with support from the COMPASS-TC Implementation Team.
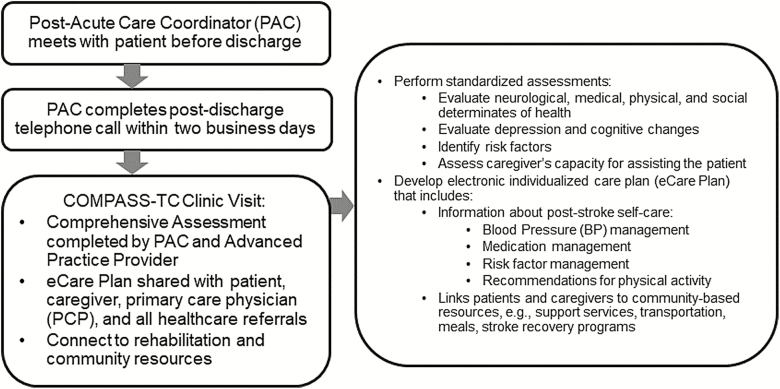
COMPASS-TC includes evidence-based elements of TC and early supported discharge services (Bushnell et al., 2018), with the primary goal of improving functional status 90 days postdischarge. COMPASS-TC combines services provided by registered nurse postacute care coordinators (PACs) and advanced practice providers (APPs), including nurse practitioners, physician assistants, and physicians. The COMPASS-TC model with associated clinician roles is included in Figure 1.
Figure 1.
Overview of the COMPASS-TC model.
COMPASS-TC assessments are aligned with Centers for Medicare and Medicaid Services (CMS) recommendations for postacute care coordination and billing requirements for TC management codes. Details on the intervention and trial methodology have been published (Andrews et al., 2018; Duncan et al., 2017, 2018; Johnson et al., 2018).
Implementing COMPASS-TC as a new standard of care required substantial changes to existing processes and structures of care within hospitals and at follow-up clinics. The pragmatic design of the COMPASS-TC Study enabled the identification of challenges in translation of COMPASS-TC into practice. In order to better understand implementation variability across intervention hospitals, we conducted a comprehensive implementation analysis of quantitative and qualitative data (Gesell et al., 2019; Glasgow, Vogt, & Boles, 1999).
In this study, findings from the analysis of qualitative data collected during Phase 1 helped to explain the variability of implementation across intervention sites (Glasgow et al., 2019; Holtrop, Rabin, & Glasgow, 2018). We discuss the challenges and facilitators that affected implementation from the perspectives of COMPASS-TC clinicians, hospital administrators, and implementation staff (Glasgow et al., 1999; Holtrop et al., 2018). We describe solutions developed by participating hospitals to address these challenges.
Methods
Study Design and Sites
Study Design
To complement our quantitative assessment of COMPASS-TC implementation (Gesell et al., 2019), we conducted a concurrent process analysis of qualitative data collected during COMPASS-TC implementation focusing on facilitators and challenges at intervention hospitals.
Sites
This article includes data from 19 of 20 COMPASS-TC intervention hospitals (2,689 patients) in Phase 1 of the study. One site was excluded from analysis because it did not enroll any patients. Fifty-eight percent of intervention hospitals had uninterrupted delivery of COMPASS-TC over 12 months, and 35% of patients enrolled at intervention hospitals completed the COMPASS-TC protocol, including a clinic visit within 30 days of discharge; 78% of these clinic visits were within 14 days. For COMPASS-TC Study Phase 1 primary results, see Duncan and colleagues (in press).
Implementation and Training Strategy
Implementation occurred in three waves (n = 5, n = 5, n = 9) starting in August 2016. Patient enrollment lasted at least 1 year, with sites completing enrollment between fall 2017 and spring 2018. Within each wave, hospital staff participated in a 2-day immersion training “boot camp” to learn the COMPASS-TC protocol, become familiar with the technology, and review standardized assessment tools and metrics. Sites also received individual in-person site visits by the Implementation Team. To link patients to community resources (e.g., mental health, social services, nutrition, and medication support), hospital staff collaborated with the study Implementation Team to identify and engage community resource providers within their service areas. Many of the training tools are available on the COMPASS-TC website: www.nccompass-study.org/.
Throughout the first year of the study, sites participated in regularly scheduled problem-solving conference calls, monthly site-specific reviews of performance data, and individualized coaching from the Implementation Team. All sites had unlimited access to online training materials and support from the Implementation Team. Sites received limited financial assistance to support patient enrollment and data entry.
Consent
Institutional Review Board (IRB) approval was granted through Wake Forest University Health Sciences (central IRB). For focus groups and interviews, participants signed informed consent. The study met the criteria for waiver of consent for the group conference calls and PAC surveys.
Data Collection
To evaluate intervention hospitals’ challenges in implementing COMPASS-TC using existing hospital and community resources, we included qualitative data from four sources during the first year of implementation. All conference calls and interviews were audio-recorded and transcribed verbatim (see Table 1 for types of data collected at each site).
Table 1.
COMPASS-TC Hospital Participation in Surveys, Group Calls, and Interviews: Ranked Highest to Lowest Based on Per Protocol Completion of the Interventiona
| Hospital performance rankeda | No. of surveys completed | No. of group calls attended | Group or individual interview | Types of data collected | |
|---|---|---|---|---|---|
| Detailed field notes | Transcribed audio recordings | ||||
| Sites performing above the mean | |||||
| 1 | 13 | 14 | X | X | X |
| 2 | 12 | 11 | X | X | X |
| 3 | 11 | 12 | X | X | X |
| 4 | 3 | 0 | |||
| 5 | 7 | 10 | X | X | X |
| 6 | 0 | 0 | |||
| Sites performing at the mean | |||||
| 7 | 4 | 5 | |||
| 8 | 7 | 11 | |||
| Sites performing below the mean | |||||
| 9 | 6 | 6 | |||
| 10 | 11 | 10 | |||
| 11ab | 11 | 5 | X | X | |
| 11bb | 8 | 10 | X | X | |
| 12 | 5 | 4 | |||
| 13 | 12 | 14 | X | X | X |
| 14 | 9 | 1 | |||
| 15 | 5 | 0 | |||
| 16 | 10 | 13 | X | X | X |
| 17 | 10 | 8 | |||
| 18 | 8 | 5 | X | X | |
| 19 | 5 | 2 |
aPerformance was ranked based on per protocol completion of the intervention. Per protocol completion is defined as % of enrolled patients who attended the COMPASS-TC clinic visit within 30 calendar days of hospital discharge (Duncan et al., in press).
bThese sites were part of the same system and were combined in the primary outcome analyses.
Group Conference Calls and Webinars
As part of program implementation, the Director of Implementation (DOI) conducted 43 regularly scheduled, structured problem-solving calls and webinars with PACs and APPs. Calls initially took place every 2 weeks, then were reduced to monthly 4–6 months after start-up. During these calls, PACs and APPs discussed implementation challenges and strategies developed to improve their performance. Participants were informed that the call transcripts would be evaluated to better understand program challenges and solutions.
To capture perceived challenges in implementation and management of these challenges, the DOI administered a survey to each site prior to the group calls (Supplementary Figure 1). Data from the surveys were used by the DOI to develop the agenda for the group conference calls. For example, if sites were experiencing challenges with the 2-day call, then that became a focus of the next group call.
Interviews With the DOI
We (B. J. Lutz, A. E. Reimold, and S. B. Gesell) conducted two semistructured interviews with the DOI, who was the primary point of contact for all sites and who spoke with each site at least monthly. As an experienced public health nurse and recognized in NC as an expert in stroke care quality and systems of care improvement, the DOI had comprehensive first-hand knowledge of the challenges and successes each site encountered with adopting COMPASS-TC for local context. Extensive field notes were also written.
Interviews With Hospitals
We also conducted semistructured group and individual interviews at 9 of the 19 intervention hospitals; 4 that were performing above the mean and 5 performing below the mean percentage of patients receiving the intervention within 30 calendar days of hospital discharge (Table 1). The interviews included 12 frontline clinicians and seven administrators (Table 1). During interviews, we reinforced that the components of COMPASS-TC and the feasibility of implementation, not the hospitals’ performance, were under evaluation. Interview questions were related to successes and challenges with implementing COMPASS-TC (Table 2) and were based on the preliminary themes identified in the group conference calls.
Table 2.
Sample Interview Questions With Participating Hospitals
| Number | Question |
|---|---|
| 1. | Please tell us about your experiences with the COMPASS-TC intervention. What worked (and should be kept)? |
| 2. | What did not work? |
| 2a. | What were the challenges? |
| 2b. | What strategies did you try to address the challenges? |
| 2c. | What competing priorities (if any) did you experience while implementing COMPASS-TC? |
| 3. | What types of challenges did you have with staffing that may have affected your ability to implement COMPASS-TC? |
| 4. | When were patients typically seen for the 7–14 day visit? |
| 5. | Were there any challenges with who got to bill for what visit? |
| 6. | What competing priorities did you experience that made implementing COMPASS-TC more difficult? |
| 7. | Why did the site decide to stop enrollment? [for sites that stopped enrolling patients] |
| 8. | What suggestions do you have on how to improve COMPASS-TC? |
Data Analysis
We conducted an iterative thematic analysis of the transcribed calls and interviews in three stages (Ryan & Bernard, 2003). First, three study team members (B. J. Lutz, A. E. Reimold, and A. K. Guzik) read and hand-coded group conference call transcripts in temporal order. Dr. Lutz has a PhD in nursing and more than 20 years of qualitative research expertise. She trained Ms. Reimold, a graduate student in psychology, and Dr. Guzik, a neurologist. Major themes were identified using open coding procedures (Charmaz, 2014; Ryan & Bernard, 2003). After general themes were established, A. E. Reimold imported transcripts into NVivo 11.0 (QSR) for data management and analysis. The study team met to discuss themes identified in the data (e.g., organizational readiness, start-up issues, patient recruitment/retention issues) and developed a provisional coding schema used for focused coding of all transcripts.
In the second stage, two study team members (B. J. Lutz and A. E. Reimold) independently recoded the transcripts using the coding schema. Coders met regularly to examine similarities and differences among sites and across time, resolve discrepancies, and revise the coding schema based on the ongoing analysis. Next, B. J. Lutz, A. E. Reimold, A. K. Guzik, and S. B. Gesell analyzed the field notes and transcripts from the DOI and hospital interviews. Themes were compared with the emerging coding schema and revised based on the ongoing analysis.
In the third stage of analysis, members of the research team (B. J. Lutz, A. E. Reimold, S. W. Coleman, A. K. Guzik, L. P. Russell, and S. B. Gesell) reviewed and compared the codes from all data sources to the domains of the RE-AIM framework (Glasgow et al., 1999). RE-AIM is commonly used to evaluate the clinical trial implementation and with interventions focused on older adults (Demiris, Oliver, Capurro, & Wittenberg-Lyles, 2014; Gitlin, Jacobs, & Earland, 2010; Glasgow et al., 2019).
Most of the themes we previously identified fit within the Reach, Adoption, and Implementation domains of the RE-AIM framework, defined as Reach of the intended patient population, Adoption of the intervention by staff and institutions, and Implementation consistency and adaptations made during delivery (Holtrop et al., 2018). We did not have themes related to Maintenance and Effectiveness of COMPASS-TC because data were collected during implementation. The validity of the placement of themes into each of the RE-AIM categories was confirmed by discussions with two Implementation Team members.
As transcripts were coded, we found themes that were conceptually distinct and did not fit the definitions of Reach, Adoption, and Implementation in the RE-AIM framework yet remained important to successful implementation. These themes were related to the construct of organizational readiness to implement change. The coding schema was revised to include “Organizational Readiness.”
Multiple strategies were used to establish trustworthiness of the findings from the analysis of the transcripts and field notes (Maher, Hadfield, Hutchings, & de Eyto, 2018). Several research team members (B. J. Lutz, S. W. Coleman, M. D. Radman, and S. B. Gesell) were involved in prolonged engagement with the COMPASS-TC sites over the course of Phase 1 implementation. Transcripts from the conference calls and interviews were coded by multiple team members. The team met regularly to discuss the ongoing analysis to reduce individual bias and come to consensus. Members of the research team (B. J. Lutz, S. W. Coleman, and S. B. Gesell) discussed findings from the preliminary analysis of the conference calls with COMPASS-TC PACs and APPs to confirm their relevance (Charmaz, 2014). Questions on the interview guide for the group and individual interviews were developed from the provisional coding of the conference call transcripts. B. J. Lutz, A. E. Reimold, and S. W. Coleman also reviewed group conference call surveys for frequencies of specific challenges identified by participating hospitals and the number of times a challenge was reported across time. These frequencies were compared with the themes to further substantiate their salience.
Results
COMPASS-TC was implemented to varying degrees of completeness across the 19 hospitals. For this analysis, per protocol implementation meant that patients attended the COMPASS-TC clinic visit within 30 calendar days of hospital discharge. Across hospitals, the range was 6% to 69% of enrolled patients who received the intervention per protocol (Duncan et al., in press). Table 3 provides a summary of the factors that influenced implementation. Table 4 includes a description of the code key for the data sources.
Table 3.
Clinician and Administrator Perspectives of Factors Influencing Implementation of COMPASS-TC
| Category | Factors influencing implementation |
|---|---|
| Facilitators | |
| Organizational readiness | Organizational vision and commitment • Administrative / clinical champions • Commitment to implementing COMPASS-TC • Making COMPASS-TC implementation a priority • Administrators communicating commitment to implementation to frontline clinical staff • Cultivating staff buy-in Organizational capacity • Dedicated and sufficient resources necessary for implementation |
| Adoption | Hiring staff with appropriate skills / relevant experience • Committed to program and improving outcomes • Autonomous, problem solvers, assertive • Case management / navigator experience |
| Challenges | |
| Reach | Case ascertainment • Incorrect / lack of diagnosis predischarge • Short lengths of stay 2-Day follow-up call to patients • Inaccurate / nonworking phone numbers |
| Implementation | Start-up issues • Technical difficulties with COMPASS-TC software • Steep learning curve from training to implementation Staffing challenges • Inadequate number of trained PACs/APPs • Insufficient back-ups • Limited time dedicated to PAC/APP role Inadequate systems to identify and track patients Integrating COMPASS-TC into existing TC programs threatened fidelity Clinic visit • Clinic location • Length of clinic visit • Lack of available clinic appointments and space • Limited transportation • Insurance co-pays • Patient preference to see own primary care provider Billing issues • Competition for Medicare TC Management billing codes • Patients without insurance / under insured Community resources • Hospital clinicians’ unfamiliarity with community resources |
Table 4.
Interview Code Key
| Data source | Code key | Example |
|---|---|---|
| Group Conference Call (GCC) | Wave #, GCC, Call #, Participant # | 1GCC1.2 |
| Director of Implementation (DOI) Interview | DOI, Interview # | DOI1 |
| Hospital Interview (HI) | Temporal Order, Participant #, HI | 1.3HI |
Organizational Readiness to Implement Change
Organizational readiness has been defined as a multilevel, multifaceted construct that reflects a shared commitment to and belief by members of an organization in its capacity for change (Weiner, 2009). It is critical to successful implementation of interventions and programs in complex organizations (Weiner, 2009). We identified administrative vision and commitment to COMPASS-TC as themes related to organizational readiness. This included having one or more “champions” identified as standout hospital administrators or clinicians who were committed to and facilitated the implementation of COMPASS-TC. Champions secured “buy-in” within the administrative structure, from the care team involved with implementation (i.e., the PACs and APPs), and from direct care support staff (e.g., staff nurses, case managers, and social workers).
Sites that were organizationally ready had the capacity to implement COMPASS-TC within the study’s timeline. They had sufficient dedicated resources, employees, and effort, with sufficient time allocated to implementation, space for the clinic visits, and systems in place to identify and track patients from recruitment through follow-up. Organizationally ready sites were able to identify challenges and develop strategies to manage them effectively. Here, the DOI describes her experience with a site that was organizationally ready to implement COMPASS-TC:
Their administration is all about improving stroke care. I mean they have a whole team of champions. This person is really very passionate … she was committed to making this happen. So, from the very beginning; front and center with education and training, [this] site visit was fantastic; had all the right people there. (DOI1)
Organizationally ready sites also tracked COMPASS-TC performance metrics (e.g., enrollment of eligible patients, receipt of 2-day call, attendance at clinic visit) in their monthly quality improvement reports so they could quickly identify and address issues with implementation.
Alternatively, there were sites where administration signed a letter of agreement (LOA) but did not inform mid-level management or clinical staff that they were participating in the COMPASS-TC Study. As a result, staff were not prepared for implementation. For instance, clinical staff from one site indicated that they were unaware they were participating in the COMPASS-TC Study until the DOI called them to prepare them for the upcoming training boot camp and site visit. Sites lacking the components of organizational readiness had higher levels of difficulty throughout the implementation process.
Adoption
Adoption focuses on the location and setting of the intervention and includes the “representativeness of settings and intervention agents (clinicians) who are willing to initiate” the intervention (RE-AIM Workgroup, 2019). Ninety-five eligible hospitals in NC were contacted to participate in the study. LOAs were received from 41 (43%) of the eligible hospitals (Johnson et al., 2018). Adoption also included hiring the “right” and sufficient staff to implement COMPASS-TC. “Right” staff needed to be committed to working collaboratively and have a “do whatever it takes” attitude. As one PAC suggested, “I am going to own this program and I am going to make it work. Yes, own it… Ownership is key.” (6.2HI). Staff needed to be adaptable problem-solvers, have a clear understanding of administrative channels and hierarchies within the hospital and/or hospital system, know what resources were available and who to contact, have the ability to build relationships, advocate for the changes in care that were part of the intervention, and work autonomously. “It does take some boldness to talk to administration; to talk to your patients; to talk to the doctor…. And autonomous. It’s a very autonomous job” (6.3HI). All five PACs from four different hospitals participating in a group interview indicated that having previous experience as a case manager, navigator, or coordinator was “huge because you know your resources and you are familiar with the staff and you know the policies and protocols” (6.2HI). They also agreed that PACs needed to be committed to patient outcomes throughout the intervention: “to follow the patient throughout the hospital and make sure that things are happening… ‘I want this patient to succeed’ and then to actually let the patient know, ‘hey, I actually know what happened to you and I am going to follow you outside and I am going to see you outside’” (6.3HI). Staff flexibility, collaboration, and commitment were key strategies that enhanced the adoption of COMPASS-TC.
Hospital administration signing the LOA without talking to direct care clinicians led to confusion, frustration, and, ultimately, challenges with implementation, indicating that buy-in must occur at all organizational levels, from administration to front-line staff. At some sites, PACs and APPs were expected to add COMPASS-TC duties to their already full-time responsibilities. As a result, sites with poor communication and low staff availability were not as successful at consistently implementing COMPASS-TC.
Reach
Reach focuses on “who” and addresses the “representativeness” of the patients who received the intervention (Glasgow et al., 2019). Issues with determining patient eligibility and patient enrollment and retention were themes in the Reach domain of the framework. Definitive and correct diagnoses in patients’ medical records prior to discharge were key to determining eligibility. Difficulties with eligibility determination happened most often with patients with a TIA or mild stroke.
…specifically with TIA patients they are hard to capture prior to discharge … sometimes the discharge summary is not completed when the patient is discharged so we are basically going by the admission diagnosis … and so we enroll them. Later on when we do our 2-day call and [are] reviewing things, we see on their discharge summary that it was vertigo instead. (1GCC1.4)
Enrolled patients whose diagnoses were changed from TIA or stroke after discharge were unenrolled from the study once the final unrelated diagnosis was determined. In many of these cases, patients were in the hospital briefly (e.g., less than 24 hr), and the PAC either was not available (e.g., during the weekend) or did not have time to see the patient due to other responsibilities. To improve eligibility determination, the COMPASS-TC Study neurologist developed an algorithm for TIA identification prior to discharge. However, in cases where the physician did not document the diagnosis in the medical record or update the diagnosis after discharge, eligibility determination was challenging.
Some sites had challenges with enrollment and retention among patients who preferred follow-up with their own primary care provider (PCP) or specialist. Sites that introduced COMPASS-TC to patients in-person prior to discharge as a component of their stroke program standard of care were generally more successful with completing the 2-day call and follow-up clinic visit. In contrast, sites that mailed COMPASS-TC information packets had poorer follow-up (Gesell, 2019).
Implementation
Staff described several challenges related to implementing COMPASS-TC consistently and adhering to all protocol components (Glasgow et al., 2019). The greatest challenges were related to resource allocation, including staffing and clinic space, and systems issues related to patient tracking and billing. Some sites had to fit COMPASS-TC into existing TC programs, which threatened fidelity of the protocol.
Staffing
Allocation of sufficient staffing resources to implement each step of COMPASS-TC was a challenge for several sites. One PAC voiced that,
We have a lot of issues with lack of resources like I took off three days in October and [name], who would have been my backup, had orientation. So I mean, we have so many different roles that we are filling [and] I don’t think we have missed an opportunity to enroll a patient yet but I am afraid we are going to because there is nobody that can consistently be doing this except me. (2GCC1.2)
Another PAC reported making follow-up phone calls while on vacation because no one else was available. Insufficient staffing resulted in inconsistencies in implementation and difficulty knowing who was ultimately accountable. Staff in some sites also described how COMPASS-TC was added to their already full-time positions, making implementation more difficult. Competing responsibilities and priorities threatened the consistency and fidelity of implementation.
Patient Tracking
Clinical staff cited difficulties tracking patients once they were enrolled. Some PACs developed strategies and tracking systems to follow patients through the steps of the intervention. One site described using electronically generated reports with a simple paper-based system to track stroke admissions and discharges.
If we go through and identify someone who has had a stroke or a TIA and/or we have gotten a consult on them, we have a folder. We can pull a report to see what our discharges were in the last 24 hours. If they have been discharged, they get pulled out of that folder and put into a discharge folder. So, we have two folders basically. One of active patients in-house and one of discharged patients. So, once they get put in the discharged folder, that folder gets looked at by that afternoon and we are making phone calls out of the discharge folder. (1GCC5.2)
Another PAC described her method of tracking patients in the hospital and those needing follow-up calls using the COMPASS-TC application and other technology.
I check-in with my charge nurses and try not to miss anyone throughout the [hospital] and then I go into my COMPASS-TC app as well as different applications that I use to track different types of patients. (1GCC5.3)
Follow-up: 2-Day Call
Completing the 2-day call was identified as a challenge by more than half of the sites on the PAC Team survey. PACs made at least two attempts to reach the patient, but some found that this was not enough, calling multiple times and leaving messages in order to get through. PACs indicated that identifying an accurate or working phone number for the patient was a frequent source of frustration. PACs also noted challenges with making the follow-up call within the timeframe due to competing demands of their jobs.
I’ve had a couple of patients in the last couple of weeks where after two calls I just don’t have the time to keep calling them back. (1GCC2.3)
Follow-up: Clinic Visit
PACs and APPs noted challenges with patient attendance at the follow-up clinic visit. Patients who chose not to attend the COMPASS-TC clinic visit often cited issues with insurance co-pays, transportation, and seeing the visit as redundant or with no added value to other appointments. PACs implemented several strategies to improve clinic attendance including: (a) communicating the importance of the clinic visit with the patient, family, and caregivers before discharge; (b) scheduling the clinic visit during hospitalization and including it in the discharge orders; (c) reminding patients of the clinic visit on the 2-day call; (d) assuring patients that the clinic visit was different from other appointments because it would help identify and address issues that may arise at home that were not identified in the hospital; and (e) assuring patients that their PCP would receive a summary of the clinic visit, including a copy of the eCare Plan.
COMPASS-TC clinic site location was an important factor in whether patients attended the clinic visit. According to hospital staff, when the visit was held in a specialty clinic (e.g., neurology) or at the hospital, rather than at a PCP clinic, patients seemed to be more willing to attend.
Some sites had difficulty finding an appropriate location for the clinic, adequate space, and time in the schedule to see patients.
Our clinic only allows us to [see COMPASS-TC patients] on Thursdays right now. That’s the only day they had rooms available for us to pull off the COMPASS-TC visits so we are not seeing patients the week of Thanksgiving because there is no other day that week that we can have clinic and Thursday is Thanksgiving and Thursday is clinic day so we just know that we are going to have to try to absorb those patients before and after that week. But again, I think that is part of what [APP name] is saying is just kind of huddling and talking about the schedules. And we are learning as we go. Again, I have a whole new appreciation for the ambulatory world after all of this. (2GC2.1)
Initially, the clinic visit often took an hour or more due to the comprehensiveness of the assessment and follow-up with the APP. Many sites only had 15- to 20-min appointment timeslots allotted for the clinic visit, so the clinic staff developed innovative strategies to use their time more efficiently while maintaining the comprehensiveness of the assessment (e.g., keeping the patient focused on the assessment). They also negotiated with administration for longer appointment times, combined multiple appointment timeslots, and added COMPASS-TC–specific timeslots.
Billing
Site orientation included instructions on using the TC management billing codes for Medicare patients; however, billing was a challenge at some sites. TC management coding was already utilized by affiliated PCP offices or was incorporated into PCP workflow at some sites, putting COMPASS-TC clinics in competition with the PCP for reimbursement. For some sites, competition for billing led to challenges as to how to cover the costs of the program implementation. Sites with higher implementation rates either anticipated this challenge and collaborated with PCP offices on billing issues or used other billing codes. In non-Medicare patients, site staff described reimbursement challenges for patients who were uninsured or whose insurance would not cover the full cost of the visit. One successful strategy was to waive the copay or cost of the visit and to find other ways to offset the cost.
Incorporating COMPASS-TC into Existing TC Programs
Several sites integrated COMPASS-TC into existing TC programs rather than implementing COMPASS-TC per protocol. One site had the PAC enroll patients in the hospital but had a nurse in the TC clinic complete the follow-up call and assessment at the clinic visit. The nurse had not been trained on COMPASS-TC, so consistency of implementation was a concern. Another site had issues with patients not returning for the clinic visit, so they integrated COMPASS-TC into their existing TC home visit program. Patients received follow-up visits at home, but follow-up percentages at this site were low because patients often would not let providers enter their homes.
Start-up
There were also implementation challenges related to “start-up.” In the first wave, in particular, sites had technical difficulties with the iPad software and the COMPASS-TC application due to unfamiliarity with the technology. The Implementation Team worked with sites to resolve these issues during Wave 1, so they did not reoccur in Waves 2 and 3. There was also a learning curve for the PACs and APPs when they first implemented the intervention. This was especially challenging for Wave 1 sites where the PACs and APPs found the time between training and start-up did not allow them to feel fully prepared to implement COMPASS-TC. This challenge was minimized by the Implementation Team by allowing more preparation time in subsequent waves.
Community Resources
An important component of COMPASS-TC was the development of community resource networks, where PACs and APPs worked with local community-based organizations to link patients to community resources. However, many PACs and APPs were not familiar with resources in their communities and struggled to identify appropriate referrals. Sites were encouraged to develop relationships with staff from local or regional Area Agencies on Aging and the NC Community Pharmacy Enhanced Services Network; postacute rehabilitation providers including home health agencies and outpatient rehabilitation facilities; local community-based services such as faith-based, mental health, and transportation services, and other human service organizations; and community paramedicine programs as available. These community resource networks helped to facilitate referrals to local health and human services for patients and caregivers and assisted PACs with procuring resources (e.g., blood pressure cuffs) for patients. Area Agencies on Aging and the NC Community Pharmacy Enhanced Services Network were particularly helpful, as these agencies had a comprehensive understanding of resources in their areas and could assist with referrals to appropriate services. For example, one PAC contacted the local pharmacist from the NC Community Pharmacy Enhanced Service Network to help negotiate getting prescriptions filled through the U.S. Department of Veteran Affairs (VA). She indicated:
If it hadn’t been for COMPASS-TC and the intervention itself and secondly, I wouldn’t have known to call [the pharmacist] and she just was a gem in getting [the problem with the VA prescriptions] solved very quickly for the patient. Since then, she has helped me with many, many patients from adherence packaging [to helping] me get blood pressure monitors for patients after they go home. Even patients that aren’t Medicaid, Medicare, she has helped me find resources for them. She is just an abundance of knowledge in all the different resources and ins and outs around the community so she kind of helps me far beyond what we think of as a pharmacist and helps me really connect the dots out in the community for our patients. (2GCC8.7)
A site in a rural community, partnered with the local paramedicine program whereby a paramedic served as the backup PAC and followed up with patients postdischarge if there were transportation issues. This creative partnership was possible because the PAC had the autonomy and support from her hospital’s administration and board to address issues as they arose.
Discussion and Implications
We thematically analyzed qualitative data from multiple sources to understand the complexities hospitals faced when redesigning their delivery of postacute stroke care to meet CMS requirements for TC management under real-world clinical practice conditions. We identified the challenges, facilitators, solutions, and contextual factors that explain the hospital-level variability in the implementation of COMPASS-TC.
Organizational readiness was the key to successful implementation. Sites that were organizationally ready had one or more champions who had the commitment to implement COMPASS-TC and the authority to allocate necessary resources to initiate and maintain implementation and address challenges as they arose. Organizational readiness has been identified as a crucial factor to successful implementation of health care interventions (Gitlin et al., 2010; Palmer et al., 2019; Weiner, 2009). For example, Palmer and colleagues (2019) found, in an implementation analysis of a pragmatic trial in nursing homes, the low performing sites had limited implementation resources and champions that were reluctant to implement the intervention. In our study, the more successful sites had the support of the top hospital administrators and stroke clinicians. In these sites, COMPASS-TC became an integral part of providing comprehensive stroke care.
Identifying dedicated resources to fund a new program is another important factor for successful implementation and sustainability (Gitlin et al., 2010). Per PCORI funding requirements, COMPASS-TC did not provide onsite support with completing study-related procedures or fund the implementation of COMPASS-TC. Several hospitals cited insufficient resources as a barrier to implementation. Major investments in time and resources were needed to ensure successful implementation and minimize burden to already overstretched frontline providers. Viewing COMPASS-TC as a critical component of comprehensive care and dedicating sufficient resources, including prioritizing clinician time and effort, so COMPASS-TC could be implemented consistently, without interruption, was necessary for successful implementation.
Research suggests that both implementation scientists and health care executives should pay attention to committed, proactive middle managers as effective change agents in health care (Birken, Lee, & Weiner, 2012; Birken, Lee, Weiner, Chin, & Schaefer, 2013). Birken and colleagues (2016) identified strategies used by middle managers for implementing innovative interventions in health care as (a) diffusing information; (b) synthesizing information; (c) mediating between the new strategy and day-to-day activities; and (d) selling innovation implementation. In our study, COMPASS-TC clinicians implemented these strategies to facilitate successful implementation. For example, PACs in some hospitals were notably adept at incorporating COMPASS-TC into their workflows and meeting challenges head-on. These clinicians tended to be flexible problem-solvers who were well-connected throughout their hospitals. They often developed solutions by leveraging preexisting relationships in the hospital and community. They also used internal performance data to justify their positions and the need for other resources (e.g., clinic space, time) to their directors or to secure backup staffing. Our analysis suggests that, when PACs were proactive, they were instrumental in successful implementation.
Incorporating performance measures is instrumental for improving quality and value in health care (Institute of Medicine, 2001). A report from the Robert Wood Johnson Foundation (Berenson, Pronovost, & Krumholz, 2013) recommends that quality should be measured strategically, at the organizational level, and that measures should “promote the concept of the rapid-learning healthcare system” (p. 2). In this study, more successful sites added COMPASS-TC metrics to their performance measures presented to their Quality Department, Stroke Team, Joint Commission Preparation Team, and other quality improvement-related teams so that COMPASS-TC was treated like any other hospital standard monitored for performance. This allowed for ongoing implementation analysis and “rapid-learning” by the hospital’s implementation team to address challenges quickly. These sites took the lead in developing and sharing their solutions to commonly encountered problems that other sites then emulated. These solutions included developing systems for identifying and tracking eligible patients within the hospital, PACs meeting with patients and families prior to discharge, explaining COMPASS-TC as part of their standard comprehensive stroke care program, and conveying the role of COMPASS-TC in the patient’s recovery.
Because they were monitoring performance metrics and related utilization outcomes, several sites reported that, after COMPASS-TC was implemented, they saw a reduction in emergency department visits and 30-day readmissions. The COMPASS-TC research team is currently analyzing Medicare and insurance claims data from all sites to determine whether there were significant differences in health care utilization between the usual care and intervention sites.
Care coordination across systems and settings over time is fundamental to improving quality of care (IOM, 2001). A key component of the COMPASS-TC was developing effective community resource networks to coordinate care and make appropriate referrals to community-based services. Often, COMPASS-TC clinicians were unaware of community-based resources available to patients prior to implementation. Community resource networks were not only a benefit to COMPASS-TC patients, but the relationships formed among the hospital-based clinicians and their community partners provided opportunities to link other, non-COMPASS-TC, patients to community resources.
Implications
In the implementation analysis, we evaluated the challenges to implementing a TC program for stroke patients discharged directly home from acute care. In Phase 2 of the study, the usual care sites (from Phase 1) implemented COMPASS-TC. To facilitate a smooth transition to the program, we included the recommendations learned from Phase 1 implementation analyses (Table 5). Many of these strategies are supported by other implementation experts. For example, Weiner (2009) recommends conducting an organizational readiness assessment and addressing gaps in readiness prior to implementation. Birken and colleagues (2012, 2013) identified the importance of middle managers and clinicians to the successful implementation of innovative interventions. Health policy experts have recommended including performance metrics to assure consistent implementation of new programs (Berenson et al., 2013; IOM, 2001).
Table 5.
Strategies to Enhance Implementation Success
| Category | Recommended strategies | Actor |
|---|---|---|
| Organizational readiness (Birken et al., 2015; Shea, Jacobs, Esserman, Bruce, & Weiner, 2014; Weiner, 2009) | • Assess organizational commitment and capacity to implement prior to implementation | • Implementation Team (which could include administrative and clinical leadership, middle management, frontline staff, and research team members, if it is a research study) |
| Adoption (Birken et al., 2012; Birken et al., 2013) | • Hire staff with appropriate skills / relevant experience • Establish vertical and horizontal support • Define intervention as new standard of care • Recognize / disseminate intervention benefits • Demonstrate commitment to caring for patients across care continuum • Identify long-term financial benefits | • Administrative and clinical leadership • Implementation Team • Administrative and clinical leadership • Clinical leadership and frontline providers • Implementation Team • Administrative and clinical leadership |
| Reach (Chhatre et al., 2018) | • Develop a system for identifying and tracking eligible and enrolled patients • Visit patient in the hospital whenever possible • Confirm questionable diagnoses as soon as possible after discharge | • Postacute care coordinator (PAC) and clinical leadership, including middle management (may involve Information Technology) • PAC or back-up frontline staff (when PAC is not available) • PAC or back-up frontline staff |
| Implementation staffing (Birken et al., 2012, Birken et al., 2013; Leeman, Birken, Powell, Rohweder, & Shea, 2017) | • Include intervention performance metrics as part of regular quality briefings • Maintain consistent and sufficient PAC and APP staff and trained back-up staff is important for fidelity and enhances shared responsibility • Train direct care staff to identify and enroll eligible patients in the absence of the PAC | • Administrative and clinical leadership (may include Performance/ Quality Improvement Team) • Implementation Team • Implementation Team |
| Patient retention (Chhatre et al., 2018) | • Include follow-up clinic visit in discharge orders and remind patient on 2-day call • Explain the importance of the follow-up clinic visit to the patient • Once identified, include a notification in the patient’s medical record indicating they are receiving the intervention • Collaborate with primary care providers and reinforce purpose of COMPASS-TC • Host follow-up clinic visit in a neurologist office; reinforce that this visit is specialty care to aid long-term recovery | • PAC or APP (may include hospitalist, discharge planners, and case managers) • PAC or APP • PAC or APP (may include hospitalist, discharge planners, and case managers) • Administrative and clinical leadership including PAC and APP • Administrative and clinical leadership |
| Develop strong community resource networks (Dreyer, 2014) | • Partner with community pharmacists to aid in postdischarge medication management • Work with specialists at the local Area Agency on Aging to identify appropriate community-based follow-up services • Work with community paramedic or other outreach programs to follow-up with “difficult to reach” patients | • PAC and APP in collaboration with administrative and clinical leadership, case managers, and discharge planners • PAC and APP • PAC and APP |
| Utilize existing web-based resources (Leeman et al., 2017) | • COMPASS-TC website provides multiple resources for patients, caregivers, and providers in NC | • PAC and APP |
Another important implication was that the RE-AIM Framework, which is commonly used for implementation analysis, did not capture all the critical constructs that influenced implementation. Other frameworks, such as the Consolidated Framework for Implementation Research (CFIR; Damschroder et al., 2009), include a readiness for implementation framework that encompasses the subconstructs of leadership engagement, available resources, and access to knowledge and information. The themes that we classified under organizational readiness align with the CFIR subconstructs. Our data suggest that organizational readiness is so overarching that lack thereof hinders full implementation across all RE-AIM dimensions.
Strengths and Limitations
Our analysis includes data from 19 diverse sites, with additional data from sites with high and low implementation performance data. Time and expense prevented us from collecting more in-depth data from all sites. However, our analysis did reach theme saturation and adds depth and meaning to understanding the variability of COMPASS-TC implementation. Rigorous analysis of qualitative data can guard against the assumption that an intervention does not work when it is truly an implementation failure and provides explanations about what implementation strategies are successful and which adaptations are beneficial (Holtrop et al., 2018). Moreover, the findings from the thematic analyses reported here align with quantitative analyses of performance data (Gesell, 2019).
Conclusion
Multifaceted models of care like COMPASS-TC are challenging to implement. The most significant factor we identified in this study is the importance of an organization’s commitment and capacity to making a change in how it delivers comprehensive stroke care and to providing care in a different, less-fragmented way. The heterogeneity of patient needs and diversity of hospital settings and resources impacted success. Nonetheless, some hospitals were able to successfully deliver this multifaceted intervention with complex patients in complex health systems. Taking responsibility for postdischarge follow-up, connecting stroke survivors to appropriate community-based resources to support long-term recovery, and communicating the follow-up plan of care with the patient’s PCP can help to provide the long-term monitoring needed to optimize recovery and prevention of a subsequent stroke (Cameron et al., 2016; Cameron, Tsoi, & Marsella, 2008; Wissel, Olver, & Sunnerhagen, 2013).
Funding
This work was supported by funding from the Wake Forest Clinical and Translational Science Award through National Center for Advancing Translational Sciences National Institutes of Health (NIH) Grant Award (UL1TR001420); Patient-Centered Outcomes Research Institute (PCORI) Contract Award (PCS-1403–14532). Support was also received from the McNeill Research Fund, University of North Carolina at Wilmington. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the NIH or PCORI, its Board of Governors, or Methodology Committee.
Conflict of Interest
Drs. P. W. Duncan and C. D. Bushnell: ownership interest in Care Directions, Inc.; Dr. B. J. Lutz: honoraria from MedBridge, Inc. All others have no conflict of interest to declare.
Supplementary Material
Acknowledgments
We thank our participating hospitals, patients, and their caregivers for making this study possible, for their commitment to this study and their patient care. We also thank our study stakeholders for their contributions to shaping the study design and intervention, in particular, stroke program managers across the state of North Carolina (NC), the NC Area Agencies on Aging, Community Pharmacy Enhanced Services Network, Justus-Warren Heart Disease and Stroke Prevention Task Force, NC Stroke Advisory Council, NC Department of Health and Human Services, FaithHealth, Carolina Center for Cognitive Rehabilitation, and the American Heart Association/American Stroke Association.
References
- Andrews J. E., Moore J. B., Weinberg R. B., Sissine M., Gesell S., Halladay J.,… Duncan P. W.; COMPASS Investigators and Stakeholders (2018). Ensuring respect for persons in COMPASS: A cluster randomised pragmatic clinical trial. Journal of Medical Ethics, 44, 560–566. doi: 10.1136/medethics-2017-104478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E. J., Muntner P., Alonso A., Bittencourt M. S., Callaway C. W., Carson A. P.,…Virani S. S.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2019). Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation, 139, e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- Berenson R. A., Pronovost P. J., & Krumholz H. M (2013). Achieving the potential of health care perrformance. Timely Analysis of Immediate Health Policy Issues. Retrieved from https://www.rwjf.org/en/library/research/2013/05/achieving-the-potential-of-health-care-performance-measures.html [Google Scholar]
- Bettger J. P., Jones S. B., Kucharska-Newton A. M., Freburger J. K., Coleman S. W., Mettam L. H.,…Rosamond W. D (2019). Meeting Medicare requirements for transitional care: Do stroke care and policy align? Neurology , 92, 427–434. doi: 10.1212/WNL.0000000000006921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettger J. P., McCoy L., Smith E., Fonarow G., Schwamm L., & Peterson E (2015). Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc, 4(2). Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4345857/pdf/jah3-4-e001038.pdf. doi: 10.1161/jaha.114.001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken S. A., DiMartino L. D., Kirk M. A., Lee S. Y., McClelland M., & Albert N. M (2016). Elaborating on theory with middle managers’ experience implementing healthcare innovations in practice. Implementation Science, 11, 2. doi: 10.1186/s13012-015-0362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken S. A., Lee S. Y., & Weiner B. J (2012). Uncovering middle managers’ role in healthcare innovation implementation. Implementation Science, 7, 28. doi: 10.1186/1748-5908-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken S. A., Lee S. Y., Weiner B. J., Chin M. H., Chiu M., & Schaefer C. T (2015). From strategy to action: How top managers’ support increases middle managers’ commitment to innovation implementation in health care organizations. Health Care Management Review, 40, 159–168. doi: 10.1097/HMR.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birken S. A., Lee S. Y., Weiner B. J., Chin M. H., & Schaefer C. T (2013). Improving the effectiveness of health care innovation implementation: Middle managers as change agents. Medical Care Research and Review: MCRR, 70, 29–45. doi: 10.1177/1077558712457427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell C. D., Duncan P. W., Lycan S. L., Condon C. N., Pastva A. M., Lutz B. J.,… Rosamond W. D.; COMPASS Trial. (2018). A person-centered approach to poststroke care: The COMprehensive post-acute stroke services model. Journal of the American Geriatrics Society, 66, 1025–1030. doi: 10.1111/jgs.15322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. I., O’Connell C., Foley N., Salter K., Booth R., Boyle R.,… Lindsay P.; Heart and Stroke Foundation Canadian Stroke Best Practice Committees (2016). Canadian Stroke Best Practice Recommendations: Managing transitions of care following Stroke, Guidelines Update 2016. International Journal of Stroke, 11, 807–822. doi: 10.1177/1747493016660102 [DOI] [PubMed] [Google Scholar]
- Cameron J. I., Tsoi C., & Marsella A (2008). Optimizing stroke systems of care by enhancing transitions across care environments. Stroke, 39, 2637–2643. doi: 10.1161/STROKEAHA.107.501064 [DOI] [PubMed] [Google Scholar]
- Charmaz K. (2014). Constructing grounded theory (2nd ed.). Thousand Oaks, CA: Sage Publications. doi: 10.1177/1049732315613982 [DOI] [Google Scholar]
- Chhatre S., Jefferson A., Cook R., Meeker C. R., Kim J. H., Hartz K. M.,… Jayadevappa R (2018). Patient-centered recruitment and retention for a randomized controlled study. Trials, 19, 205. doi: 10.1186/s13063-018-2578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damschroder L. J., Aron D. C., Keith R. E., Kirsh S. R., Alexander J. A., & Lowery J. C (2009). Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implementation Science, 4, 50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiris G., Parker Oliver D., Capurro D., & Wittenberg-Lyles E (2014). Implementation science: Implications for intervention research in hospice and palliative care. The Gerontologist, 54, 163–171. doi: 10.1093/geront/gnt022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer T. (2014). Care transitions: Best practices and evidence-based programs. Retrieved from https://chrt.org/publication/care-transitions-best-practices-evidence-based-programs/
- Duncan P. W., Abbott R. M., Rushing S., Johnson A. M., Condon C. N., Lycan S. L.,… Bushnell C. D.; COMPASS Investigative Team (2018). COMPASS-CP: An electronic application to capture patient-reported outcomes to develop actionable stroke and transient ischemic attack care plans. Circulation. Cardiovascular Quality and Outcomes, 11, e004444. doi: 10.1161/CIRCOUTCOMES.117.004444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. W., Bushnell C., Jones S., Psioda M., Gesell S., D’Agostino R. Jr.,…Rosamond W. D (in press). A randomized pragmatic trial of stroke transitional care: The COMPASS Study. Circulation. Cardiovascular Quality and Outcomes. [DOI] [PubMed] [Google Scholar]
- Duncan P. W., Bushnell C. D., Rosamond W. D., Jones Berkeley S. B., Gesell S. B., D’Agostino R. B. Jr.,…Vetter B (2017). The Comprehensive Post-Acute Stroke Services (COMPASS) study: Design and methods for a cluster-randomized pragmatic trial. BMC Neurology, 17, 133. doi: 10.1186/s12883-017-0907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fens M., van Heugten C. M., Beusmans G. H., Limburg M., Haeren R., Kaemingk A., & Metsemakers J. F (2013). Not as transient: Patients with transient ischaemic attack or minor stroke experience cognitive and communication problems; an exploratory study. The European Journal of General Practice, 19, 11–16. doi: 10.3109/13814788.2012.715147 [DOI] [PubMed] [Google Scholar]
- Gesell S. B., Bushnell C. D., Jones S. B., Coleman S. W., Levy S. M., Xenakis J. G.,…Duncan P. W (2019). Implementation of a billable transitional care model for stroke patients: The COMPASS study. BMC Health Services Research, 19, 978. doi: 10.1186/s12913-019-4771-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesell S. B., Klein K. P., Halladay J., Bettger J. P., Freburger J., Cummings D. M.,…Duncan P. W (2017). Methods guiding stakeholder engagement in planning a pragmatic study on changing stroke systems of care. Journal of Clinical and Translational Science, 1, 121–128. doi: 10.1017/cts.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L. N., Jacobs M., & Earland T. V (2010). Translation of a dementia caregiver intervention for delivery in homecare as a reimbursable Medicare service: Outcomes and lessons learned. The Gerontologist, 50, 847–854. doi: 10.1093/geront/gnq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R. E., Harden S. M., Gaglio B., Rabin B., Smith M. L., Porter G. C.,…Estabrooks P. A (2019). RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Frontiers in Public Health, 7, 64. doi: 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R. E., Vogt T. M., & Boles S. M (1999). Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health, 89, 1322–1327. doi: 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik A., & Bushnell C (2017). Stroke epidemiology and risk factor management. Continuum (Minneapolis, Minn), 23(1, Cerebrovascular Disease), 15–39. doi: 10.1212/CON.0000000000000416 [DOI] [PubMed] [Google Scholar]
- Higashida R., Alberts M. J., Alexander D. N., Crocco T. J., Demaerschalk B. M., Derdeyn C. P.,… Wood J. P.; American Heart Association Advocacy Coordinating Committee (2013). Interactions within stroke systems of care: A policy statement from the American Heart Association/American Stroke Association. Stroke, 44, 2961–2984. doi: 10.1161/STR.0b013e3182a6d2b2 [DOI] [PubMed] [Google Scholar]
- Holtrop J. S., Rabin B. A., & Glasgow R. E (2018). Qualitative approaches to use of the RE-AIM framework: Rationale and methods. BMC Health Services Research, 18, 177. doi: 10.1186/s12913-018-2938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (2001). Crossing the quality chasm: A new health system for the 21st century. Washington DC: The National Academies Press. doi: 10.17226/10027 [DOI] [PubMed] [Google Scholar]
- Johnson A. M., Jones S. B., Duncan P. W., Bushnell C. D., Coleman S. W., Mettam L. H.,… Rosamond W. D (2018). Hospital recruitment for a pragmatic cluster-randomized clinical trial: Lessons learned from the COMPASS study. Trials, 19, 74. doi: 10.1186/s13063-017-2434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr M. M. H., van der Zee D. J., Luijckx G. J., & Buskens E (2020). Optimising acute stroke care organisation: A simulation study to assess the potential to increase intravenous thrombolysis rates and patient gains. BMJ Open, 10, e032780. doi: 10.1136/bmjopen-2019-032780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman J., Birken S. A., Powell B. J., Rohweder C., & Shea C. M (2017). Beyond “implementation strategies”: Classifying the full range of strategies used in implementation science and practice. Implementation Science, 12, 125. doi: 10.1186/s13012-017-0657-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Cushman J. T., Shah M. N., Kelly A. G., Rich D. Q., & Jones C. M. C (2020). Prehospital time intervals and management of ischemic stroke patients. The American Journal of Emergency Medicine. Advance online publication. doi: 10.1016/j.ajem.2020.02.006 [DOI] [PubMed] [Google Scholar]
- Lutz B. J., Young M. E., Cox K. J., Martz C., & Creasy K. R (2011). The crisis of stroke: Experiences of patients and their family caregivers. Topics in Stroke Rehabilitation, 18, 786–797. doi: 10.1310/tsr1806-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. J., Young M. E., Creasy K. R., Martz C., Eisenbrandt L., Brunny J. N., & Cook C (2017). Improving stroke caregiver readiness for transition from inpatient rehabilitation to home. The Gerontologist, 57, 880–889. doi: 10.1093/geront/gnw135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher C., Hadfield M., Hutchings M., & de Eyto A (2018). Ensuring rigor in qualitative data analysis: A design research approach to coding combing NVivo with traditional methods. International Journal of Qualitative Methods, 17, 1–13. doi: 10.1177/1609406918786362 [DOI] [Google Scholar]
- Miller J. B., Heitsch L., Madsen T. E., Oostema J., Reeves M., Zammit C. G.,… Schrock J. W (2019). The extended treatment window’s impact on emergency systems of care for acute stroke. Academic Emergency Medicine, 26, 744–751. doi: 10.1111/acem.13698 [DOI] [PubMed] [Google Scholar]
- Nicol M. B., & Thrift A. G (2005). Knowledge of risk factors and warning signs of stroke. Vascular Health and Risk Management, 1, 137–147. doi: 10.2147/vhrm.1.2.137.64085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. A., Parker V. A., Barre L. R., Mor V., Volandes A. E., Belanger E.,…Mitchell S. L (2019). Understanding implementation fidelity in a pragmatic randomized clinical trial in the nursing home setting: A mixed-methods examination. Trials, 20, 656. doi: 10.1186/s13063-019-3725-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RE-AIM Workgroup (2019). Adoption of health behavior interventions. Retrieved from http://www.re-aim.org/about/what-is-re-aim/adoption/
- Ryan G., & Bernard H (2003). Techniques to identify themes. Field Methods, 15(1), 85–109. doi: 10.1177/1525822X02239569 [DOI] [Google Scholar]
- Shea C. M., Jacobs S. R., Esserman D. A., Bruce K., & Weiner B. J (2014). Organizational readiness for implementing change: A psychometric assessment of a new measure. Implementation Science, 9, 7. doi: 10.1186/1748-5908-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner B. J. (2009). A theory of organizational readiness for change. Implementation Science, 4, 67. doi: 10.1186/1748-5908-4-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein C. J., Stein J., Arena R., Bates B., Cherney L. R., Cramer S. C.,… Zorowitz R. D.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research (2016). Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 47, e98–e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- Wissel J., Olver J., & Sunnerhagen K. S (2013). Navigating the poststroke continuum of care. Journal of Stroke and Cerebrovascular Diseases, 22, 1–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.021 [DOI] [PubMed] [Google Scholar]
- Yousufuddin M., & Young N (2019). Aging and ischemic stroke. Aging, 11, 2542–2544. doi: 10.18632/aging.101931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.