Abstract
Objective:
Carney complex (CNC) is a rare autosomal dominant multiple neoplasia syndrome characterized by the presence of endocrine and non-endocrine tumors. More than 125 different germline mutations of the protein Kinase A type 1-α regulatory subunit (PRKAR1A) gene have been reported. We present a novel PRKAR1A gene germline mutation in a patient with severe osteoporosis and recurrent vertebral fractures.
Design:
Clinical case report.
Case report:
A 53 year-old male with a medical history of surgically removed recurrent cardiac myxomas was evaluated for repeated low-pressure vertebral fractures and severe osteoporosis. Physical examination revealed spotty skin pigmentation of the lower extremities and papules in the nuchal and thoracic region. The presence of hypercortisolism due to micronodular adrenal disease and the history of cardiac myxomas suggested the diagnosis of CNC; the patient underwent detailed imaging investigation and genetic testing.
Methods:
Standard imaging and clinical testing; DNA was sequenced by the Sanger method.
Results:
Sequence analysis from peripheral lymphocytes’ DNA revealed a novel heterozygous point mutation at codon 172 of exon 2 (c.172G>T) of the PRKAR1A gene, resulting in early termination of the PRKAR1A transcript [p.Glu58Ter (E58X)].
Conclusion:
We report a novel point mutation of the PRKAR1A gene in a patient with CNC who presented with significant osteoporosis and fractures. Low bone mineral density along with recurrent myxomas should prompt the diagnosis of CNC.
Keywords: Carney complex, PRKAR1A gene, PPNAD, hypercortisolism, osteoporosis
Introduction
Carney complex (CNC) is a rare disease with an autosomal dominant inheritance, characterized by the presence of myxomas, spotty skin pigmentation and endocrine overactivity.1 CNC is associated with a variety of endocrine and non-endocrine abnormalities, the most frequent being primary pigmented nodular adrenocortical disease (PPNAD) that usually leads to adrenocorticotropin (ACTH) -independent Cushing’s syndrome (CS). Other common endocrine abnormalities include cystic or nodular thyroid disease in about 75% of patients, subtle hyperprolactinemia (64%), acromegaly (in up to 15%) and large-cell calcifying Sertoli tumors (41% of affected males). Non-endocrine abnormalities include skin myxomas (80% of patients), lentiginosis (80%), breast fibroadenomas or myxomas (50%), cardiac myxomas (20–40%), cutaneous myxomas (20–30%) and psammomatous melanotic schwannomas (8%).2
CNC is a genetically heterogeneous disease, with linkage analysis so far identifying two independent loci [17q22–24 (CNC1), 2p16 (CNC2)]; a third locus was erroneously linked to CNC, reflecting the oddity of a single family with myosin mutations and the concurrent occurrence of myxomas.3,4,5 The most common genetic cause of CNC is a defect in the PRKAR1A gene (at the CNC1 locus). PRKAR1A encodes for the 1-α regulatory subunit (RI-α) of protein Kinase A (PKA) and functions as a tumor suppressor gene (TSG).6 Heterozygous inactivating PRKAR1A mutations lead to CNC with a penetrance close to 98% by the age of 50 years; these mutations have been reported in 73% of CNC patients.2,7,8 The majority of these mutations results in frame-shift, nonsense or splice site variants that lead to premature stop-codon generation.9 Mutant mRNA is unstable and degraded by nonsense-mediated mRNA decay (NMD). This leads to the loss of the mutant protein and a 50% reduction of the total RI-α protein levels since only the wild type allele is translated.6 RI-α protein reduction stimulates protein kinase A (PKA) activity by cyclic adenosine monophosphate (cAMP) thus interfering in the regulation of cell glucose and lipid metabolism pathways.2 Until recently, about 750 CNC patients have been diagnosed worldwide by the National Institute of Health, the Mayo Clinic (U.S.A), the Cochin Hospital (France) and elsewhere with more than 125 PRKAR1A gene mutations identified to date (online database: http:prkar1a.nichd.nih.gov).9 Despite the genetic heterogeneity and the large number of PRKAR1A mutations spread along the length of the gene, no direct correlation between all PRKAR1A mutations and the various CNC phenotypes has yet been established. However, recent data report potential associations between specific mutations and CNC manifestations.2,10–13
In this article, we present a CNC case with a novel germline PRKAR1A mutation that was diagnosed after recurrent vertebral fractures presumably due to hypercortisolemia and we provide a brief review of the existing literature concerning genotype and phenotype associations in CNC.
Case report
A 53 year-old male (weight 69 kg, height 1.72 cm, blood pressure 110/70 mmHg) was admitted in our Department for evaluation of deteriorating severe osteoporosis (left femoral neck T-score: −4.2, right femoral neck T-score: −4.0, L2-L4 T-score: −5.2), resulting in low-pressure fractures of lumbar spine vertebrae and muscle weakness. Radiologic imaging revealed T8-T10 and L3-L5 vertebral compression fractures. The patient had a history of prior T12-L2 spondylodesis due to a low-pressure L1 vertebra osteoporotic fracture and was treated with denosumab and calcium (500mg bd) for two years without any bone mass improvement. In the last three decades he underwent three cardiac operations for recurrent atrial and ventricular peduncular myxomas (left atrium and right ventricle) with his last serial echocardiography not revealing the presence of any myxoma. He had also undergone polipectomy following two episodes of intestinal blood loss. No family history of endocrine or non-endocrine tumors was identified.
On admission he had muscle weakness and incapacitating back pain. Physical examination revealed spotty skin pigmentation (lentigines) on both legs and two 2 cm brownish papules on the preauricolar and lower right thoracic region, consistent with cutaneous myxomas. Hormonal work-up revealed hypercortisolism [(08.00 morning cortisol levels: 389 nmol/L (NR: 138–690 nmol/L), adrenocorticotropic hormone (ACTH): 5.5 pg/ml (NR: 9–52 pg/ml), urinary cortisol concentration (UFC): 210 μg/24h (NR: 20–90 μg/24h), cortisol levels following a low dose dexamethasone suppression test (LDDST): 412 nmol/L and paradoxical increase of UFC (303 μg/24h) following dexamethasone administration]. Secondary hyperparathyroidism [parathyroid hormone (PTH): 140 pg/ml (NR: 11–62 pg/ml), corrected plasma calcium: 8.6 mg/dl, plasma phosphorus: 3 mg/dl, 25-OHD3: 15 ng/ml] was also evident. Prolactin [(PRL: 13.9 ng/ml (NR: 3.46–19.4)], free thyroxin [(FT4: 14.8 pmol/L (NR: 9.01–21 pmol/L)], thyrotropin [(TSH: 1.6 μIU/ml (NR: 0.35–4.94μIU/ml)], FSH: (7 mU/ml), LH (4.7 mU/ml), and SHBG levels [38nmol/L (NR: 30–100 nmol/L)] were normal. Growth hormone (GH: 2.9 ng/ml) and GH response to a 75gr oral glucose load (nadir of GH: 1 ng/ml) were also normal. Testosterone [1.7 ng/ml (NR: 2.67–10.12 ng/ml)], DHEA-S [468 ng/ml (NR: 518–4707 ng/ml)] and insulin-like growth factor-1(IGF-1) levels [117 ng/ml (NR for sex and age: 180–406 ng/ml)] were decreased.
Adrenal computed tomography (CT) showed micronodular adrenal disease (Figure 1). Ultrasonography demonstrated multiple thyroid nodules and multiple microcalcifications of the testes, whereas echocardiography did not reveal any recurrent cardiac myxomas. Pituitary magnetic resonance imaging (MRI) was also normal.
Figure 1.
Adrenal computed tomography revealing bilateral nodularity of the adrenal glands.
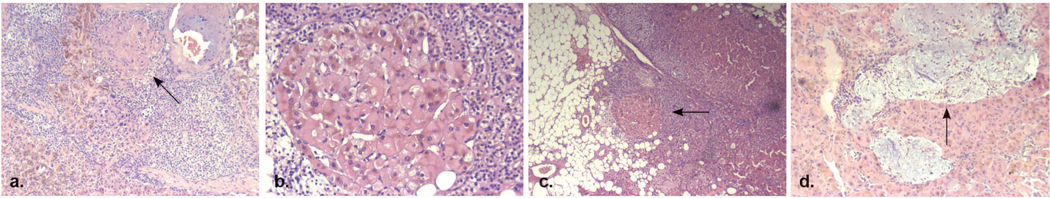
Denosumab was discontinued and the patient was prescribed ketoconazole (400mg/day) and metopyrone (1,5gr/day) while on the waiting list for bilateral adrenalectomy. Histopathology revealed multiple, 0.1–0.5cm sized, pigmented nodules surrounded by atrophic cortex. Histological results were consistent with the diagnosis of primary pigmented nodular adrenocortical disease (PPNAD) (Figure 2). Most of the nodule cells were large and globular with granular eosinophilic cytoplasm that included lipofuscin. Myxomatous areas were also detected within the nodules.
Figure 2.
Macroscopic appearance of the surgically removed PPNAD adrenal gland. Multiple pigmented micronodules can be seen at the cross section.
Sequencing analysis
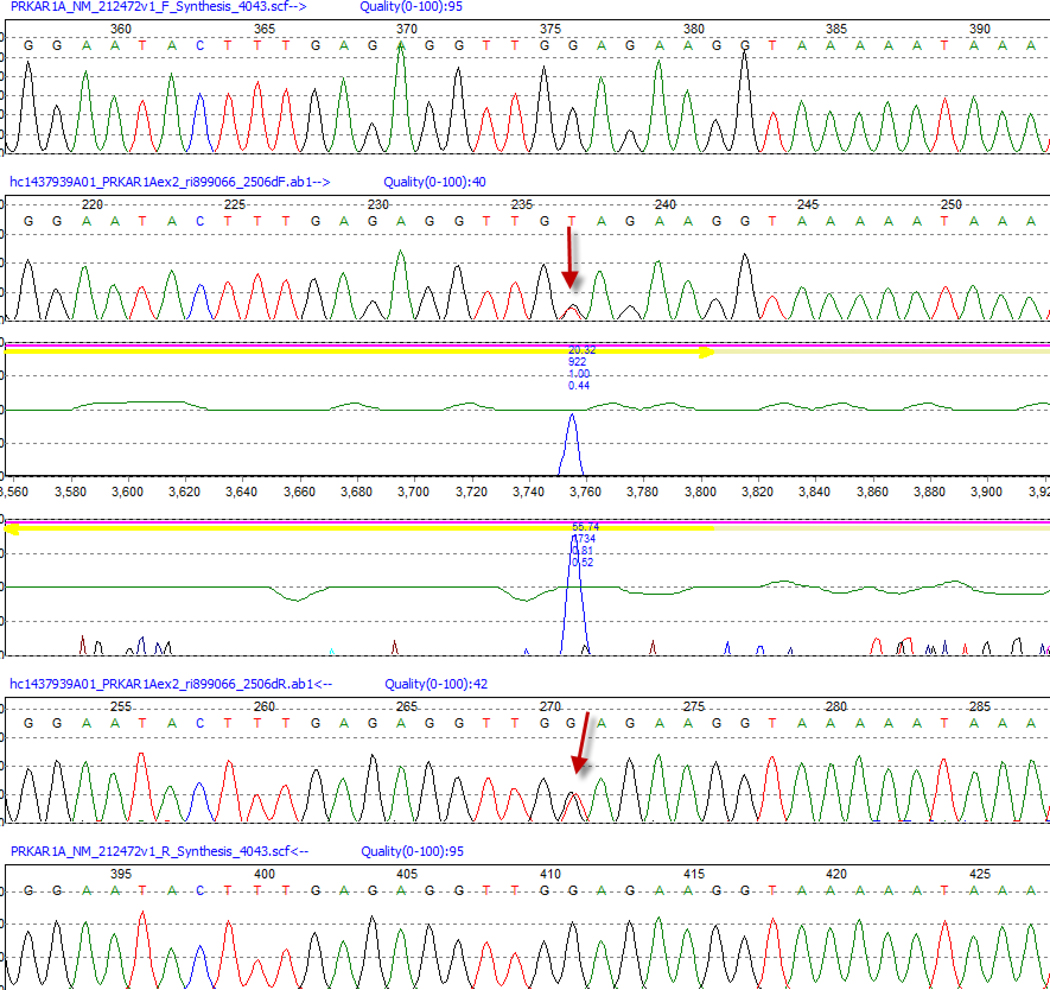
A peripheral blood sample was drawn after obtaining informed consent from the patient. DNA was extracted from peripheral leucocytes and subjected to polymerase chain reaction (PCR) followed by bidirectional DNA sequence analysis of the PRKAR1A gene was performed as described previously.6,14 The nucleotide sequence was compared with the published cDNA PRKAR1A sequence. A novel c.172G>T heterozygous point mutation at codon 172, of exon 2 was identified that resulted in direct stop-codon generation and in early termination of PRKAR1A transcript (Figure 3).
Figure 3.
Pathology of the adrenal glands. a. Classic appearance of PPNAD (x50) b. Nodular cortical cells containing large nucleus and eosinophilic cytoplasm with lipofuscin (x100). c. Expansion of cortical nodules to the adjacent adipose tissue (x25). d. Myxomatous areas within the nodule; hematoxylin and eosin stain (x50).
Discussion
CNC is a rare endocrine syndrome which is most frequently caused by PRKAR1A gene mutations. We report a novel germline nonsense mutation of the PRKAR1A gene in a patient with clinical features as well as laboratory and histological findings of CNC. Our patient had developed a wide, heterogeneous spectrum of CNC-associated manifestations including hypercortisolism with PPNAD and secondary osteoporosis, skin lentigines and myxomas, multiple recurrent cardiac myxomas and nodular thyroid disease. We also found a paradoxical increase of cortisol secretion after dexamethasone administration, which is characteristic of CNC and particularly useful for the diagnosis of patients with normal baseline cortisol levels and subclinical or cyclic CS.15
Osteoporotic bone changes are often found in CNC patients. Glucocorticoids’ excess accelerates bone resorption and reduces bone formation by direct action on bone cells. In addition, glucocorticoids decrease intestinal calcium absorption, by opposing the action of vitamin D, and by decreasing the expression of calcium channels in the duodenum leading to secondary hyperparathyroidism.16 They also inhibit IGF-1and serum testosterone production as observed in our patient).16, 17 Apart from the secondary osteoporosis due to PPNAD-associated cortisol hypersecretion, the osteogenic potential may be also be influenced by PRKAR1A gene ablation that can interfere with signaling pathways which are necessary for osteoblast differentiation, as shown in experimental data from mouse and human cell lines.18 Osteoporotic bone changes, fractures and secondary hyperparathyroidism that were found in our CNC patient, indicated the long duration of the disease.
Recurrent cardiac myxomas occur in much younger age in CNC patients compared to sporadic occurring myxomas).19 Interestingly, up to 30% of non-CNC myxomas can also bear PRKAR1A mutations, suggesting a potential role of the gene and the PKA pathway in the development of both syndromic and isolated cardiac myxomas.20 The PRKAR1A mutation found in our patient, caused lack of mutant protein detection due to degradation, similarly to other PRKAR1A mutations reported so far. Although genetic screening was not been performed in the close relatives of our patient, the absence of any characteristics of the disease suggests that this mutation is a de novo one. This finding is in accordance with previous studies that described de novo mutation in high frequency (85%) in sporadic cases with CNC.6
In contrast to RET mutations in MEN2 syndrome, it is widely accepted that PRKAR1A gene mutations do not appear to correlate consistently with a specific clinical phenotype. However, overall data indicate that CNC patients bearing PRKAR1A mutations (CNC1) have a more severe disease with earlier presentation and higher frequency of myxomas, thyroid and gonadal tumors, schwannomas, and lentigines compared to PRKAR1A negative patients with a mutation mapped in the 2p16, CNC2 locus.2,4,14 In addition, a small number of PRKAR1A missense mutations whose mRNA escape from NMD and express R I-α mutant proteins are associated with a more severe phenotype.21,22 Two PRKAR1A mutations (a deletion c.709-7del6 and a mutation in the initiation codon of PRKAR1A, M1V c.1A>G/p.M1Vsubstitution) are associated with low-penetrance, early life isolated PPNAD and CS.23, 24 Moreover, in some cases of isolated PPNAD, a small intronic deletion in PRKAR1A has been associated with a lower penetrance and mild phenotype. 25 In general, patients with exonic PRKAR1A mutations seem to present at a younger age and to manifest cardiac myxomas, lentigines, schwannomas and acromegaly, more often compared to patients with intronic ones.
In the study of Salpea et al, a significant number (21.6%) of CNC patients had haploinsufficiency due to large 17q24.2-q24.3 deletions surrounding the PRKAR1A gene.10 These deletions were not detected by Sanger sequencing (PRKAR1A mutation-negative) but with array-based comparative genomic hybridization (array-CGH). It is worth noting that apart from the usual CNC-related manifestations, some of these patients shared skeletal abnormalities and global developmental delay. Moreover, the average age of presentation was younger (14 years) and morbidity more severe than in typical PRKAR1A mutation-positive CNC patients. In addition, in a family of CNC patients, a spectrum of disease including adrenal carcinoma due to a S147G point mutation in PRKAR1A gene that escapes NMD was identified. This mutation led to decreased cAMP and catalytic subunit binding by R I-α and increased PKA activity in vitro.11
Lately, genes coding for other PKA subunits were identified to be responsible for CNC-linked phenotypes (but not the full syndrome). Duplication of the main catalytic subunit of the cAMP-dependent PKA, Cα (PRKACA), may result in Cushing syndrome caused by bilateral micronodular adrenal hyperplasia.12 In a single patient, triplication of the catalytic subunit Cβ (the PRKACB gene) was associated with skin pigmentation, acromegaly and myxomas.13
All the aforementioned genetic data could have implications on our understanding of the CNC phenotype and more importantly for counseling CNC patients. Genetic analysis is a powerful tool aiding the investigation, confirmation and early identification of tumors and endocrine disorders related to CNC. Younger age of onset and potential diverse prognosis according to the genotype could help us properly counsel patients’ family members and descendants. Even though there is yet no obvious and straightforward correlation between genotype findings and the clinical characteristics of the disease, there is growing evidence that a genotype-phenotype association is plausible for at least certain PRKAR1A defects.
In conclusion, we have identified a novel PRKAR1A nonsense mutation in a sporadic case of CNC. The data add to what we know about PRKAR1A and human disease. Recognizing CNC is important to identify early and address the many associated comorbidities including acromegaly, atrial fibrillation and stroke due to atrial myxomas, diabetes mellitus, hypertension, osteoporosis and fractures due to hypercortisolism, and the risk of various tumors and certain cancers. PRKAR1A mutation analysis should be undertaken in suspected cases of CNC in order to confirm the diagnosis, provide close monitoring and follow-up.
Figure 4.
Direct sequencing of leukocytes’ RT-PCR products demonstrating the p.E58Xc.172 GAG>TAG mutation of the PRKAR1A gene.
Acknowledgements/Funding
This work was supported by the Intramural Research Program (IRP) of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD 20892 USA.
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to disclose
References
- 1.Carney JA, Gordon H, Carpenter PC, et al. , 1985. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64: 270–283. [DOI] [PubMed] [Google Scholar]
- 2.Bertherat J, Horvath A, Groussin L, et al. , 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94: 2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey M, Mah C, Merliss AD, et al. , 1998. Identification of a novel genetic locus for familial cardiac myxomas and Carney complex. Circulation 98: 2560–2566. [DOI] [PubMed] [Google Scholar]
- 4.Stratakis CA, Carney JA, Lin JP, et al. , 1996. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest 97: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veugelers M, Bressan M, McDermott DA, et al. , 2004. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. N Engl J Med 351: 460–469. [DOI] [PubMed] [Google Scholar]
- 6.Kirschner LS, Carney JA, Pack SD, et al. 2000. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 26: 89–92. [DOI] [PubMed] [Google Scholar]
- 7.Veugelers M, Wilkes D, Burton K, et al. , 2004. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A 101: 14222–14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groussin L, Kirschner LS, Vincent-Dejean C, et al. , 2002. J Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet 71: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath A, Bertherat J, Groussin L, et al. , 2010. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat 31: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salpea P, Horvath A, London E, et al. , 2014. Deletions of the PRKAR1A locus at 17q24.2-q24.3 in Carney complex: genotype-phenotype correlations and implications for genetic testing. J Clin Endocrinol Metab 99: E183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anselmo J, Medeiros S, Carneiro V, et al. , 2012. A large family with Carney complex caused by the S147G PRKAR1A mutation shows a unique spectrum of disease including adrenocortical cancer. J Clin Endocrinol Metab 97: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beuschlein F, Fassnacht M, Assié G, et al. , 2014. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med 370: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forlino A, Vetro A, Garavelli L, et al. , 2014. PRKACB and Carney complex N Engl J M 370: 1065–1067. [DOI] [PubMed] [Google Scholar]
- 14.Kirschner LS, Sandrini F, Monbo J, et al. , 2000. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet 9: 3037–3046. [DOI] [PubMed] [Google Scholar]
- 15.Stratakis CA, Sarlis N, Kirschner LS, et al. , 1999. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 131: 585–591. [DOI] [PubMed] [Google Scholar]
- 16.Canalis 2007, Huybers S, Naber TH, et al. , 2007 Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol 292: G92. [DOI] [PubMed] [Google Scholar]
- 17.MacAdams MR, White RH, Chipps BE 1986. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med 104: 648. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Manchanda PK, Wu D, et al. , 2014. Knockdown of PRKAR1A, the gene responsible for Carney complex, interferes with differentiation in osteoblastic cells. Mol Endocrinol 28: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzam R, Abdelbar A, Yap KH, et al. , 2014 Carney complex: fourth time excision of recurrent atrial myxoma via left thoracotomy. BMJ Case Rep 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maleszewski JJ, Larsen BT, Kip NS, 2014. PRKAR1A in the development of cardiac myxoma: a study of 110 cases including isolated and syndromic tumors. Am J Surg Pathol 38: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 21.Greene EL, Horvath AD, Nesterova M, et al. , 2008. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat 29: 633–639. [DOI] [PubMed] [Google Scholar]
- 22.Meoli E, Bossis I, Cazabat L, et al. 2008. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res 68: 3133–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groussin L, Jullian E, Perlemoine K, et al. , 2002. Mutations of the PRKAR1A gene in Cushing’s syndrome due to sporadic primary pigmented nodular adrenocortical disease.J Clin Endocrinol Metab 87: 4324–4329. [DOI] [PubMed] [Google Scholar]
- 24.Pereira AM, Hes FJ, Horvath A, et al. , 2010. Association of the M1V PRKAR1A mutation with primary pigmented nodular adrenocortical disease in two large families. J Clin Endocrinol Metab 95: 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groussin L, Horvath A, Jullian E,et al. , 2006. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab 91: 1943–1949. [DOI] [PubMed] [Google Scholar]