Abstract
Background
Despite the increasingly recognized impact of novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), on many aspects of health in adults and children, its effects on neonates born to infected mothers remain unclear. We conducted this study to investigate the outcomes of neonates born to mothers with COVID-19.
Methods
We searched the medical databases from inception to March 31, 2020 to perform a systematic review of outcomes in neonates born to mothers with COVID-19. Data were pooled using a random effects regression model. Primary and secondary outcomes were neonatal clinical outcomes and infectious status, respectively.
Results
Fourteen studies involving 105 neonates fulfilling the study criteria were identified. The rates of preterm neonates and those small for gestational age (SGA) were 25 (23.8%) and 10 (11.2%), respectively. Among 91 neonates who were tested, 8 (8.8%) were positive for nucleic acids or antibodies for SARS-CoV-2. Additionally, 28 (26.7%) of the neonates were symptomatic and two test-negative neonates died, including one stillbirth. Between test-positive and test-negative groups, the rates of SGA, preterm delivery, duration between maternal symptom onset and delivery, and perinatal complication were not significantly different; but the rate of symptomatic after birth reached significant difference (62.5% vs 20.5%, p = 0.008).
Conclusions
Most neonates born to infected mothers had favorable outcomes. Although direct evidences of intrauterine infection were scarce, the risk of intrauterine infection should be considered based on a positive test in 8.8% of the neonates. Symptomatic neonates born to infected mothers should receive tests for SARS-CoV-2 to initiate appropriate treatment and quarantine. Further studies are warranted to assess the outcomes of COVID-19 in neonates.
Keywords: Novel coronavirus, COVID-19, SARS-CoV-2, Vertical transmission, Intrauterine infection, Neonate
Introduction
Coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) is an emerging disease with a massive disease burden.1, 2, 3, 4 The main manifestations of COVID-19 include fever and respiratory symptoms; however, the clinical presentations are protean. Infected patients might have gastrointestinal symptoms, anosmia, hyposmia, or dysgeusia5 , 6 or may otherwise be asymptomatic; thus, controlling the disease spread is difficult. Approximately 20% of infected patients have severe disease, and the approximate case-fatality rate is 5%–6%.6, 7, 8 Older individuals and those with underlying diseases are at a higher risk for poor outcomes. Conversely, the effect of COVID-19 on pregnancy remains largely unclear although pregnant women are susceptible to various viral infections such as influenza virus and SARS.
In a retrospective study of 38 SARS-CoV-2-infected mothers, Schwartz et al. reported that the clinical manifestations of infected mothers were similar to those who were not pregnant.9 No additional pregnancy risk was found in patients with COVID-19, and there was no evidence of intrauterine infection.9 However, in a study of 33 neonates, Zeng et al. reported that three neonates had a positive nucleic acid test for SARS-CoV-2.10 The complete features of SARS-CoV-2-infected pregnant women and neonates born to infected mothers remain largely unclear. Therefore, we conducted a systematic review to investigate the clinical features of neonates born to mothers with COVID-19.
Methods
Study design and study selection
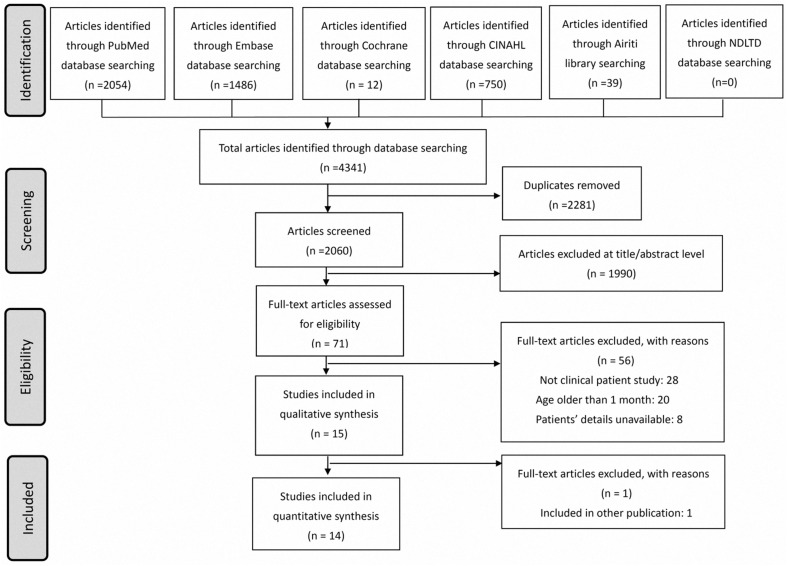
Our study was approved by the Institutional Review Board of the MacKay Memorial Hospital, Taipei, Taiwan (approval number: 20MMHIS140e). We performed the present systematic review and meta-analysis in accordance with the PRISMA guidelines (Fig. 1 , Supplementary Table 1).11
Figure 1.
Schematic illustration of the literature search and the study-selection criteria; assessed on 01Apr2020. Abbreviations: Airiti database: Art Image Indexing Service on the Internet Database (Chinese database); CINAHL database: the cumulative index to nursing and allied health literature; NDLTD database: National Digital Library of Theses and Dissertations in Taiwan.
Websites; Airiti: https://www.airitilibrary.com/.
NDLTD: https://etds.ncl.edu.tw/cgi-bin/gs32/gsweb.cgi/ccd=oLFRX3/webmge?switchlang=en.
In the present systematic review, we used comprehensive keywords with Boolean operators and MeSH terms to perform electronic search of the following medical databases from inception to March 31, 2020, supplemented with hand searching: PubMed/Medline, EMBASE, Cumulative Index to Nursing and Allied Health Literature, National Digital Library of Theses and Dissertations in Taiwan database, Art Image Indexing Service on the Internet Database (Chinese database), and the Cochrane database. The search was performed independently by two authors, and disagreements were resolved through discussion with the third author. Briefly, the electronic search included the keywords “COVID-19,” “severe acute respiratory syndrome coronavirus 2,” “2019-nCoV,” “SARS-CoV-2,” and “Wuhan.” No constraints were placed on language, year of publication, and participant characteristics to ensure a comprehensive search and identify the maximum number of potential articles. Authors of specific articles were contacted to obtain additional information if necessary.
Data extraction, quality assessment, and data synthesis and analysis
Studies investigating “pregnancy,” “pregnant,” “mother,” “neonates,” “infant,” or “children” were analyzed. Exclusion criteria were duplicate publications, irrelevant articles, studies where the status of children was not clearly defined, studies that did not evaluate clinical outcomes, and review articles. Primary and secondary outcomes were neonatal clinical outcomes and infectious status, respectively.
Furthermore, two authors independently appraised the selected articles and extracted the following data: name of the first author, study country, participant population, gestational age, perinatal complications, mode of delivery, neonatal outcomes, site of sampling, and COVID-19 test results. Preterm delivery was defined as gestational age less than 37 complete weeks. Poor outcomes of mothers or neonates were defined as requiring intensive care, mechanical ventilator or inotropic agents use, multiple organ failure, extracorporeal membrane oxygenation use, or mortality. In case of a disagreement between two authors, consensus was reached through discussion with the third author. The quality assessment was conducted independently by two authors based on the domains of selection, ascertainment, causality and reporting.12
Statistical analyses
If continuous outcomes were assessed, data were analyzed using odds ratios with 95% confidence intervals. For categorized variables, comparison was performed using Student's t test and the chi-square test. If meta-analysis was performed, we used a random-effects regression model assuming that the true effect size was not the same.13 Heterogeneity was further quantified using the Cochran Q test and I 2 statistics.14 A p value less than 0.05 was regarded as statistically significant. Review Manager v5.3.5 (Cochrane Community, London, UK) and SPSS version 23.0 (SPSS, Chicago, IL, USA) were used for statistical analyses.
Results
Description of studies and quality assessment
The search process is illustrated in Fig. 1. As of March 31, 2020, 2060 articles were identified by the medical database search. The titles and abstracts of all articles were screened and 71 full-text articles were carefully reviewed. Finally, 14 studies fulfilling the inclusion and exclusion criteria were included in the final systematic review.10 , 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27
Clinical features and neonatal outcomes
The clinical features of the included studies are summarized in Table 1 . Most studies had good quality. All studies were conducted in China, and 107 mothers with confirmed COVID-19 were identified. Three mothers had not delivered at the publication time of the original study, and there were a total of 105 neonates including one set of twins. The mothers were aged between 22 and 40 years, and 43 mothers developed perinatal complications including preeclampsia, placenta previa, placenta abruptio, fetal distress, premature rupture of membranes, and uterine scarring. Most mothers had uneventful recovery except for one mother who developed acute respiratory distress syndrome and multiple organ failure requiring extra-corporeal membrane oxygen.20 Majority of the mothers (87.6%) underwent cesarean section (CS), and 25 (23.8%) neonates were delivered prematurely. 76 (83.5%) mothers had fever or respiratory symptoms. Among mothers with clear records of onsets, 8 (22.2%) mothers developed symptoms after delivery. After birth, 28 (26.7%) neonates exhibited symptoms including fever, tachypnea, shortness of breath, and vomiting. There was one stillbirth, and one neonate died following gastric bleeding and multiple organ failure.20 , 26
Table 1.
Clinical characteristics of the included studies.
| Study ref | Maternal population (N) | Neonatal population (N) | Maternal age (years) | Preterm delivery (N) | Perinatal complicationsa (N) | CS (N) | SGA (N) | Maternal symptoms? (N) | Maternal symptoms onset to delivery (days)b | Maternal symptoms onset to delivery (test-positive) | Neonatal symptomsc (N) | Intensive care (N) | Neonatal poor outcomes (N) | Maternal poor outcomes (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al.15 | 9 | 9 | 26–40 | 4 | 5 | 9 | 2 | 9 | 1, 1, 2, 2, 3, 4, 4, 6, 7 | 0 | 0 | 0 | 0 | |
| Chen et al.16 | 3 | 3 | 23–34 | 1 | 3 | 3 | 1 | 3 (2 after delivery) | NR | 1 | 0 | 0 | 0 | |
| Chen et al.27 | 5 | 5 | 25–31 | 0 | 3 | 2 | 0 | 5 (3 after delivery) | −1, −1, −1, 1, 10 | 0 | 0 | 0 | 0 | |
| Dong et al.17 | 1 | 1 | 29 | 0 | 0 | 1 | 0 | 1 | 25 | 25 | 0 | 0 | 0 | 0 |
| Fan et al.18 | 2 | 2 | 29, 34 | 1 | 0 | 2 | 0 | 2 | 7, 11 | 2 | 0 | 0 | 0 | |
| Li et al.19 | 1 | 1 | 30 | 1 | 1 | 1 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | |
| Liu et al.20 | 13 | 10 | 22–36 | 6 | 5 | 10 | NR | 9 | NR | 0 | 0 | 1 | 1 | |
| Wang et al.21 | 1 | 1 | 34 | 0 | 0 | 1 | 0 | 1 | 0.5 | 0.5 | 1 (vomiting) | 0 | 0 | 0 |
| Wang et al.22 | 1 | 1 | 28 | 1 | 1 | 1 | 1 | 1 | 6 | 0 | 0 | 0 | 0 | |
| Yu et al.23 | 7 | 7 | 29–34 | 0 | 3 | 7 | 0 | 7 | NR | NR | 1 | 0 | 0 | 0 |
| Zeng et al.24 | 6 | 6 | NR | 0 | NR | 6 | NR | 6 | NR | NR | 0 | 0 | 0 | 0 |
| Zeng et al.10 | 33 | 33 | NR | 4 | 3 | 26 | 3 | 22 | 1, 2, 4 | 1, 2, 4 | 4 | 1 | 0 | 0 |
| Zhang et al.25 | 16 | 16 | 24–34 | 1 | 12 | 16 | 1 | NR | NR | 6 | 0 | 0 | 0 | |
| Zhu et al.26 | 9 | 10 | 25–35 | 6 | 7 | 7 | 2 | 9 (3 after delivery) | −3, −2, −1, 0, 0, 1, 3, 3, 4, 6 | 9 | 1 | 1 | 0 |
| Study ref | Neonatal population (N) | Neonates tested (N) | Positive Ab (N) | Positive PCR (N) | Sites of PCR sampling | Suggestive vertical transmission? |
|---|---|---|---|---|---|---|
| Chen et al.15 | 9 | 6 | 0 | Amniotic fluid, cord blood, throat swab, breastmilk | No | |
| Chen et al.16 | 3 | 3 | 0 | Pharyngeal swab, placenta | No | |
| Chen et al.27 | 5 | 5 | 0 | Pharyngeal swab | No | |
| Dong et al.17 | 1 | 1 | 1 | 0 | Negative PCR: swab; positive IgG/IgM | Yes |
| Fan et al.18 | 2 | 2 | 0 | Newborn's nasopharyngeal swab, maternal serum, placenta, cord blood, amniotic fluid, vaginal swabs, and breast milk | No | |
| Li et al.19 | 1 | 1 | 0 | Amniotic fluid, cord blood, breastmilk, pharyngeal swab, blood, feces, and urine samples | No | |
| Liu et al.20 | 10 | 10 | 0 | No clinical or serologic evidence suggestive of vertical transmission of SARS-Co-V2 | No | |
| Wang et al.21 | 1 | 1 | 1 | Pharyngeal swab at 36 h; negative cord blood, placenta, breastmilk | Possible | |
| Wang et al.22 | 1 | 1 | 0 | Amniotic fluid, placenta, cord blood, gastric juice, throat swab, and stool sample | No | |
| Yu et al.23 | 7 | 3 | 1 | Throat swab at 36 h; negative cord blood and placenta | Possible | |
| Zeng et al.24 | 6 | 6 | 2 | 0 | Negative PCR: throat, serum; positive IgG/IgM | Yes |
| Zeng et al.10 | 33 | 33 | 3 | Nasopharyngeal and anal swabs on days 2 and 4 | Yes | |
| Zhang et al.25 | 16 | 10 | 0 | Pharyngeal swab | No | |
| Zhu et al.26 | 10 | 9 | 0 | Pharyngeal swab | No |
Abbreviations: Ab, antibody; CS, cesarean section; NR, not reported; PCR, polymerase chain reaction; SGA, small for gestational age.
Perinatal complications included preeclampsia, placenta previa, placenta abruptio, fetal distress, premature rupture of membrane, and uterine scarring. Poor outcomes of mothers or neonates were defined as requiring intensive care, morbidity mechanical ventilator or inotropic agents use, multiple organ failure, extracorporeal membrane oxygenation use, or mortality.
Days referred to duration between maternal symptom onset and delivery; a negative day meant symptom onset after delivery.
Neonatal symptoms included fever, tachypnea, shortness of breath, and vomiting.
Risk of vertical transmission and tests for SARS-CoV-2
Considering the risk of vertical transmission, 91 (86.7%) neonates were tested for SARS-CoV-2 by a nucleic acid or antibody test. Five neonates were positive by the nucleic acid test, and three neonates were positive for SARS-CoV-2 antibodies. The authors of three enrolled studies thought the risk of vertical transmission existed10 , 17 , 24; two studies found positive nucleic acid at 36 h that postnatal infections were unable to be completely excluded.21 , 23 No evidence of vertical transmission was found in the rest of the studies.
Of the 105 neonates, 91 neonates were tested for SARS-CoV-2 and 8 (8.8%) neonates had a positive nucleic acid or antibody test result. Further comparison of the clinical manifestations between the test-positive and test-negative neonates (Table 2 ) revealed that the rates of neonates who were small for gestational age (SGA) and those exhibiting symptoms after birth were higher among those with a positive test. However, the rates of preterm delivery and perinatal complications were lower among the test-positive neonates. Between both groups, the rates of SGA, preterm delivery, duration between maternal symptom onset and delivery, and perinatal complication were not significantly different but the rate of symptomatic after birth reached significant difference.
Table 2.
Comparison of clinical characteristics between test-positive and test-negative neonates.
| Clinical variablesa | Preterm delivery | Perinatal complications | Maternal symptom onset to delivery (days) | CS | SGA | Symptomatic neonates | Positive PCR or Ab |
|---|---|---|---|---|---|---|---|
| All neonates (105) | 25 (23.8) | 43 (42.6) | 3.32 | 92 (87.6) | 10 (11.2) | 28 (26.7) | 8 (8.8) |
| Test-negative (83) | 22 (26.5) | 34/79 (43) | 2.75 ± 3.5 | 73 (88) | 7/69 (10.1) | 17 (20.5) | |
| Test-positive (8) | 1 (12.5) | 1/6 (16.7) | 6.5 ± 10.4 | 8 (100) | 1/6 (16.7) | 5 (62.5) | |
| p | 0.384 | 0.189 | 0.469 | 0.383 | 0.6 | 0.008 |
Ab, antibody; CS, cesarean section; PCR, polymerase chain reaction; SGA, small for gestational age.
Presented as numbers (%).
Discussion
The present systematic review evaluating the currently unknown impact of SARS-CoV-2 on neonates born to mothers with COVID-19 revealed that most neonates had a favorable outcome. Importantly, 8.8% of the tested neonates were positive for SARS-CoV-2, indicating that the risk of vertical transmission should be considered. Compared with the test-negative neonates, the rates of SGA, preterm delivery, duration between maternal symptom onset and delivery, and perinatal complication were not significantly different but the rate of symptomatic after birth reached significant difference between both groups. Therefore, further studies are warranted to elucidate the impact of COVID-19 on pregnancy and neonates.
Pregnant women have unique physiologic changes and are susceptible to several viral infections such as influenza and SARS.28 , 29 The present systemic review to address the concerns regarding COVID-19 infection in pregnant women revealed that the clinical manifestations of COVID-19 were similar between mothers and non-pregnant individuals. All but one mother had relatively favorable outcomes.20 Pregnant women had similar clinical presentations and risk of severe complications compared with non-pregnant individuals of the same age group.5 , 6 The low risk of severe COVID-19 in pregnant women might be due to the relatively younger patient age and the absence of underlying diseases. However, the expression of angiotensin-converting enzyme 2, a predicted SARS-CoV-2 receptor, was reported to be increased during pregnancy.30 , 31 Moreover, the increased expression of angiotensin-converting enzyme 2 was observed in adolescents with preterm delivery in previous report and preterm neonates might have higher risk of COVID-19.32 We found a lower rate of preterm delivery in test-positive neonates but the difference was not statistically significant. Despite the increasing number of studies investigating COVID-19, the exact underlying pathophysiology has not been elucidated and further studies are warranted to clarify the relationship between SARS-CoV-2 and pregnancy.
When taking care of expectant mothers with COVID-19, the risk of intrauterine infection and vertical transmission was an important concern. Eight of the studies included in the systematic review found no evidence of vertical transmission and could not detect the virus in amniotic fluid, cord blood, breast milk, serum, feces, placenta, nasopharyngeal, rectal, or vaginal swabs.15 , 16 , 18, 19, 20 , 22 , 25 , 26 However, two of the included studies found elevated levels of SARS-CoV-2 immunoglobulin (Ig) G and IgM antibodies in three neonates; however, the nucleic acid tests were negative, and the evidence was not convincing.17 , 24 IgM is a larger molecule that does not usually transfer from mother to fetus and does not appear until 3–7 days after infection. The presence of neonatal SARS-CoV-2 IgM raises the possibility of intrauterine infection. However, there are several kinds of antibody tests against SARS-CoV-2 and the concerns regarding the sensitivity and specificity of present available antibody tests exist. Maternal IgM may reach fetal circulation in case of placental inflammation. These studies with positive neonatal IgM provide evidence for additional investigation. Furthermore, SARS-CoV-2 nucleic acids were detected in five neonates.10 , 21 , 23 However, the strength of this direct evidence might be challenged by the sampling time of 24–36 h after birth thus postnatal infection cannot be completely excluded. Naturally the fetal circulation bypass lungs and virus might be not lodged in the airway mucosa before delivery. However, the authors claimed strict infectious control measurements had been implemented for neonates after birth thus the risk of postnatal infection was minimal.10 , 21 , 23 Further nucleic acid tests performed right after delivery were valuable to elucidate the origin of infection and the route of infection remained largely unclear based on present evidences. Additionally, our analyses determined that the rate of neonates exhibiting symptoms after birth was higher among the test-positive neonates than in the test-negative neonates (Table 2, 62.5% vs 20.5%, p = 0.008), which might reflect the possible role of congenital infections. We also observed a longer duration between maternal symptom onset and delivery in test-positive neonates (Table 2, 6.5 vs 2.75 days, p = 0.469). Patients with COVID-19 might have deteriorated clinical conditions in approximate 7 days after symptom onset.33 The coincidence of 6.5 and 7 days caught our attention but we failed to identify the significant predictive factors of neonatal infection, including maternal fever, respiratory symptoms, laboratory findings, or perinatal conditions. Despite the controversies, the present systematic review demonstrated that 8.8% of the neonates had a positive nucleic acid or antibody test and that the risk of vertical transmission should be considered. Further studies utilizing both nucleic acid and antibody tests will be valuable to provide further evidence for correlations between clinical symptoms and viral tests. Further direct evidences from amniotic fluid, placenta, umbilical cord and neonates are valuable to draw a definite conclusion.
We found that 26.7% of the neonates born to mothers with COVID-19 were symptomatic after birth and that most of the symptoms were mild. Additionally, 25 neonates were delivered preterm and cesarean section was performed in 87.6% of the cases. Reasons of CS included uncertainty about the risk of intrapartum mother-to-child transmission by vaginal delivery, severe pre-eclampsia, fetal distress, and previous CS. Neonates with preterm delivery and CS are at a higher risk of transient tachypnea of newborn, and determining whether the observed symptoms were due to SARS-CoV-2 infection is difficult. Although the rate of symptomatic neonates was higher among those with a positive test, the casual relationship could not be clarified. Moreover, cesarean section was performed in the majority of mothers to control perinatal complications and infections, and 13 neonates who were delivered via normal spontaneous labor were negative for SARS-CoV-2. Further studies are warranted to investigate the need for cesarean section to reduce the rate of perinatal infections including SARS-CoV-2.
The present study has several limitations that should be acknowledged. First, the results of laboratory tests, inflammatory markers, and imaging studies were not available in some studies. Although most neonates had favorable outcomes, details of infectious survey are necessary for a better evaluation of the COVID-19 infection status and to obtain a more complete picture of neonatal COVID-19. Second, the situation of COVID-19 pandemic changed rapidly and bigger cohorts will be helpful for a better assessment of COVID-19's impact on neonates, including the risk of vertical transmission and breastfeeding, differences based on gestational age, and impact of the duration between exposure and delivery. Third, although the quality of most studies were good, case series didn't provide high-ranked evidences. Randomized controlled studies were appreciated but it's ethically impossible to conduct a randomized control study. Finally, all enrolled studies were conducted in China, the neonatal outcomes may differ across races, geographic regions, healthcare resources, and time. Further studies are necessary to address these important questions.
Conclusions
In conclusion, the present systematic review of 14 studies comprising 105 neonates born to mothers with COVID-19 revealed that the outcome was favorable in most neonates. However, 8.8% neonates had a positive nucleic acid or antibody test. Although direct evidences of vertical transmission remained scarce, the risk of vertical transmission should be considered. Neonates with a positive test had a higher rate of symptoms after birth, a finding warranting further investigation. Symptomatic neonates born to mothers with COVID-19 should receive testing for SARS-CoV-2 to initiate appropriate quarantine and treatment.
Funding
We received no funding and we thanked everyone's efforts to combat COVID-19.
Ethical approval
This study was approved by the ethical committee of MacKay Memorial Hospital (No.: 20MMHIS140e).
Declaration of competing interest
All authors had no competing interests to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2020.07.024.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P.I., Hsueh P.R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020;53:365–367. doi: 10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.M., Tan T.W., Ho T.C., Chen C.C., Su T.H., Lin C.Y. COVID-19: Taiwan's epidemiological characteristics and public and hospital responses. PeerJ. 2020;8 doi: 10.7717/peerj.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2648. [published Online First: 25/02/20] [DOI] [PubMed] [Google Scholar]
- 7.WHO Coronavirus disease (COVID-19) outbreak situation 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from:
- 8.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. [published Online First: 18/03/20] [DOI] [PubMed] [Google Scholar]
- 10.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 12.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 17.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. J Am Med Assoc. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020:ciaa226. doi: 10.1093/cid/ciaa226. [published Online First: 18/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26:1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [published Online First: 08/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [published Online First: 13/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. [published Online First: 03/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W. Antibodies in infants born to mothers with COVID-19 pneumonia. J Am Med Assoc. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [published Online First: 30/03/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamieson D.J., Honein M.A., Rasmussen S.A., Williams J.L., Swerdlow D.L., Biggerstaff M.S. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 30.Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953–R1961. doi: 10.1152/ajpregu.90592.2008. [DOI] [PubMed] [Google Scholar]
- 31.Devaux C.A., Rolain J.-M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.South A.M., Nixon P.A., Chappell M.C., Diz D.I., Russell G.B., Jensen E.T. Association between preterm birth and the renin-angiotensin system in adolescence: influence of sex and obesity. J Hypertens. 2018;36:2092–2101. doi: 10.1097/HJH.0000000000001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.