Abstract
Background
Sanguinarine (SAG), a benzophenanthridine alkaloid, occurs in Papaveraceas, Berberidaceae and Ranunculaceae families. Studies have found that SAG has antioxidant, anti-inflammatory, and antiproliferative activities in several malignancies and that it exhibits robust antibacterial activities. However, information reported on the action of SAG against Providencia rettgeri is limited in the literature. Therefore, the present study aimed to evaluate the antimicrobial and antibiofilm activities of SAG against P. rettgeri in vitro.
Methods
The agar dilution method was used to determine the minimum inhibitory concentration (MIC) of SAG against P. rettgeri. The intracellular ATP concentration, intracellular pH (pHin), and cell membrane integrity and potential were measured. Confocal laser scanning microscopy (CLSM), field emission scanning electron microscopy (FESEM), and crystal violet staining were used to measure the antibiofilm formation of SAG.
Results
The MIC of SAG against P. rettgeri was 7.8 μg/mL. SAG inhibited the growth of P. rettgeri and destroyed the integrity of P. rettgeri cell membrane, as reflected mainly through the decreases in the intracellular ATP concentration, pHin and cell membrane potential and significant changes in cellular morphology. The findings of CLSM, FESEM and crystal violet staining indicated that SAG exhibited strong inhibitory effects on the biofilm formation of P. rettgeri and led to the inactivity of biofilm-related P. rettgeri cells.
Keywords: Sanguinarine, Providencia rettgeri, Antimicrobial, Antibiofilm
Introduction
Providencia rettgeri (P. rettgeri) is a common gram-negative bacilli from the Enterobacteriaceae family and is often isolated from wounds, the urinary tract, reptile faces, and human blood (Wie, 2015). It may cause gastroenteritis and bacteremia (Wie, 2015), which are considered the primary causes of travelers’ diarrhea (Yoh et al., 2005). P. rettgeri is considered a hospital pathogen that often causes urinary tract infections (Armbruster et al., 2014). The first infection of multidrug-resistant P. rettgeri in a neonate reportedly caused late-onset neonatal sepsis (Sharma, Sharma & Soni, 2017), which was resistant to ampicillin, polymyxins, and first-generation cephalosporins (Magiorakos et al., 2012); moreover, the treatment of P. rettgeri infection was challenging. In recent, P. rettgeri has been the focus of researchers due to the emergence of new multidrug-resistant clinical strain (Mbelle et al., 2020), which have threatened public health worldwide. More importantly, at the beginning of the 21st century, P. rettgeri caused two food poisoning outbreaks, as reported by the Chinese Center for Disease Control (Murata et al., 2001). This report revealed that the risk of exposure to P. rettgeri extended beyond individuals who traveled, were were hospitalized, or interacted with the general public.
Biofilms increase food safety risks by increasing the resistance of embedded bacteria to stress that is often encountered in food processing and by acting as a persistent source of microbial contamination (Giaouris et al., 2014). Thus, another major reason why P. rettgeri causes food contamination is the robustness of bacterial biofilms. Therefore, there is an urgent need to increase the supply of the ideal preservative against P. rettgeri in food, preferably a plant extract, given that food safety is crucial.
Sanguinarine (13-methyl(1,3)benzodioxolo(5,6-c)-1,3-dioxolo(4,5)phenanthridinium) (SAG) is an isoquinoline derivative and a benzophenanthridine alkaloid, with a molecular formula of C2H15NO5 (Yatoo et al., 2018). SAG occurs in Papaveraceas, Berberidaceae and Ranunculaceae families (Och et al., 2019). Previous studies have revealed that SAG has antimicrobial, antioxidant, and anti-inflammatory abilities (Firatli et al., 1994), as well as cytotoxic effects on soma cancer cells through its action on the Na+/K+-ATPase transmembrane protein. It is known that Na+/K+-ATPase with normal physiological functions maintains the resting potential and regulates cellular volume by pumping sodium out of cells and potassium into cells, both against their concentration gradients (Singh & Sharma, 2018). SAG is mainly utilized in veterinary clinic to promote the growth of livestock and poultry and prevent diarrhea. Of course, more efforts should be made to further evaluate its effectiveness and safety to meet its clinical application. A previous study reported that SAG possesses antimicrobial acidity against Psoroptes cuniculi (Miao et al., 2012).
Although the activity of SAG against some bacteria has been reported, to the best of our knowledge, its effect on P. rettgeri has not been reported. Therefore, the present study aimed to investigate the antimicrobial and antibiofilm activities of SAG against P. rettgeri.
Materials and Methods
Reagents
Sanguinarine (CAS:5578-73-4; Nanjing, China) was purchased from Nanjing Zelang Meditech Ltd., with a purity level of 98%. For all experiments, 10 mg/mL SAG in dimethyl sulfoxide (DMSO; Sigma–Aldrich, MI, USA) was employed as a stock concentration, and a 10-fold dilution of the stock was prepared in DMSO before addition to culture suspensions to obtain the required SAG concentrations.
Bacterial and culture condition
Providencia rettgeri can form normal biofilms. The clinical P. rettgeri strains used in this study included P. rettgeri-1, P. rettgeri-2, P. rettgeri-3, P. rettgeri-4, P. rettgeri-5, P. rettgeri-6 and P. rettgeri-7—the seven P. rettgeri isolates originally derived from the hydrothorax and urine of patients. The strains were routinely grown in tryptic soy broth (TSB) at 37 °C in a shaking incubator. The cell suspensions were diluted according to 0.5 McFarland standards to prepare inoculum densities of 2 × 106 colony-forming units (CFU)/mL for the antibacterial activity assay and those of 5 × 108 CFU/mL for the biofilm assay.
Minimum inhibitory concentration test
Evaluation of the minimum inhibitory concentration (MIC) was performed by following the broth microdilution (European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology & Infectious Dieases (ESCMID), 2000). The P. rettgeri culture was diluted into 96-well plate (Costar, Corning, NY, USA) using TSB at 106 CFU/mL. Tryptic soy agar (TSA) was mixed with SAG with final concentrations ranging from 0 to 500 μg/mL in the culture dish. After mixing, the tested bacteria were spotted on the media and incubated at 37 °C for further 24 h. The MIC value was regarded as the lowest SAG concentration at which no visibility of bacteria growth was sighted. The assays were performed independently thrice.
Growth curves
The method of Xu et al. (2017) for establishing growth curves was slightly modified to assess the antimicrobial effect of SAG on P. rettgeri. Then, the log-phase bacterial suspension (100 μL; OD600 = 0.5) was diluted 100-fold, and treated with SAG at different concentrations of 0, 1/8, 1/4, 1/2, 1 and 2 MIC. Subsequently, the samples were incubated at 37 °C with gentle shaking at 150 rpm. The cultures were sampled at 1 h-intervals and plated on TSA after adequate serial dilutions. Finally, the resulting plates were incubated at 37 °C for 24 h, and then the colonies were manually counted. The dynamic growth or inactivation curves were sets up for the viable cell counts.
Measurement of ATP levels
The method described by Sianglum et al. (2018) was employed to determine the intracellular ATP concentration of P. rettgeri with minor modifications. Briefly, P. rettgeri suspension (1 × 105 CFU/mL) was treated with 0, 1 and 2 MIC of SAG at 37 °C for 1, 2 and 3 h, respectively. Then, the cells were harvested by centrifugation (8,000×g, 10 min). Simultaneously, the supernatants were filtered and transferred to a test tube to measure the extracellular ATP concentration. The bacterial precipitates were washed twice with 1 mM phosphate-buffered saline (PBS; pH 7.0), suspended in the same PBS, and then placed on ice. Finally, the intracellular ATP was extracted by DMSO. ATP was measured using the ATP Assay Kit (Beyotime Bioengineering Institute, Shanghai, China), according to the manufacturer’s instructions.
Intracellular pH test
The intracellular pH (pHin) was evaluated according to the method presented by Qian et al. (2020). 300 μL overnight cultures of P. rettgeri were transferred to 30 mL TSB and then incubated at 37 °C for 8 h. After incubation, the cells were centrifuged (8,000×g, 10 min), rinsed twice with 50 mM HEPES and five mM EDTA (mixed solution at pH 8.0), and resuspended in 20 mL mixed solution. Subsequently, 3 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Beyotime Bioengineering Institute) was added to the sample. The sample was incubated at 37 °C for 20 min, then washed once with the mixture solution (pH 7.0; 50 mM PBS and 10 mM MgCl2), and resuspended in the buffer. To eliminate nonconjugated CFDA-SE, glucose (10 mM final concentration) was added and incubated at 37 °C for another 30 min. Cells were then washed twice, resuspended with 50 mM potassium phosphate buffer (pH 7.0), and stored in ice.
Fluorescently stained cell suspensions were treated with SAG (0, 1 and 2 MIC) and transferred into a black opaque 96-well flat-bottomed plate (Nunc, Copenhagen, Denmark, Europe). After treatment for 20 min, fluorescence intensity was assessed at excitation wavelengths of 440 and 490 nm and emission wavelength of 520 nm, where the excitation slit width was 9 nm and the emission were 20 nm. pHin was defined as the ratio of the fluorescence signal at the pH-sensitive wavelength of 490 nm to the fluorescence signal at the pH-insensitive wavelength of 440 nm. The measurements were performed by a fluorescence microplate reader (Thermo Fisher Scientific, Finland, Europe). During the experiment, the system was maintained at 25 °C, and the fluorescence of the control group was measured and subtracted from the value of the treated suspension.
The calibration curves were established for CFDA-SE-loaded cells with mixed solutions of different pH values. The mixture including glycine (50 mM), citric acid (50 mM), Na2HPO42H2O (50 mM) and KCl (50 mM). The pH was adjusted to various values (2–10) with NaOH or HCl, and valinomycin (10 μM) and nigrosine (10 μM) were added to adjust the pHin and pHout. Finally, fluorescence intensity was evaluated at 25 °C.
Determination of membrane potential
The membrane potential was determined according to the method by Wang et al. (2018), with minor modifications. The fluorescent probe bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) was used to determine the changes in bacterial membrane potential. Bacterial suspensions (1 × 105 CFU/mL) were centrifuged at 4,000×g for 10 min and washed twice with 10 mM PBS (pH 7.0). The cells were treated with different concentrations of SAG (0, 1 and 2 MIC) and incubated at 37 °C for 2 h. Then, the resulting samples were incubated with DiBAC4(3) at 25 °C for 10 min in the dark and washed once with PBS. The fluorescence microplate reader was utilized to determine the florescence intensity at excitation/emission wavelengths of 492/515 nm. The excitation and emission slit widths were 3 and 5 nm, respectively.
Evaluation of bacterial membrane integrity
The modified method of Liu et al. (2018) was applied to assess the cell membrane integrity of P. rettgeri cells using confocal laser scanning microscopy (CLSM; Zeiss LSM 880 with Airyscan, Bonn, Germany). Overnight cultures were diluted in TSB medium a concentration of 1 × 105 CFU/mL and treated with SAG (0, 1 and 2 MIC) for 2 h. Subsequently, the cells were harvested by centrifugation at 8,000×g for 5 min, and then resuspended with 10 mM PBS of equivalent volume. Then, the samples were mixed with SYTO 9 and propidium iodide (PI) at 25 °C for 15 min to visualize the cells. Cells were collected and washed with 10 mM PBS to remove excess dyes, and finally measured with a CLSM.
Transmission electron microscopy
Transmission electron microscopy (TEM) (G2 F20 S-Twin; FEI, Hillsboro, OR, USA) was used to analyze cell morphology as described by Joung et al. (2016) with some modification. Overnight cultures of P. rettgeri (1 × 105 CFU/mL) were diluted and exposed to three concentrations of SAG (0, 1, and 2 MIC) for 4 h at 37 °C. Thereafter, the cultures were centrifuged (10,000×g, 8 min), and washed twice with 0.85% NaCl. The suspension was removed, and cell pellets were fixed with 2.5% glutaraldehyde for 12 h at 37 °C. Then, cells were dehydrated through graded alcohols (20%, 50%, 70%, 80%, 90% and 100%) for 15 min; the resulting cell pellets were embedded in resin. Ultrathin samples were incised by ultramicrotome, and uranyl acetate stain was used for TEM.
Field emission scanning electron microscopy
After treatment with SAG at different concentrations, field emission scanning electron microscopy (FESEM) (Nova Nano SEM-450; FEI, Eindhoven, Netherlands, Europe) findings have revealed changes in the morphology of bacteria, as described previously (Shi et al., 2018). The log-phase cells were exposed to SAG at three concentrations (0, 1 and 2 MIC) and incubated for 4 h at 37 °C. The cultures were washed with 10 mM PBS (pH 7.0), and fixed with 2.5% glutaraldehyde for 12 h at 4 °C. The cells were dehydrated by sequential treatment with ethanol concentrations ranging from 30% to 100% for 10 min. Finally, the cells were fixed to the FESEM scaffold, gold sputtering was used to coat the cells under vacuum conditions, and FESEM was used to examine cell morphology.
Inhibition of the biofilm formation
Effects of SAG on the biofilm formation of P. rettgeri were quantitatively analyzed by crystal violet staining method, as described by Qian et al. (2020). P. rettgeri cells were incubated in the presence of SAG at 0, 1/32, 1/16, 1/8, 1/4, 1/2, 1 and 2 MIC for 24 h in a 96-well plate. After incubation, the cells washed twice with distilled H2O to remove the planktonic cells and loosen the adherent cells in the plate. For crystal violet staining assay, the biofilms were stained with 1% crystal violet (100 μL), incubated for 15 min at 28 °C, and washed twice with distilled H2O. Then, the absorbance was measured at 570 nm by a microplate reader. The specific biofilm formation index was determined by attaching and stained bacteria (OD570) normalized with the cell growth (OD630).
Moreover, the effects of SAG on the biofilm formation were qualitatively evaluated by FESEM and CLSM, as described previously (Qian et al., 2020). The slides of the cells were treated with different concentrations at 0, 1/16, 1/8 and 1/4 MIC, incubated for 24 h at 37 °C, washed thrice with 0.85% NaCl, fixed with 2.5% glutaraldehyde in 200 mM phosphate buffer for 4 h at 25 °C, dehydrated with different concentrations of ethanol gradient (30%, 60%, 70%, 80%, 90% and 100%) for 20 min for each concentration, and treated with gold spray. The biofilm formation of P. rettgeri was finally subjected to the examination under an FESEM.
Providencia rettgeri cells grown on the slides for 12-h were exposed to SAG (0, 1/16, 1/8 and 1/4 MIC) and cultured for 24 h at 37 °C, and then washed twice with 0.85% NaCl. The samples of P. rettgeri biofilm were stained with CFDA-SE and then incubated for 15 min at 25 °C for direct visual observation of the biofilm formation using a CLSM. Fluorescence intensity of the cells was measured at excitation/emission wavelengths of 488/542 nm for CFDA-SE.
Biofilm matrix by CLSM
The changes in major biofilm matrix levels within 24-h-old biofilms of mono or dual species in the presence of SAG were detected by CLSM of the biofilm matrix combined with different dyes. Briefly, biofilms were prepared using the procedure described in the crystal violet biofilm assay section. The resulting samples were washed thrice with 1 mM sterile PBS (pH 7.4) and labeled with three fluorescent dyes: (1) 25% (v/v) SYPRO Ruby, which stains most classes of proteins, and 2 μM SYTO9 dye; (2) 5 μg/mL WGA dye, which labels polysaccharides, and 5 μg/mL water-soluble FM® dyes; and (3) 2 μM PI dye and 2 μM SYTO9 dye. Subsequently, the biofilm was washed twice with 1 mM PBS to remove all planktonic bacteria, where the excitation/emission wavelengths were 450/610 nm for SYPRO Ruby (red), 498/517 nm for SYTO9 (green), 488/617 nm for PI (red), 495/519 nm for WGA (green) and 479/565 nm for water-soluble FM® dyes (red). Color confocal images were observed with CLSM.
Diffusion bioassay for gatifloxacin within biofilms
To evaluate the diffusion of antibiotics into biofilms, the diffusion of gatifloxacin into biofilms was identified based on the intrinsic fluorescence of CLSM. The biofilms described above were placed on glass coverslips inside the 24-well plate for 48 h at 37 °C in the presence of SAG at 0, 1/16, 1/8 and 1/4 MIC, withdrawn, and gently washed thrice with 10 mM PBS, added with gatifloxacin at a final concentration of 0.4 mg/mL, and further incubated at 37 °C for 4 h. Then, 3 μM SYTO 9 was added and incubated for 15 min to observe gatifloxacin diffusion within biofilms. The samples were washed with 10 mM PBS thrice and observed with CLSM. The emission peak of gatifloxacin at 495 nm was recorded upon excitation at 291 nm. At least three random fields were visualized for each biofilm, and representative images were displayed.
Statistical analysis
All experiments in this study were conducted thrice independently. Each biological replicate was needed to conduct two technical replicates. Data are expressed as mean ± standard deviation (SD). Results were analyzed using SPSS 8.0 software and Origin 8.5 statistics. Analysis of variance (ANOVA) was performed to determine any significant differences (P ≤ 0.01).
Results
MIC of SAG against P. rettgeri
According to the MIC results presented in Table 1, P. rettgeri manifested antibiotic resistance, as evidenced by the MIC of ampicillin (32 μg/mL), cefepime (16–64 μg/mL), ertapenem (8 μg/mL), imipenem (16 μg/mL), tobramycin (8–16 μg/mL), amikacin (2–64 μg/mL), piperacillin/tazobactam (64–128 μg/mL) and nitrofurantoin (32–512 μg/mL). SAG exhibited excellent antibacterial activity against P. rettgeri 1-7, with the MIC values of 7.8, 7.8, 7.8, 7.8, 7.8, 3.9 and 3.9 μg/mL, respectively.
Table 1. Minimum inhibitory concentration of nine kinds of drugs and antibiotics against seven P. rettgeri strains.
| Stains (#) | MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAG | AMP | FEP | ETP | IPM | TOB | AMK | TZP | F | |
| 1 | 7.8 | >=32 | >=64 | >=8 | >=16 | >=16 | >=64 | >=128 | 32 |
| 2 | 7.8 | >=32 | >=64 | >=8 | >=16 | >=16 | >=64 | >=128 | 32 |
| 3 | 7.8 | >=32 | >=64 | >=8 | >=16 | >=16 | 16 | 64 | 512 |
| 4 | 7.8 | >=32 | >=64 | >=8 | >=16 | >=16 | 8 | >=128 | 512 |
| 5 | 7.8 | >=32 | >=64 | >=8 | >=16 | >=16 | 8 | >=128 | 512 |
| 6 | 3.9 | >=32 | >=64 | >=8 | >=16 | 4 | <=2 | >=128 | 256 |
| 7 | 3.9 | >=32 | 16 | >=8 | >=16 | 8 | 4 | >=128 | 256 |
Note:
AMP (Ampicillin); FEP (Cefepime); ETP (Ertapenem); IPM (Imipenem); TOB (Tobramycin); AMK (Amikacin); TZP (Piperacillin/tazobactam); F (Nitrofurantoin).
Effects of SAG on the growth kinetics of P. rettgeri cells
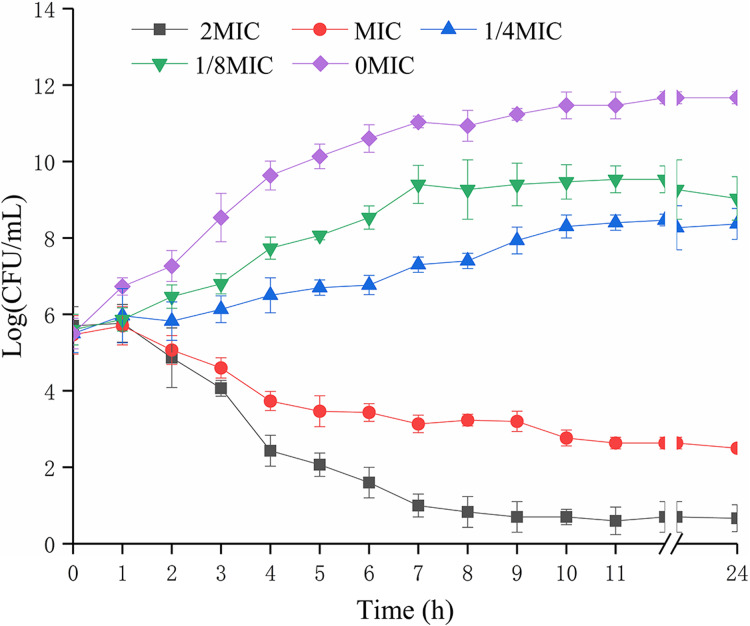
The biomass of P. rettgeri was decreased when treated with SAG at MIC in media (Fig. 1); however, cells treated with SAG at 2 MIC exhibited a significant decline in the number of viable cells, which was lower than the detection limit after 24 h. However, the growth curves exhibited weaker increases and lower growth rates at concentrations of 1/8 and 1/4 MIC than those at other MIC. Higher concentrations of SAG resulted in a significant reduction in the relative number of viable cells, while the opposite has been found in lower SAG concentrations.
Figure 1. Effects of SAG on the growth kinetics of P. rettgeri cells.
Effect of SAG on the growth of P. rettgeri. Bacterial cells were incubated and grown in TBS with 0, 1/8, 1/4, 1 and 2 MIC of SAG at 37 °C. Error bars are SD of three replicates.
SAG treatment decreased intracellular ATP concentrations, pHin and membrane potential of P. rettgeri
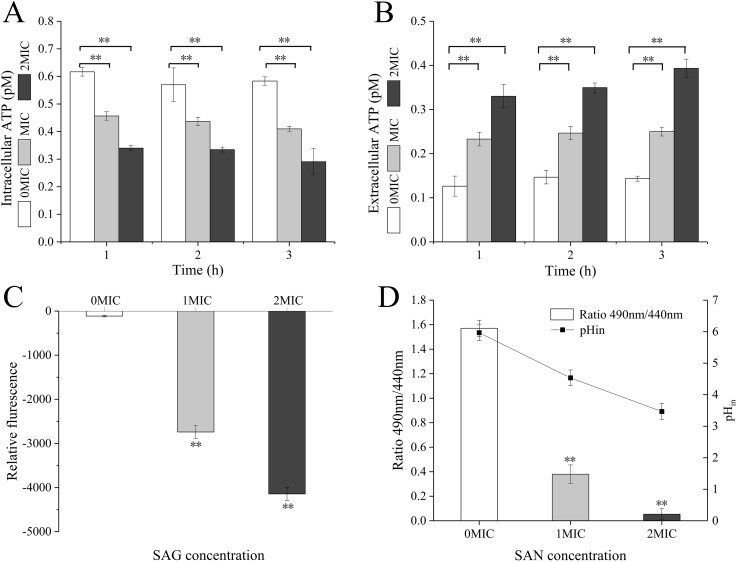
When P. rettgeri cells were treated with SAG at 1 and 2 MIC, the intracellular ATP concentration was significantly decreased (P < 0.01) in the SAG-treated group compared with the untreated control group (Fig. 2A); however, there were no significant differences between the cells treated with SAG at 1 and 2 MIC, but the decrease in intracellular ATP concentration was positively followed by the increase in SAG concentration. Simultaneously, the extracellular ATP concentrations of P. rettgeri cells increased in a concentration-dependent manner, and its action was in a time-dependent manner (Fig. 2B).
Figure 2. SAG treatment led to a decrease in the intracellular ATP concentrations, pH and membrane potential of P. rettgeri.
Effects of SAG on P. rettgeri: (A) intracellular and (B) extracellular ATP levels, (C) membrane potential, and (D) pHin. Data are expressed as mean ± SD. **P < 0.01 vs. 0 MIC.
The pHin of P. rettgeri after SAG treatment changed significantly, as presented in Fig. 2C. In this study, the original pHin of P. rettgeri was 5.97 ± 0.25, and treatment with SAG at 1 and 2 MIC decreased this pHin significantly to 4.53 ± 0.25 (P < 0.01) and 3.47 ± 0.25 (P < 0.01), respectively.
Moreover, the fluorescence intensities of P. rettgeri treated with SAG at 1 MIC for 2 h were less than those of the untreated group (Fig. 2D). Consistently, with an increasing concentration of SAG from 1 to 2 MIC, the membrane potential decreased significantly (P < 0.01).
SAG treatment increased the cell membrane permeability of P. rettgeri
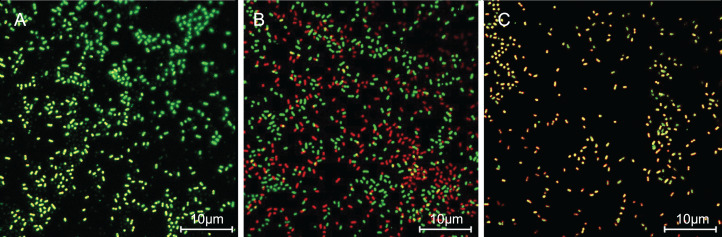
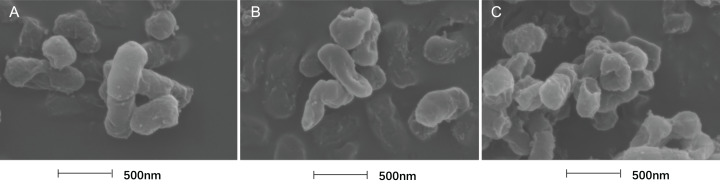
The cells with intact membranes stained with CFDA-SE exhibited bright green fluorescence, but membrane-damaged cells loaded with PI exhibited red fluorescence. In Fig. 3, P. rettgeri cells of the untreated control group exhibited bright green fluorescence, thereby revealing the physical integrity of the cell membrane. However, the results demonstrated significantly reduced green fluorescence and increased red fluorescence when treated with 1 MIC of SAG. As the SAG concentrations increased, green fluorescence declined, and red fluorescence gradually increased.
Figure 3. SAG treatment increased the cell membrane permeability of P. rettgeri.
Effects of SAG on the cell membrane integrity of P. rettgeri cells through CLSM: (A) P. rettgeri cells exposed to 1% DMSO; (B) P. rettgeri cells exposed to SAG at 1 MIC, and (C) P. rettgeri cells exposed to SAG at 2 MIC.
Treatment with SAG led to changes in the cell morphology of P. rettgeri
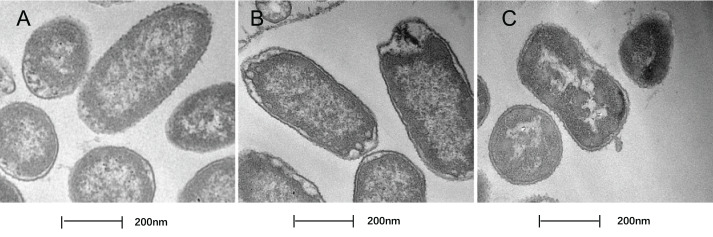
In this study, TEM was used to assess the level of cell wall damage and intracellular modification in SAG-treated P. rettgeri. The P. rettgeri cells without treatment exhibited visible outline and the peptidoglycan layer (Fig. 4A), while P. rettgeri cells treated with SAG at 1 MIC were malformed, damaged, and out of proportion (Fig. 4B). However, P. rettgeri cells treated with SAG at 2 MIC showed a wavy contour of the cytoplasmic membrane and dense, undifferentiable cellular content, indicating an obvious shrinkage of the cytoplasm (Fig. 4C). Consequently, SAG-treated P. rettgeri could damage the cell membrane and cell wall outline.
Figure 4. Treatment with SAG led to changes in the cell morphology of P. rettgeri.
Effects of SAG on the cell structure of P. rettgeri through TEM. (A) P. rettgeri cells treated with 1% DMSO; (B) P. rettgeri cells treated with SAG at 1 MIC, and (C) P. rettgeri cells treated with SAG at 2 MIC.
The cell morphology after treatment with SAG presented significant changes using FESEM. Compared with the normal smooth cell surface of the untreated group (Fig. 5A), cells treated with SAG at 1 MIC exhibited significant enlargement, uneven size, and rough surface (Fig. 5B). In addition, cells treated with SAG at 2 MIC exhibited significant surface collapse and expansion on the cell membrane as a result of the destruction of the bacterial cell wall (Fig. 5C), which indicated a positive correlation between the increase in the concentrations of SAG and the damage degree of cell membrane.
Figure 5. Treatment with SAG led to changes in the cell morphology of P. rettgeri.
Effects of SAG on the cell morphology of P. rettgeri using FESEM: (A) P. rettgeri cells exposed to 1% DMSO; (B) P. rettgeri cells exposed to SAG at 1 MIC, and (C) P. rettgeri cells exposed to SAG at 2 MIC.
Inhibitory effect of SAG on the biofilm formation of P. rettgeri
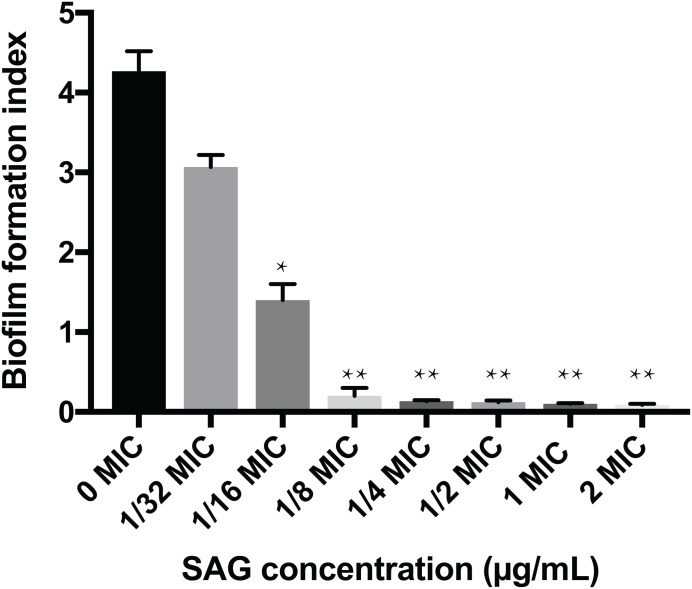
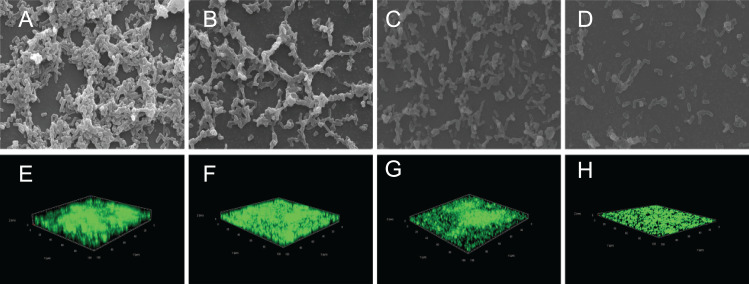
The crystal violet assay, FESEM and CLSM were used to analyze the inhibitory effects of SAG on the biofilm formation of P. rettgeri. As presented in Fig. 6, SAG exhibited a significant inhibitory effect on the biofilm formation of P. rettgeri at different concentrations (P < 0.01). The biofilm formation index of the untreated control group was approximately 3.6. Biofilm formation was inhibited by 68% in the presence of 1/16 MIC SAG; furthermore, when the SAG concentration was higher than 1/4 MIC, the biofilm formation in P. rettgeri was suppressed by more than 95%. The results of FESEM and CLSM further showed that biofilm formation was significantly inhibited at 1/16, 1/8 and 1/4 MIC in the SAG-treated group compared with the untreated group (Fig. 7).
Figure 6. Inhibitory effects of SAG on the biofilm formation of P. rettgeri.
The biofilm formation index was tested by crystal violet staining with different concentrations of SAG in 96-well plates. Data are expressed as mean ± SD. *P < 0.05 vs. 0 MIC, **P < 0.01 vs. 0 MIC.
Figure 7. Effect of SAG on the biofilm formation of P. rettgeri.
Images of FESEM (A–D; magnification, 10,000′×g) and CLSM (E–H). (A and E) P. rettgeri cells exposed to 1% DMSO; (B and F) P. rettgeri cells exposed to SAG at 1/16 MIC; (C and G) P. rettgeri cells exposed to SAG at 1/8 MIC, and (D and H) P. rettgeri cells exposed to SAG at 1/4 MIC.
Inactivation effect of SAG on the biofilm of P. rettgeri cells
The inactivation effect of SAG against 36-h-old biofilm-associated P. rettgeri cells is presented in Fig. 8. The untreated group was almost entirely green, as observed with CLSM (Figs. 8A and 8E), indicating that most of the cell membranes of P. rettgeri cells embedded in biofilms were integrated and viable. However, when P. rettgeri cells within biofilms were exposed to 16 MIC SAG, the CLSM observation displayed abundant red florescence, revealing that most of the cell membranes of P. rettgeri within biofilms were impaired, as presented in Figs. 8D and 8H. Furthermore, with an increasing concentration of SAG from 4 to 8 MIC, the green signal on the membrane disappeared gradually and the red signal increased gradually (Figs. 8B, 8C, 8F and 8G), indicating that intact and viable biofilms changed with the increasing concentration of SAG. These results suggested that SAG presented the killing effect on the P. rettgeri cells within biofilms.
Figure 8. Inactivation effect of SAG on the biofilm of P.rettgeri cells.
Inactivation effect of SAG on P. rettgeri cells within biofilms. 1D (A–D) and 3D (E–H) images of CLSM. (A and E) P. rettgeri cells within biofilms unexposed to SAG. (B and F) P. rettgeri cells within biofilms exposed to SAG at 1 MIC. (C and G) P. rettgeri cells within biofilms exposed to SAG at 2 MIC. (D and H) P. rettgeri cells within biofilms exposed to SAG at 4 MIC.
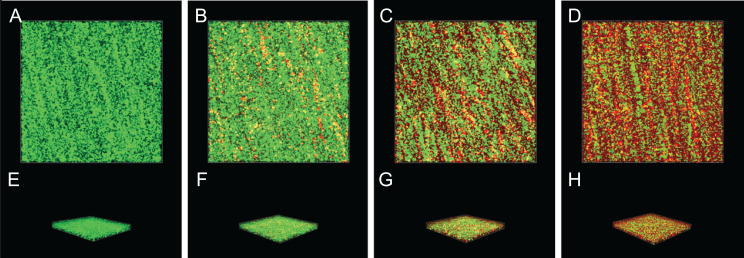
SAG could change the biofilm matrix composition of P. rettgeri cells
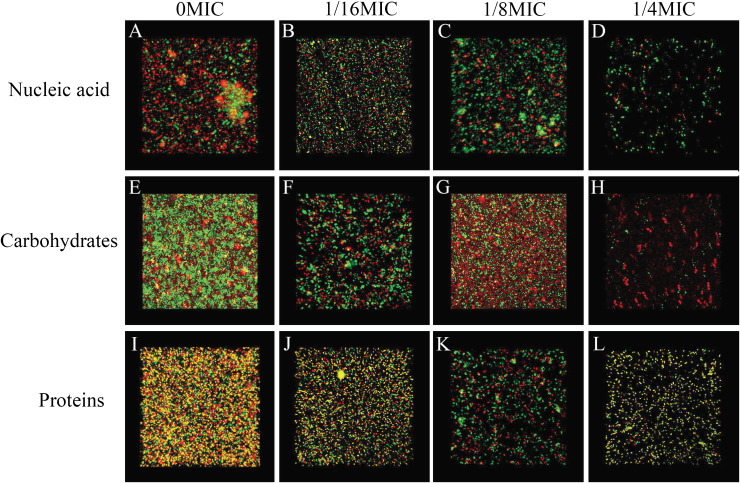
The biofilm matrix commonly comprises proteins, nucleic acid (environmental DNA (eDNA)) and carbohydrates, which provide structural rigidity and protection from the external environment to control gene regulation and nutrient adsorption (Hobley et al., 2015). Thus, the changes in the biofilm matrix composition could affect biofilm formation. Therefore, the effects of SAG on the proteins, nucleic acid (eDNA) and carbohydrates of P. rettgeri were investigated. The changes in major biofilm matrix levels within 24-h-old biofilms of mono or dual species in the presence of SAG were detected by CLSM in Fig. 9. Different reagents were used to mark the eDNA and proteins red and the carbohydrates green. The untreated groups of nucleic acids and proteins exhibited almost red florescence, as observed with CLSM; with the increasing concentration of SAG, the red signal was gradually reduced (Figs. 9A and 9C). The untreated group of carbohydrates exhibited green florescence, as observed with CLSM; with an increasing concentration of SAG, the green signal gradually disappeared (Fig. 9B), indicating that nucleic acid, protein, and carbohydrate contents decrease with an increasing concentration of SAG.
Figure 9. SAG could change the biofilm matrix composition of P. rettgeri cells.
Effects of different concentrations of SAG on the levels of carbohydrates, extracellular proteins, and extracellular DNA inside P. rettgeri biofilms. (A–D) eDNA labeled red (PI). (E–H) Carbohydrates labeled green (WGA). (I–L) Proteins labeled red (SYPRO Ruby). Scale bar, 10 μm.
Effects of SAG on biofilm diffusion
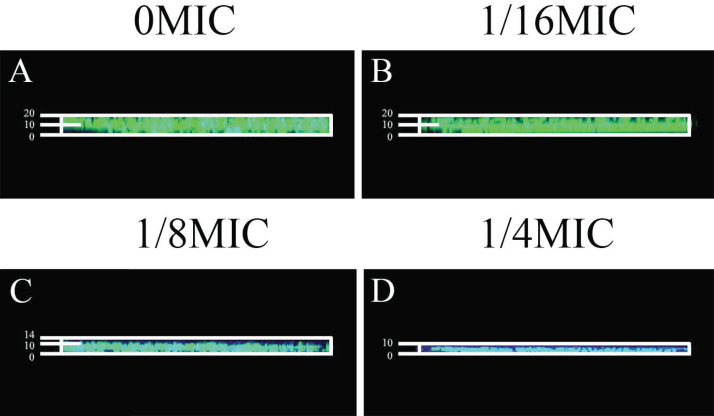
Gatifloxacin was used to verify the effects of SAG on biofilm diffusion, which was monitored using fluorescence CLSM. Providencia rettgeri biofilms were grown with different SAG concentrations for 48 h and then added with gatifloxacin for 4 h. Formed biofilms were stained with SYTO9 to allow the visualization of nucleic acid (green fluorescence).
Gatifloxacin diffused significantly at 0 MIC (Fig. 10A). However, in mixed biofilms with 1/4 MIC of SAG, the gatifloxacin signal was minimally detected (Fig. 10D). With an increasing concentration of SAG, the signal diffusion became weaker, thus proving that SAG inhibits biofilm formation.
Figure 10. Effect of SAG on biofilms of diffusion.
(A) P. rettgeri cells treated with 0 MIC and gatifloxacin; (B) P. rettgeri cells treated with 1/16 MIC and gatifloxacin; (C) P. rettgeri cells treated with 1/8 MIC and gatifloxacin; (D) P. rettgeri cells treated with 1/4 MIC and gatifloxacin.
Discussion
To the best of our knowledge, the present study is the first to evaluate the antibacterial activity and mechanism of SAG against P. rettgeri. The findings of TEM, CLSM, and FESEM revealed significant effects of SAG against P. rettgeri biofilms, including its inhibitory effect on biofilm substance expression and formation as well as on biofilm inactivation. We found that SAG could decrease intracellular ATP concentration and changes in pHin and reduce the membrane potential.
Here SAG demonstrated strong activity against P. rettgeri biofilms. SAG not only inhibited biofilm formation but also destroyed the intact and viable biofilm. At 1/16 MIC, SAG inhibited biofilm formation by approximately 68%, whereas at 1/4 MIC, more than 95% of the biofilm was inhibited, thus an outstanding antibacterial effect of SAG was observed on P. rettgeri.
ATP depletion is a common biological change associated with cell damage, suggesting that ATP is used as a potential indicator of the effects of antimicrobial agents on the intact and viable cell membrane. These results presented that SAG-treated P. rettgeri exhibited a remarkable decrease in intracellular ATP concentrations, and changes in intracellular and extracellular ATP balance were observed in P. rettgeri cells, suggesting cell membrane damage. These results were generally consistent with the results of other analyses (Qian et al., 2020).
Intracellular pH has a critical influence on cell physiology, including DNA replication, RNA transcription, protein synthesis and enzyme activity. Moreover, pHin is positively related to intact and viable cell membranes (Gonelimali et al., 2018). Consequently, the cell membrane damage can be reflected by the changes in the pHin. In the present study, changes in the pHin of P. rettgeri were significantly related to SAG concentrations, wherein the addition of 2 MIC SAG led to a decrease in the pHin from 5.97 ± 0.25 to 3.47 ± 0.25. Hence, as described in the previous literature for plant-derived products, the effect of SAG on P. rettgeri could be cleared as the breaking to intact and viable cell membrane. In addition, when SAG entered into cells, it could cause hyperpolarization across the cell membrane, thus making the internal membrane potential more negative. Compared with that in the untreated control group, when the SAG concentration increased from 1 to 2 MIC, the membrane potential significantly decreased in the SAG-treated group. Similarly, Zhong et al. (2017) reported that SAG could decrease the membrane potential of Candida albicans cells. Taken together, these results suggest that SAG causes plasma membrane hyperpolarization fin determined bacteria, which possibly resulting in cell metabolic activity disruption and cell death.
Our studies have also indicated that P. rettgeri cells exhibit significant membrane dissolution, as observed with CLSM, whereas leakage of cytoplasmic components has been reported after exposure to SAG, as observed with TEM. Herein, CLSM revealed that the permeability of cell membrane increased after treatment with SAG. The 2 MIC-exposed P. rettgeri cells were apparently entirely red, indicating that the majority of cell membranes were destroyed and that some substances could move allodially in and out of the cell. Therefore, FESEM images of P. rettgeri cells exposed to SAG at 2 MIC revealed that the cells had severe morphological alterations, whereas MIC-exposed cells were visibly extended and had a rough surface. The physical and morphological changes in P. rettgeri cells may be due to the effect of SAG on membrane permeability and integrity. However, dense undifferentiated intracellular materials in SAG-exposed cells were observed with TEM. Moreover, CLSM revealed that almost all bacterial cells were damaged after exposure to SAG at 2 MIC. As reported by Matijašević et al. (2016), FESEM and TEM were used to observe the morphological changes in S. aureus cells treated with Coriolus versicolor methanol extract.
Previous studies have verified that biofilms could enhance bacterial resistance to adverse environmental pressures, including resistance to antibiotics and antimicrobial agents (Jakobsen, Tolker-Nielsen & Givskov, 2017). We found that exopolysaccharides, proteins and extracellular DNA formed bacterial cells in biofilms, which were embedded in extracellular polymeric substances. In this study, the effects of SAG on biofilm formation and biofilm-associated cell inactivation was further illustrated, and the results of crystal violet staining revealed that SAG at different concentrations presented inhibitory effects on the biofilm formation of P. rettgeri. Moreover, FESEM and CLSM results illustrated that SAG-exposed cells exhibited substantially decreased adhesion and survival, indicating that biofilm formation was significantly inhibited by SAG. These results were in agreement with the effectiveness of antistaphylococcal lysin CF-301 on the inactivation of 95 S. aureus strains within biofilms, as described by Schuch et al. (2017). In addition, CLSM further revealed that SAG exhibited robust impaired effects on biofilm-associated P. rettgeri cells. CLSM images of P. rettgeri cells within biofilms exposed to 4 MIC of SAG displayed dark red florescence, proving that most of the membranes of cells within the biofilm were damaged. Our study were divided into two main parts, one part demonstrated SAG had antimicrobial activity against P. rettgeri through causing cell membrane dysfunction and changes in cellular morphology, similarlgy a study by Qian et al. (2020), and the other part was the effect of SAG on P. rettgeri biofilm, which showed that different concentrations of SAG could inhibit the formation of P. rettgeri biofilm by changing the biofilm matrix while also killing through mature biofilms in chronic infections. The results were in line with previous reports that showed that SAG inhibits biofilm formation of Candida albicans and Staphylococcus aureus and synchronously reducing extracellular proteins, polysaccharides, and eDNA levels in a dose-dependent manner (Qian et al., 2020). These findings revealed that SAG exhibits potent antibacterial and antibiofilm activity against P. rettgeri, and thus has potential to be exploited as a natural preservative to control the P. rettgeri associated infections.
Conclusions
In the present study, we confirmed the efficacy of SAG in inactivating both the biofilm formation and integrity of P. rettgeri cells. SAG could induce cell lysis, leading to cell membrane damage and intracellular component leakage in P. rettgeri cells. In addition, 2 MIC SAG could inactivate the biofilm formation in P. rettgeri cells, indicating its potential as a natural antibacterial agent to control the negative impact of P. rettgeri in the food industry.
Supplemental Information
The growth curve, extracellular ATP, the intracellular ATP and membrane potential.
The different concentrations were 0, 1/32, 1/16, 1/8, 1/4, 1/2, 1, 2 MIC respectively.
The effect of different concentration of SAG on PR was observed by crystal violet. SAG concentrations were 0, 1/16, 1/8 and 1/4 MIC respectively.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Nos. 81902067 and 81300028). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Qian Zhang conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yansi Lyu performed the experiments, prepared figures and/or tables, and approved the final draft.
Jingkai Huang performed the experiments, prepared figures and/or tables, and approved the final draft.
Xiaodong Zhang analyzed the data, prepared figures and/or tables, and approved the final draft.
Na Yu analyzed the data, prepared figures and/or tables, and approved the final draft.
Ziping Wen analyzed the data, prepared figures and/or tables, and approved the final draft.
Si Chen conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.
References
- Armbruster et al. (2014).Armbruster CE, Smith SN, Yep A, Mobley HLT. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. Journal of Infectious Diseases. 2014;209(10):1524–1532. doi: 10.1093/infdis/jit663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology & Infectious Dieases (ESCMID) (2000).European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID) EUCAST definitive document E.DEF 3.1, June 2000: determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2000;6(9):509–515. doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Firatli et al. (1994).Firatli E, Unal T, Onan U, Sandalli P. Antioxidative activities of some chemotherapeutics: a possible mechanism in reducing gingival inflammation. Journal of Clinical Periodontology. 1994;21(10):680–683. doi: 10.1111/j.1600-051X.1994.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Giaouris et al. (2014).Giaouris E, Heir E, Hébraud M, Chorianopoulos N, Langsrud S, Møretrø T, Habimana O, Desvaux M, Renier S, Nychas G-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Science. 2014;97(3):298–309. doi: 10.1016/j.meatsci.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Gonelimali et al. (2018).Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Frontiers in Microbiology. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobley et al. (2015).Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiology Reviews. 2015;39(5):649–669. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen, Tolker-Nielsen & Givskov (2017).Jakobsen T, Tolker-Nielsen T, Givskov M. Bacterial biofilm control by perturbation of bacterial signaling processes. International Journal of Molecular Sciences. 2017;18(9):1970. doi: 10.3390/ijms18091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung et al. (2016).Joung D-K, Mun S-H, Choi S-H, Kang O-H, Kim S-B, Lee Y-S, Zhou T, Kong R, Choi J-G, Shin D-W, Kim Y-C, Lee D-S, Kwon D-Y. Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism. Experimental and Therapeutic Medicine. 2016;12(3):1579–1584. doi: 10.3892/etm.2016.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu F, Wang F, Du L, Zhao T, Doyle MP, Wang D, Zhang X, Sun Z, Xu W. Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control. 2018;84:442–448. doi: 10.1016/j.foodcont.2017.09.004. [DOI] [Google Scholar]
- Magiorakos et al. (2012).Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Matijašević et al. (2016).Matijašević D, Pantić M, Rašković B, Pavlović V, Duvnjak D, Sknepnek A, Nikšić M. The antibacterial activity of coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella enteritidis. Frontiers in Microbiology. 2016;7(222):1707. doi: 10.3389/fmicb.2016.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbelle et al. (2020).Mbelle NM, Osei Sekyere J, Amoako DG, Maningi NE, Modipane L, Essack SY, Feldman C. Genomic analysis of a multidrug-resistant clinical Providencia rettgeri (PR002) strain with the novel integron ln1483 and an A/C plasmid replicon. Annals of the New York Academy of Sciences. 2020;1462(1):92–103. doi: 10.1111/nyas.14237. [DOI] [PubMed] [Google Scholar]
- Miao et al. (2012).Miao F, Yang X-J, Ma Y-N, Zheng F, Song X-P, Zhou L. Structural modification of sanguinarine and chelerythrine and their in vitro acaricidal activity against Psoroptes cuniculi. Chemical & Pharmaceutical Bulletin. 2012;60(12):1508–1513. [PubMed] [Google Scholar]
- Murata et al. (2001).Murata T, Iida T, Shiomi Y, Tagomori K, Akeda Y, Yanagihara I, Mushiake S, Ishiguro F, Honda T. A large outbreak of foodborne infection attributed to Providencia alcalifaciens. Journal of Infectious Diseases. 2001;184(8):1050–1055. doi: 10.1086/323458. [DOI] [PubMed] [Google Scholar]
- Och et al. (2019).Och A, Zalewski D, Komsta Ł, Kołodziej P, Kocki J, Bogucka-Kocka A. Cytotoxic and proapoptotic activity of sanguinarine, berberine, and extracts of Chelidonium majus L. and Berberis thunbergii DC. toward hematopoietic cancer cell lines. Toxins. 2019;11(9):485. doi: 10.3390/toxins11090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian et al. (2020).Qian W, Wang W, Zhang J, Liu M, Fu Y, Li X, Wang T, Li Y. Sanguinarine inhibits mono- and dual-species biofilm formation by Candida albicans and Staphylococcus aureus and induces mature hypha transition of C. albicans. Pharmaceuticals. 2020;13(1):13. doi: 10.3390/ph13010013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Qian et al. (2020).Qian W, Yang M, Wang T, Sun Z, Liu M, Zhang J, Zeng Q, Cai C, Li Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant enterobacter hormaechei. Journal of Food Protection. 2020;83(4):576–583. doi: 10.4315/JFP-19-469. [DOI] [PubMed] [Google Scholar]
- Schuch et al. (2017).Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrobial Agents and Chemotherapy. 2017;61(7):95. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, Sharma & Soni (2017).Sharma D, Sharma P, Soni P. First case report of Providencia rettgeri neonatal sepsis. BMC Research Notes. 2017;10(1):534. doi: 10.1186/s13104-017-2866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2018).Shi C, Che M, Zhang X, Liu Z, Meng R, Bu X, Ye H, Guo N. Antibacterial activity and mode of action of totarol against Staphylococcus aureus in carrot juice. Journal of Food Science and Technology. 2018;55(3):924–934. doi: 10.1007/s13197-017-3000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sianglum et al. (2018).Sianglum W, Saeloh D, Tongtawe P, Wootipoom N, Indrawattana N, Voravuthikunchai SP. Early effects of rhodomyrtone on membrane integrity in methicillin-resistant Staphylococcus aureus. Microbial Drug Resistance (Larchmont, N.Y.) 2018;24(7):882–889. doi: 10.1089/mdr.2016.0294. [DOI] [PubMed] [Google Scholar]
- Singh & Sharma (2018).Singh N, Sharma B. Toxicological effects of berberine and sanguinarine. Frontiers in Molecular Biosciences. 2018;5:218. doi: 10.3389/fmolb.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang J, Ma M, Yang J, Chen L, Yu P, Wang J, Gong D, Deng S, Wen X, Zeng Z. In vitro antibacterial activity and mechanism of monocaprylin against Escherichia coli and Staphylococcus aureus. Journal of Food Protection. 2018;81(12):1988–1996. doi: 10.4315/0362-028X.JFP-18-248. [DOI] [PubMed] [Google Scholar]
- Wie (2015).Wie S-H. Clinical significance of Providencia bacteremia or bacteriuria. Korean Journal of Internal Medicine. 2015;30(2):167–169. doi: 10.3904/kjim.2015.30.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2017).Xu Y, Shi C, Wu Q, Zheng Z, Liu P, Li G, Peng X, Xia X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathogens and Disease. 2017;14(5):282–287. doi: 10.1089/fpd.2016.2226. [DOI] [PubMed] [Google Scholar]
- Yatoo et al. (2018).Yatoo MI, Gopalakrishnan A, Saxena A, Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K, Iqbal HMN. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—a review. Recent Patents on Inflammation & Allergy Drug Discovery. 2018;12(1):39–58. doi: 10.2174/1872213X12666180115153635. [DOI] [PubMed] [Google Scholar]
- Yoh et al. (2005).Yoh M, Matsuyama J, Ohnishi M, Takagi K, Miyagi H, Mori K, Park K-S, Ono T, Honda T. Importance of Providencia species as a major cause of travellers’ diarrhoea. Journal of Medical Microbiology. 2005;54(Pt. 11):1077–1082. doi: 10.1099/jmm.0.45846-0. [DOI] [PubMed] [Google Scholar]
- Zhong et al. (2017).Zhong H, Hu D-D, Hu G-H, Su J, Bi S, Zhang Z-E, Wang Z, Zhang R-L, Xu Z, Jiang Y-Y, Wang Y. Activity of sanguinarine against Candida albicans Biofilms. Antimicrobial Agents and Chemotherapy. 2017;61(5):293. doi: 10.1128/AAC.02259-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The growth curve, extracellular ATP, the intracellular ATP and membrane potential.
The different concentrations were 0, 1/32, 1/16, 1/8, 1/4, 1/2, 1, 2 MIC respectively.
The effect of different concentration of SAG on PR was observed by crystal violet. SAG concentrations were 0, 1/16, 1/8 and 1/4 MIC respectively.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.