Abstract
Objectives
To analyze whether frailty and comorbidities are associated with in-hospital mortality and discharge to home in older adults hospitalized for coronavirus disease 2019 (COVID-19).
Design
Single-center observational study.
Setting and Participants
Patients admitted to geriatric care in a large hospital in Sweden between March 1 and June 11, 2020; 250 were treated for COVID-19 and 717 for other diagnoses.
Methods
COVID-19 diagnosis was clinically confirmed by positive reverse transcription polymerase chain reaction test or, if negative, by other methods. Patient data were extracted from electronic medical records, which included Clinical Frailty Scale (CFS), and were further used for assessments of the Hospital Frailty Risk Score (HFRS) and the Charlson Comorbidity Index (CCI). In-hospital mortality and home discharge were followed up for up to 25 and 28 days, respectively. Multivariate Cox regression models adjusted for age and sex were used.
Results
Among the patients with COVID-19, in-hospital mortality rate was 24% and home discharge rate was 44%. Higher age was associated with in-hospital mortality (hazard ratio [HR] 1.05 per each year, 95% confidence interval [CI] 1.01‒1.08) and lower probability of home discharge (HR 0.97, 95% CI 0.95‒0.99). CFS (>5) and CCI, but not HFRS, were predictive of in-hospital mortality (HR 1.93, 95% CI 1.02‒3.65 and HR 1.27, 95% CI 1.02‒1.58, respectively). Patients with CFS >5 had a lower probability of being discharged home (HR 0.38, 95% CI 0.25‒0.58). CCI and HFRS were not associated with home discharge. In general, effects were more pronounced in men. Acute kidney injury was associated with in-hospital mortality and hypertension with discharge to home. Other comorbidities (diabetes, cardiovascular disease, lung diseases, chronic kidney disease and dementia) were not associated with either outcome.
Conclusions and Implications
Of all geriatric patients with COVID-19, 3 out of 4 survived during the study period. Our results indicate that in addition to age, the level of frailty is a useful predictor of short-term COVID-19 outcomes in geriatric patients.
Keywords: COVID-19, frailty, comorbidity, geriatrics, survival, aging
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus pandemic, which causes coronavirus disease 2019 (COVID-19), has particularly high morbidity among the older segment of the population. COVID-19 mortality rates show sharp increase with increasing age; the vast majority of deaths in Sweden are reported among those aged 70 years and older. There seems to be an increasing age-gradient and sex-difference among the intensive care unit-treated patients,1 but the reasons for these variations in the clinical outcomes are not clear. It has been speculated that certain somatic conditions like diabetes, hypertension, and obesity are risk factors for worse outcomes.2 Because of the difficulties in identifying the underlying risk factors, restrictive recommendations have been directed broadly to people older than 70 years of age irrespective of health and activity status.
The older population is characterized by large heterogeneity in terms of health and vigor. Depending on factors like life-style, socioeconomic status, and genetic predisposition, health trajectories develop differently between individuals. Irrespective of chronological age and concurrent disease, some age faster and become vulnerable and susceptible to disease and disability earlier than others. To recognize this condition, the concept of frailty has been introduced over the last decades.3 World Health Organization defines frailty as “a progressive age-related decline of body functions resulting in vulnerability and reduced resilience to physical and mental stressors with an increased risk of negative health outcomes.”4 Frailty can be assessed using various approaches, such as scales that take fitness and dependency into account. The Clinical Frailty Scale (CFS) is one such tool that classifies subjects according to a 9-graded scale, from very fit (=1) to terminally ill (=9).5 Another measure developed for identification of older hospitalized patients with frailty is the Hospital Frailty Risk Score (HFRS), based on diagnostic codes in the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10).6 Although not yet confirmed in larger samples, there is preliminary evidence that the susceptibility to COVID-19 and the increased mortality risk in older people is linked to frailty.7 This is partly supported by the high death tolls in residents of sheltered housing (eg, nursing homes), where most residents are frailer than community-dwelling older individuals. Other factors, such as group dining and staff challenges may as well have contributed to the high death rates.
The Stockholm area has around 2 million inhabitants and was hit by COVID-19 in early spring 2020 with more than 2000 casualties within the first 2 months of the outbreak. The aim of this article is to use data from a large hospital in Stockholm with 967 patients treated at a geriatric ward unit during the first three months of the COVID-19 outbreak, whereof 250 were diagnosed with COVID-19. The primary hypothesis is that frailty according to CFS and the recently developed HFRS6 are stronger risk factors for negative outcomes than chronological age and comorbidities.
Methods
Study Sample
Patients admitted to the geriatric care unit at a large hospital in Stockholm, Sweden, from March 1 to June 11, 2020 were included in the study. In total, 967 individuals with 250 patients with COVID-19, 232 (92.3%) confirmed (ICD-10 code U07.1) and 18 (7.2%) suspected (ICD-10 code U07.2), were studied for short-term outcomes (ie, in-hospital mortality or being discharged to home). Most patients came directly from home and transitioned through the emergency section before being admitted to geriatric care. Follow-up was done through electronic medical records throughout the study period, resulting in different follow-up times for different patients. At a maximum, patients were followed up for up to 25 days for in-hospital mortality and up to 28 days for discharge to home. Patient demographics, diagnoses and death data were collected through electronic health records in the Take Care system, where all deaths occurring in patients with COVID-19 were assumed to be due to COVID-19.
COVID-19 Diagnosis
A clinical COVID-19 diagnosis was determined using reverse transcriptase polymerase chain reaction (RT-PCR) using extracts from nasopharyngeal swabs. Clinical diagnosis in patients with a negative RT-PCR was made in collaboration with infection disease specialists if the patient had typical symptoms and typical findings on a computed tomography scan with no other explanation of the symptoms (ie, bacterial infection). As stated above, 18 of 250 (7.2%) of the patients with COVID-19 were tested negative in the RT-PCR. In a preprint systematic review, the false negative rate has been estimated between 2% and 29%.8 The most likely reasons for false negatives include too low amount of the virus for the test to detect (eg, the swab not inserted deep enough in the nose), nonoptimal sample type, a person is tested too early or too late in the infection, technical issues in the assay or if the sample sits too long before being tested, allowing the viral RNA to degrade.9 , 10 The false positive rate has also been shown to vary with time from exposure and symptom onset,10 making decision-making based on other assessments, such as the computed tomography scan pivotal.
Frailty
Frailty was defined using the CFS and HFRS. The CFS score was assigned by a geriatrician trained in scoring with the CFS scale within 1-2 hours of admission. A chart review and face-to-face assessments with patients and families were used to determine the CFS level as it was some time before admission. The CFS scale ranges from 1 (very fit) to 9 (terminally ill).5 The CFS scores are commonly divided into the following 3 categories: nonfrail (CFS 1–4), mild-to-moderately frail (CFS 5–6), and severely frail (CFS 7–9). However, in the present study, we wished to test different cut-offs to determine the most meaningful categorization for COVID-19–related outcomes and used dichotomization at CFS of >5.
HFRS was assessed according to the model by Gilbert et al6 using all available ICD-10 diagnostic codes that were documented for the sample. The HFRS is a composite score based on 109 ICD-10 diagnostic codes that have been associated with the risk of frailty.6 Each of the 109 ICD-10 diagnostic codes are assigned with a weight, ranging from 0.1 to 7.1, based on how strongly they associate with frailty, and the weights are summed across the ICD-10 codes to yield the HRFS. In our analyses, we used the HFRS as continuous measure.
Comorbidity
The different comorbidities were defined using ICD-10 codes for diabetes (E10, E11), cardiovascular disease (I25), hypertension (I10-I15), asthma (J45), chronic obstructive pulmonary lung disease (J44), acute kidney injury (AKI; N17), chronic kidney disease (N18), as well as dementia diagnoses (F00-04, F05.1, G30, G31, A81.0). The Charlson Comorbidity Index (CCI) was calculated based on weighted ICD-10 diagnosis codes according to the protocol.11
Statistical Methods
Differences across the study variables between patients with COVID-19 and other patients, as well as between men and women, were assessed using t-test, Mann-Whitney test, and χ2 test statistics as appropriate. To analyze whether the CFS, HFRS, CCI, and comorbidities (listed in Table 1 ) were predictive of in-hospital mortality we performed Cox proportional hazard model analyses. For being discharged to home, proportional subdistribution hazards model analysis,12 where death is treated as a competing risk, was used. We first tested the CFS, HFRS, CCI, and the comorbidities individually in univariate Cox models adjusted for age and sex. Those predictors that were significant in the univariate models were further entered in multivariate models, again adjusting for age and sex. All the reported variables were tested for the proportional hazard assumption. The Harrell C was used to assess the overall predictive accuracy (discrimination) of each Cox proportional hazard model for mortality. R version 3.6.3 and the packages “survival” and “cmprsk” were used in the analysis. Significance level was set to α value of <0.05.
Table 1.
Characteristics of the Patients Admitted to Geriatric Care in Stockholm During the SARS-CoV-2 Outbreak Between March 1 and June 11, 2020
| Patients with COVID-19 | Patients without COVID-19 | P for Difference∗ | |
|---|---|---|---|
| Patients, n (%) | 250 (26) | 717 (74) | |
| Age y, mean (SD) | 81.01 (8.56) | 82.79 (8.77) | P < .01 |
| Men, n (%) | 120 (48) | 297 (41) | .08 |
| Deaths, n (%) | 59 (24) | 29 (4) | P < .001 |
| Discharged to home, n (%) | 110 (44) | 423 (59) | P < .001 |
| Comorbidity | |||
| CCI, median (range) | 1 (0-8) | 1 (0-7) | P < .001 |
| Diabetes, n (%) | 78 (31) | 158 (22) | P < .01 |
| CVD, n (%) | 19 (8) | 47 (7) | .68 |
| Hypertension, n (%) | 144 (58) | 295 (41) | P < .001 |
| Asthma, n (%) | 15 (6) | 21 (3) | .04 |
| COPD, n (%) | 47 (19) | 87 (12) | .01 |
| CKD, n (%) | 36 (14) | 89 (12) | .49 |
| AKI, n (%) | 16 (6) | 27 (4) | .12 |
| Dementia, n (%) | 38 (15) | 143 (20) | .12 |
| CFS, n (%) | |||
| 1-5 | 120 (48) | 266 (37) | P < .001 |
| 6-9 | 95 (38) | 239 (33) | |
| Missing | 35 (14) | 212 (30) | |
| HFRS, median (range) | 2.8 (0-17) | 2.3 (0-15.4) | .04 |
AKI, acute kidney injury; CCI, charlson comorbidity index; CFS, clinical frailty score; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; HFRS, hospital frailty risk score; SD, standard deviation.
t-test for age, Wilcoxon rank-sum test for HFRS and CCI and χ2 test for other variables.
Ethical Statement
The study was approved by the Swedish Ethical Review Authority in Stockholm on April 14, 2020 with Dnr 2020-01497.
Results
Sample Characteristics
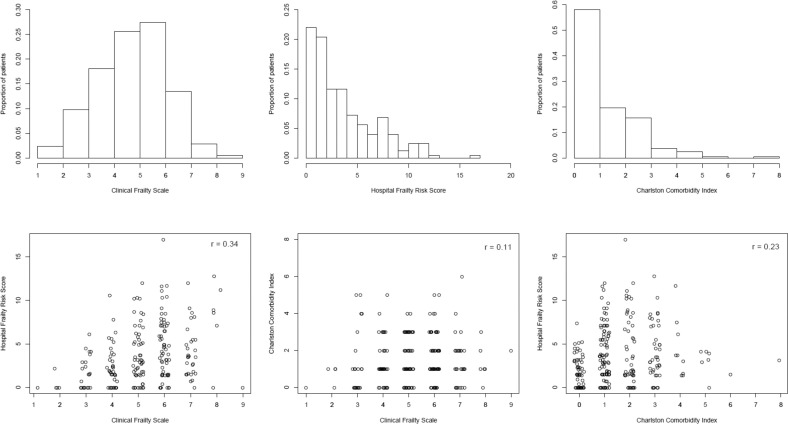
Characteristics of the study sample are presented in Table 1. Proportions of in-hospital mortality in the patients with COVID-19 was 24% (59/250) and for being discharged to home 44% (110/250). Mortality rate was higher among the patients with COVID-19, and they were younger, more frail, less likely to be discharged to home, and had higher prevalence of diabetes and hypertension, compared with the patients without COVID-19. Sex-stratified characteristics of the patients with COVID-19 are presented in Supplementary Table 1. Distributions and correlation plots of CFS, HFRS, and CCI are shown in Supplementary Figure 1. Only few among patients with COVID-19 and without COVID-19 had CFS 1-3 (10% and 7%, respectively), whereas 38% and 30% had CFS 4-5, 35 and 31% CFS 6-7, and 3 and 2% CFS 8-9, respectively. The CFS and HFRS were correlated in patients with COVID-19 (Spearman r = 0.34, P < .001) and moderately correlated in other patients (Spearman r = 0.12, P = .005). The CFS and CCI were not significantly correlated, whereas HFRS and CCI were correlated in both patients with COVID-19 (Spearman r = 0.23, P < .001) and in other patients (Spearman r = 0.31, P < .001).
Supplementary Fig. 1.
Distributions and correlations of frailty and comorbidity in patients with COVID-19.
Survival and Discharge to Home Regression Analyses
Higher age was predictive of in-hospital mortality (Table 2 ) and decreased the probability of being discharged to home (Table 3 ). Being frail, corresponding to a CFS >5, and higher comorbidity index were both associated with in-hospital mortality (Table 2). Being frail (CFS >5) was also associated with a decreased probability of being discharged to home (Table 3), whereas CCI was not (Table 3). Higher HFRS was not associated with in-hospital mortality (Table 2), but it was associated with a decreased probability of being discharged to home (Table 3). With the exception of AKI, which was associated with in-hospital morality (Table 2), and hypertension, which was associated with being discharged to home (Table 3), none of the comorbidities – diabetes, cardiovascular disease, chronic obstructive pulmonary disease, asthma, chronic kidney disease, and dementia – were associated with either of the outcomes. Stratifying patients based on usage of antihypertensive drugs (ATC code C09) demonstrated a nonsignificant trend for users of ACE inhibitors and related drugs to have a lower mortality and a higher probability of discharge home than non-users (data not shown). Sex was not associated with in-hospital mortality or discharge to home (Tables 2 and 3). Sex-stratified analyses for in-hospital mortality are presented in Supplementary Table 2. CFS >5 and CCI conferred a relatively greater risk in men, whereas AKI was a significant risk factor in women only. The Harrell C indices for the models indicated that age and sex (Table 2) or age alone (Supplementary Table 2) performed poorly in predicting the outcomes, and adding the frailty measures, CCI and AKI increased the models' predictive accuracies to a fair level.
Table 2.
Survival Analysis of Short-Term In-Hospital Mortality in Patients With COVID-19
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
Model 5 HR (95% CI) |
Model 6 HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Age | 1.05 (1.01‒1.08)∗ | 1.04 (1.00‒1.08)∗ | 1.05 (1.01‒1.09)∗ | 1.05 (1.01‒1.09)∗ | 1.06 (1.02‒1.10)∗ | 1.05 (1.02‒1.09)∗ |
| Male sex | 1.43 (0.79‒2.60) | 1.42 (0.78‒2.57) | 1.64 (0.79‒2.60) | 1.34 (0.74‒2.43) | 1.21 (0.65‒2.25) | 1.11 (0.60‒2.06) |
| CFS >5 | 1.93 (1.02‒3.65)∗ | 1.85 (0.97‒3.52) | ||||
| HFRS | 1.00 (0.91‒1.10) | 0.94 (0.85‒1.04) | ||||
| CCI | 1.27 (1.02‒1.58)∗ | 1.32 (1.07‒1.65)∗ | ||||
| AKI | 3.79 (1.66‒8.63)∗ | 4.02 (1.68‒9.60)∗ | ||||
| Harrell C | 0.65 | 0.67 | 0.65 | 0.66 | 0.71 | 0.73 |
CI, confidence interval; HR, hazard ratio.
Model 1‒5: univariate models adjusted for age and sex, Model 6: multivariate model.
P < .05.
Table 3.
Survival Analysis for Discharged to Home in Patients With COVID-19
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
Model 5 HR (95% CI) |
Model 6 HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Age | 0.97 (0.95‒0.99)∗ | 0.97 (0.94‒0.99)∗ | 0.97 (0.95‒0.99)∗ | 0.97 (0.94‒0.99)∗ | 0.96 (0.94‒0.99)∗ | 0.97 (0.94‒0.99)∗ |
| Male sex | 0.88 (0.59‒1.3) | 0.85 (0.57‒1.25) | 0.89 (0.59‒1.33) | 0.88 (0.59‒1.32) | 0.91 (0.61‒1.35) | 0.90 (0.60‒1.34) |
| CFS >5 | 0.38 (0.25‒0.58)∗ | 0.42 (0.28‒0.65)∗ | ||||
| HFRS | 0.92 (0.85‒0.99)∗ | 0.93 (0.87‒1.01) | ||||
| CCI | 0.94 (0.81‒1.10) | 0.95 (0.82‒1.11) | ||||
| Hypertension | 1.92 (1.27‒2.88)∗ | 1.83 (1.23‒2.71)∗ |
CI, confidence interval; HR, hazard ratio.
Model 1‒5: univariate models adjusted for age and sex, Model 6: multivariate model
P < .05.
Discussion
The results of this study in hospitalized geriatric patients with COVID-19 show that frailty assessed as CFS >5 and CCI were predictive of in-hospital mortality, whereas HFRS was not. Both CFS >5 and higher HFRS were associated with decreased probability of being discharged back to home, but similar to the former association, CFS presented the strongest effect. Apart from AKI, which was predictive of in-hospital mortality, and hypertension, which was predictive of discharged to home, none of the individual comorbidities were associated with either outcome. Higher age was also associated with in-hospital mortality and decreased probability of being discharged to home; however, the model including only age and sex (model 1) had a poor predictive accuracy. Adding CFS >5, CCI and AKI to the model for in-hospital mortality increased its predictive accuracy to a fair level. A sex-stratified analysis further showed that the CFS >5 and CCI were associated with greater risk of in-hospital mortality in men, whereas AKI was a significantly greater risk factor in women. However, because of the relatively low power in the sex-stratified analysis, larger studies are needed to confirm whether some of the risk factors for COVID-19-related outcomes are sex-specific.
To the best of our knowledge, our study is the first one to analyze the joint associations of frailty and comorbidity–measured as CCI and individual disease diagnoses–on COVID-19 outcomes. Our results on frailty are in line with those of De Smet et al who reported that CFS was associated with COVID-19 survival in hospitalized older adults in Belgium.7 However, in contrast to De Smet et al who used the CFS as a continuous measure, we tested different cut-offs for the CFS to identify the clinically most meaningful cut-off for increased risk. We observed that the risk for in-hospital mortality showed most prominent increase from scores more than 5. Our results on CCI are in accordance with those of Bezzio et al who analyzed COVID-19 outcomes in an Italian sample of patients (median age of 48 years) with inflammatory bowel disease. They found that in addition to older age and active inflammatory bowel disease, CCI score >1 was associated with negative outcomes, such as pneumonia, hospitalization, respiratory therapy, and death.13 CCI was also assessed in a sample of patients hospitalized with COVID-19 in the New York City area.14 Although the prognostic value of CCI on the COVID-19 outcomes was not modeled, the authors reported a median CCI score of 4 in the patients, which is seemingly higher than in our sample, and reflects a significant comorbidity burden in hospitalized patients with COVID-19. It should be noted, however, that our data included comorbidities only if they were of relevance to the current admission. Hence, it is possible that our study has underreported other diagnoses, leading to less likelihood of detecting effects from comorbidities on COVID-19 outcomes. Nevertheless, we found AKI to be associated with in-hospital mortality, with stronger effects in women. However, the acute state of AKI is likely an indicator of the COVID-19 severity rather than a marker of an underlying (kidney) disease.
In a recent analysis on population vulnerability to COVID-19, Sweden was identified among the high-risk countries. This was concluded based on the high proportions of older adults, and high rates of years lived with disability because of medical conditions considered risk factors for severe COVID-19.15 Frailty has also been highlighted as one of the conditions posing an increased risk in older individuals with COVID-19.16 , 17 It is, therefore, pertinent to set the focus on identifying those factors that predict COVID-19-related outcomes in older populations. Especially for a new disease like COVID-19, studies improving prognosis are urgently needed because there are no evidence-based clinical guidelines to follow.18 Although the overall COVID-19 death rate in our sample was high compared with the patients hospitalized for other diagnoses (24% vs 4%), and higher age was an independent risk factor for mortality, not all old individuals had the same risk. That is, three-quarters of the geriatric patients with COVID-19 survived and 44% were able to return home directly after hospitalization. Our results, thus, suggest that both CFS and CCI can be used to complement risk assessments and identify geriatric patients who are in need of more focused care. However, as noted in a recent commentary on frailty in the face of COVID-19,17 frailty is not synonymous to end-of-life. Hence, the knowledge now accumulating on how to treat hospitalized patients with COVID-19 should be used to improve survival of the most vulnerable individuals.
Our study has several strengths. Compared with other studies published on this topic, we use a large sample of hospitalized geriatric patients including both those diagnosed with and without COVID-19. We also include 2 different assessments of frailty, both the established CFS, and the more recently developed HFRS, and assess comorbidity not only as individual disease diagnoses but also using the CCI. In addition, we perform multivariate statistical modeling taking into account the competing risk of death on discharged to home, and analyze the added predictive value of frailty and comorbidity using the Harrell C statistic that facilitates interpretations.
Our study also comes with some limitations, and the results need to be interpreted in light of these limitations. First, the population under study is drawn from a geriatric hospitalized sample of older adults in need of hospital care. Hence, the results are not generalizable to the wider population of Sweden and other older individuals. Second, because of the selected sample, some part of the analysis may suffer from selection bias and effects such as collider bias (when a risk factor is interpreted as a protective factor in multivariate models because of underlying correlations19) may be in place. For example, we observe in our data that hypertension shows a protective effect on COVID-19 survival, although this may be an artifact from correlations with the usage of antihypertensives or other factors. Third, we only report short-term outcomes as our data are collected from in-hospital records only. A longer follow-up with record linkage is planned as a continuous study, and adding more variables to the models may also improve the prognostic C-statistic value. Forth, as the CFS is assessed at admission, recall bias may be an issue here. Finally, as alluded to above, diagnoses available in the electronic medical records were only those related to the current admission. Hence, it is likely that we missed a number of comorbidities in the patients with COVID-19, resulting in a lower CCI and less ability to test other diseases as risk factors.
Conclusions
In conclusion, the results in this study show that 76% of patients with COVID-19 survived, indicating that providing hospital level care to frail older patients with COVID-19 is not futile. Although higher age is also associated with in-hospital mortality and decreased probability of discharged back to home in geriatric patients, including frailty and comorbidity assessments to the models improves their predictive accuracy. Therefore, such assessments can help to better identify older patients with COVID-19 who are at risk of adverse outcomes and, thus, in need of a more multi-dimensional care, both from an acute care as well as from a rehabilitation care perspective.
Footnotes
S.H. and J.J. shared first author position.
The authors are supported by grants from the Swedish Research Council, the Stockholm County Council (ALF Project and SU-Region Stockholm Project), Stiftelse Stockholms Sjuhem, Knut and Alice Wallenberg Foundation, Karolinska Institutet, King Gustaf V:s and Queen Victorias Freemason Foundation, Osterman Foundation, the Strategic Research Program in Epidemiology at the Karolinska Institutet. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
The authors declare no conflicts of interest.
Appendix
Supplementary Table 1.
Characteristics of the Patients With COVID-19 Admitted to Geriatric Care in Stockholm During the SARS-CoV-2 Outbreak Stratified by Sex
| Men | Women | P for Difference∗ | |
|---|---|---|---|
| Patients, n (%) | 120 (48) | 130 (52) | |
| Age y, mean (SD) | 79.98 (8.32) | 81.96 (8.7) | .07 |
| Deaths, n (%) | 31 (26) | 28 (22) | .51 |
| Discharged to home, n (%) | 48 (40) | 62 (48) | .27 |
| Comorbidity | |||
| CCI, median (range) | 1 (0‒8) | 1 (0-5) | .09 |
| Diabetes, n (%) | 44 (37) | 34 (26) | .10 |
| CVD, n (%) | 13 (11) | 6 (5) | .10 |
| Hypertension, n (%) | 66 (55) | 78 (60) | .50 |
| Asthma, n (%) | 2 (2) | 13 (10) | .01 |
| COPD, n (%) | 26 (22) | 21 (16) | .34 |
| CKD, n (%) | 22 (18) | 14 (11) | .13 |
| AKI, n (%) | 11 (9) | 5 (4) | .14 |
| Dementia, n (%) | 13 (11) | 25 (19) | .09 |
| CFS, n (%) | |||
| 1‒5 | 63 (52) | 57 (44) | .38 |
| 6‒9 | 41 (34) | 54 (42) | |
| Missing | 16 (13) | 19 (15) | |
| HFRS, median (range) | 2.8 (0-12.8) | 2.85 (0-17) | .80 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; SD, standard deviation.
P for difference between men and women COVID-19 patients using t-test for age, Wilcoxon rank-sum test for HFRS and CCI and χ2 test for other variables.
Supplementary Table 2.
Survival Analysis of Short-Term In-Hospital Mortality in COVID-19
| Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
Model 5 HR (95% CI) |
Model 6 HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Men | ||||||
| Age | 1.05 (1‒1.1) | 1.04 (0.99‒1.09) | 1.05 (1‒1.1) | 1.03 (0.98‒1.09) | 1.05 (1‒1.11) | 1.02 (0.97‒1.08) |
| CFS>5 | 2.2 (0.87‒5.56) | 2.59 (1‒6.73) | ||||
| HFRS | 0.99 (0.86‒1.15) | 0.94 (0.81‒1.1) | ||||
| CCI | 1.58 (1.09‒2.28)∗ | 1.75 (1.21‒2.53)∗ | ||||
| AKI | 1.96 (0.65‒5.92) | 1.86 (0.58‒6.03) | ||||
| Harrell C | 0.68 | 0.67 | 0.68 | 0.73 | 0.70 | 0.77 |
| Women | ||||||
| Age | 1.05 (1‒1.11) | 1.04 (0.99‒1.1) | 1.05 (1‒1.11) | 1.05 (1‒1.11)∗ | 1.07 (1.01‒1.13)∗ | 1.08 (1.02‒1.14)∗ |
| CFS >5 | 1.66 (0.69‒3.98) | 1.53 (0.59‒3.97) | ||||
| HFRS | 1 (0.89‒1.13) | 0.9 (0.78‒1.04) | ||||
| CCI | 1.13 (0.83‒1.54) | 1.19 (0.86‒1.64) | ||||
| AKI | 13.09 (3.91‒43.8)∗ | 20.02 (4.63‒86.51)∗ | ||||
| Harrell C | 0.62 | 0.66 | 0.62 | 0.62 | 0.73 | 0.76 |
CI, confidence interval; HR, hazard ratio.
P < .05.
References
- 1.The Swedish Intensive Care Registry. https://www.icuregswe.org/en/data--results/covid-19-in-swedish-intensive-care/ Available at:
- 2.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clegg A., Young J., Iliffe S., et al. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva: 2017. WHO clinical consortium on healthy ageing: Topic focus: frailty and intrinsic capacity: report of consortium meeting, 1–2 December 2016 in Geneva, Switzerland. [Google Scholar]
- 5.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert T., Neuburger J., Kraindler J., et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Smet R., Mellaerts B., Vandewinckele H., et al. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932.e1. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., et al. False-negative results of initial rt-pcr assays for COVID-19: A systematic review. medRxiv. 2020 doi: 10.1371/journal.pone.0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahamtan A., Ardebili A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev Mol Diagn. 2020;20:453–454. doi: 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kucirka L.M., Lauer S.A., Laeyendecker O., et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson M.E., Pompei P., Ales K.L., et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 13.Bezzio C., Saibeni S., Variola A., et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut. 2020;69:1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 14.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyper G.M.A., Assuncao R., Cuschieri S., et al. Population vulnerability to COVID-19 in Europe: A burden of disease analysis. Arch Public Health. 2020;78:47. doi: 10.1186/s13690-020-00433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moug S., Carter B., Myint P.K., et al. Decision-making in COVID-19 and frailty. Geriatrics (Basel) 2020;5:30. doi: 10.3390/geriatrics5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard R.E., Maier A.B., Hilmer S.N., et al. Frailty in the face of COVID-19. Age Ageing. 2020;49:499–500. doi: 10.1093/ageing/afaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart J.C. Making the call. N Engl J Med. 2020;383:e46. doi: 10.1056/NEJMp2014108. [DOI] [PubMed] [Google Scholar]
- 19.Griffith G., Morris T.T., Tudball M., et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. medRxiv. 2020 doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]