Graphical abstract

Keywords: Network pharmacology, Rhizoma polygonati, Molecular docking, New coronavirus pneumonia, COVID-19
Abbreviations: COVID-19, corona virus disease-2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; PPI, protein-protein interaction network; ACE2, angiotensin converting enzyme II; S, spike protein; M, membrane; E, envelope; N, nucleocapsid; RdRp, RNA dependent RNA polymerase; 3CL, 3C-like proteinase
Abstract
Rhizoma Polygonati (huangjing in Chinese, 黄精) is a medicine food homology herb used as a component of traditional Chinese medicine treating COVID-19 in the current pandemic emergency in China but the mechanisms remain elusive. Here using TCMSP and Swiss Target Prediction databases to sort out the potential targets of the main chemical components and GenCLiP3, NCBI, and GeneCard databases to search for COVID-19 related targets, the chemical compound-target-pathway network was analyzed. Each component was molecularly docked with host cell target angiotensin converting enzyme II, SARS-CoV-2 targets Spike protein, RNA-dependent RNA polymerase, or 3CL hydrolase. Our results showed a higher affinity of the compound diosgenin and (+)-Syringaresinol-O-beta-D-glucoside binding to the three SARS-CoV-2 proteins compared to the other compounds tested. Thus, our data suggest that potential compounds in Rhizoma Polygonati may act on different targets with viral and cancer related signaling and have a great potential in treatment of COVID-19.
1. Introduction
New coronavirus (SARS-CoV-2) has been continue spreading and causing huge mortalities. According to World Health Organization, the disease induced by SARS-CoV-2, i.e. “COVID-19” (WHO, 2020), is highly infectious, leading to pandemic all over the word. The main symptoms of COVID-19 are fever, fatigue, dried cough, breathe difficulty and a small number of patients may be accompanied without any symptoms (WHO, 2020). The genome of the SARS-CoV-2 has been identified by sequencing with coding 4 proteins of spike (S), membrane (M), envelope (E), nucleocapsid (N) for structure, RNA dependent RNA polymerase (RdRp) and 3CL proteinase for life cycle and infection (Wu et al., 2020).
According to the information obtained by the experts of traditional Chinese medicine (TCM) against new coronary pneumonia based on the clinical frontline, this infectious disease is designated as “cold and damp disease” (New Coronavirus Pneumonia Collaboration Group, 2020). The pathogenesis can be summarized as dampness, poison, blood stasis, and deficiency function of lung (New Coronavirus Pneumonia Collaboration Group, 2020). The medicine inducing intense warmth and dryness, disperse the qi and dispel the cold, then keep the knee open to clear the cold, may restore normal physiological function, and finally the disease may be healed. Therefore, some TCM multiple compound, such as Lianhua Qingwen with main compound of Emodin has been suggested and applied to against the SARS-CoV-2 virus (Runfeng et al., 2020).
Rhizoma Polygonati, also named as huangjing, which rhizome is used as medicine, is one of medicine food homology herbal from plant belonging to the family Liliaceae Polygonatum. According to the “Flora of China”, there are about 40 species of the RP plant in the global northern temperate zone, with 31 species in China, distributed throughout the country, and some species are unique to specific regions. In ancient times, Rhizoma Polygonati has been used as alternative food for shortage of food as it contains polysaccharide. It has been recorded in the ancient pharmacology books that other unknown nutrition could help weight loss, anti-fatigue, and reduce hair whiten. Moreover, modern and ancient pharmacological records showed that Rhizoma Polygonati has a wide pharmacological function in antibacterial, antiviral, immunity enhancement, anti-ageing, anti-cancer, anti-diabetes, anti-fatigue, and anti-heart disease (Zhao et al., 2018), suggesting the multiple compounds are enriched in Rhizoma Polygonati. For example, Zhao et al. isolated a low molecular weight Polygonatum polysaccharide with anti-herpes simplex virus of type I and type II activity, in eyes (Zhao et al., 2018). Most importantly, Rhizoma Polygonati has been recommended as treatment of patients with symptoms of deficiency of lung, spleen and weakness of qi (Zhejiang University School of Medicine, 2020).
Network pharmacology in TCM combines with multidisciplinary technologies, such as systems biology, and computational biology in order to build a complex network between drug-target-diseases, and elucidate the mechanism of drugs in treatment (Luo et al., 2020). Molecular docking in TCM is also a computer-based drug design technology that simulates the geometric structure of molecules and the interaction force between molecules through stoichiometric calculation methods (Wang et al., 2019, Wang et al., 2019). The aim is to seek for the best binding mode of small molecule drugs and large molecules (proteins) with known structures. Using both techniques, some Chinese medicine compounds have been identified to have potential treatment effect on COVID-19 or other diseases (Wang et al., 2020, Yu et al., 2020).
Here we performed a network pharmacology analysis of the targets in Rhizoma Polygonati and COVID-19, aiming to identify the biological pathways of Rhizoma Polygonati which may be used for targeting SARS-CoV-2 entry, RNA genome replication and enzymes. Furthermore, molecular docking approach was performed to link the main components of drug to COVID-19 targets and evaluated the potential functions of Rhizoma Polygonati in the inhibition of COVID-19.
2. Materials and methods
2.1. Virtual TCMSP screening of the compounds of Rhizoma Polygonati
By searching the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) with Analysis Platform (Ru et al., 2014), the active compound of Rhizoma Polygonati and the potential targets were obtained. In details, we selected the “Herb name” option on the TCMSP website (https://tcmspw.com/), entered the keyword “Huangjing” in the search box to search, and the molecule name of the 38 compounds contained in Rhizoma Polygonatum was displayed. At the same time, the search results will provide pharmacokinetic information for each compound, such as oral bioavailability (OB). The OB at least 30% and drug-like (DL) at least 0.18 were set up as sorting parameters (Ahmed and Ramakrishnan, 2012, Xu et al., 2012) to search active compounds as potential drugs, and the targets of the main active compounds were obtained by searching TCMSP and Swiss Target Prediction databases (Daina, Michielin, & Zoete, 2019).
2.2. COVID-19 target collection and potential target Prediction
GeneCards database (Stelzer et al., 2016) NCBI gene database (National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/), GenCLiP3 database (Wang et al., 2019, Wang et al., 2019), were used to search COVID-19 encoding genes with “Novel coronavirus” as the key word as described in literature (Wang et al., 2020). Venny2.1.0 (Sun et al., 2019) was used to map drug targets of Rhizoma Polygonati to the disease targets of the COVID-19.
2.3. Protein-protein interaction (PPI) map and compound-target-Pathway map
The potential targets are used to construct through STRING database (Szklarczyk et al., 2019) to obtain the targets-PPI network. Cytoscape 3.7.2 (Shannon et al., 2003) software was applied to construct the drug-compound-target-pathway network.
2.4. Gene ontology (GO) enrichment and KEGG analysis
The disease-related targets obtained from screening were input into the DAVID database (Huang, Sherman, & Lempicki, 2009) by entering the list of target gene names and selecting the species as “homo sapiens ”. All target gene was named to their official gene symbol. To perform GO enrichment, threshold was set to the p< 0.01 (or equal) and the WeChat online mapping website was used to visualize the analysis results. Further, the core target was imported into the KOBAS3.0 database (Wu, Mao, Cai, Luo, & Wei, 2006), and KEGG function was used to do pathway analysis. Taking p < 0.05 (or equal) was used to sort out pathways and the top 20 KEGG pathways that meet the conditions were selected.
2.5. Molecular docking technology
The 3D structure of the active substance of Rhizoma Polygonati was searched through the pubchem website (Kim, Chen, & Cheng, 2019), and then Open Babel 2.3.2 software was used to convert the SDF file into a PDB file. The receptor proteins were searched from the Protein Data Bank database (Goodsell, Zardecki, & Di Costanzo, 2020). PyMOL software (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.) was used to perform dehydration/ligand/receptor analysis and Autodock 4.2.6 software was used to perform hydrogenation/charge calculation on proteins. Parameters of the receptor protein docking site was set to include the active pocket sites where small molecule ligands may bind. The receptor protein and ligand small molecules were converted into pdbqt format, and AutoDock Vina 1.1.2 (Trott & Olson, 2010) was used to dock the three receptor proteins with ten ligand of small molecules.
3. Results
3.1. Screening of the main active compounds and druggable targets of Rhizoma Polygonati
From TCMSP, a total of 38 compound components in Rhizoma Polygonati were obtained. Further, 12 compounds was selected to meet parameter which was set as an OB at least 30% and DL at least 0.18 as threshold. From these 12 potential active ingredients, 10 compounds were finally identified as the active chemical compound of Rhizoma Polygonati (Table 1 and Fig. 1 ). Then, a total of 129 targets corresponding to the above active compound were identified from the TCMSP database and the Swiss-TargetPrediction database.
Table 1.
Basic information of main compounds in Rhizoma Polygonati.
| Node | Mol ID | Molecule Name | Molecular Formula | Molecular Weight | OB/(%) | DL |
|---|---|---|---|---|---|---|
| 1 | MOL000546 | Diosgenin | C27H42O3 | 414.69 | 80.88 | 0.81 |
| 2 | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | C15H12O4 | 256.27 | 71.12 | 0.18 |
| 3 | MOL002959 | 3′-Methoxydaidzein | C16H12O5 | 284.28 | 48.57 | 0.24 |
| 4 | MOL006331 | 4′,5-Dihydroxyflavone | C15H10O4 | 254.25 | 48.55 | 0.19 |
| 5 | MOL009763 | (+)-Syringaresinol-O-beta-D-glucoside | C28H36O13 | 580.64 | 43.35 | 0.77 |
| 6 | MOL000358 | Beta-sitosterol | C29H50O | 414.79 | 36.91 | 0.75 |
| 7 | MOL000359 | Sitosterol | C29H50O | 414.79 | 36.91 | 0.75 |
| 8 | MOL003889 | Methylprotodioscin_qt | C52H86O22 | 446.74 | 35.12 | 0.86 |
| 9 | MOL002714 | Baicalein | C15H10O5 | 270.25 | 33.52 | 0.21 |
| 10 | MOL001792 | DFV | C15H12O4 | 256.27 | 32.76 | 0.18 |
Fig. 1.
The structure of main compounds in Rhizoma Polygonati.
3.2. Potential targets of Rhizoma Polygonati in anti-COVID-19
The 48, 346, 33 targets of COVID-19 were obtained after sorting from NCBI, GeneCards, and GenCLiP3 databases respectively. After removing duplicated targets, 363 treatment targets were found for COVID-19 or the virus. Further, by using Venny 2.1 drawing software, the 129 druggable targets of corresponding active ingredients of Rhizoma Polygonati were mapped to 363 COVID-19-related disease targets. Then finally 23 druggable targets of corresponding active ingredients of Rhizoma Polygonati was identified to be potential Rhizoma Polygonati targets in treatment of COVID-19 (Fig. 2 ).
Fig. 2.
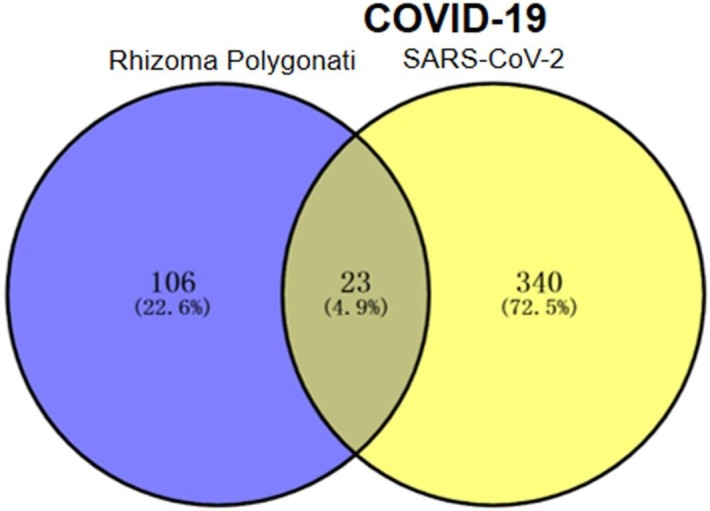
Venn diagram of Rhizoma Polygonati and COVID-19 targets.
3.3. The PPI map of the targets of the intersection of Rhizoma Polygonati and COVID-19
The 23 intersection targets obtained above were further analyzed on the STRING platform by PPI network, then the data was input into Cytoscape 3.7.2 to obtain the PPI map, which contains 23 nodes and 122 edges (Fig. 3 ). The nodes in the network graph represent proteins, and the degree value was represented by the number of lines connected to the same node, meaning the importance of each node in the network. The larger, and the darker color were in the PPI network, the greater the value was. Each edge represents the interaction between proteins in the PPI network. The more lines there were in the PPI, the greater association were found in the drug and the virus. The thickness of the line represents combine-score in the PPI network. In our result, the average node value of the target protein is 11.09, and there were 11 target proteins which had more than the average degree value. For example, CASP3, TP53, MAPK14, PTGS2, CAT, FOS, PPARG, CASP8, BCL2L1, IL2, RELA, whose value were significantly higher than that of the other targets, suggesting essential roles in the PPI map. Among them, CASP3 and TP53 had larger nodes and the darkest colors, indicating they were probably the most important potential targets in COVID-19 which Rhizoma Polygonati could work on.
Fig. 3.
PPI network of Rhizoma Polygonati and COVID-19 intersection targets.
3.4. GO enrichment analysis
The data obtained from TCMSP website were input into the DAVID database for GO analysis. Total 84 GO term were selected according to p-value parameter (p ≤ 0.01). Among them, 64 GO term are biological processes (BP), 6 GO term are cellular components (CC), and 14 are molecular functions (MF). From those GO term, it suggests that Rhizoma Polygonati compounds are involved in regulating the activities of peroxidase, prostaglandin-endoperoxide synthase, channel protein in host-virus interaction. They may function in regulation of apoptosis, DNA damage repair, redox, cell metabolism, and inflammatory response (Fig. 4 ).
Fig. 4.
GO enrichment analysis of intersection targets of Rhizoma Polygonati.
3.5. KEGG enrichment and pathway-target-component network construction
To perform KEGG pathway enrichment, the key targets of the intersection of diseases and drugs were imported into the KOBAS 3.0 database. From data analysis, a total of 188 pathways were enriched. By setting up parameter, p ≤ 0.05, 179 channels was selected, and the top 20 channels were displayed (See Table 2 ). Data analysis shows that targets were significantly enriched in multiple pathways such as cancer siganling, tuberculosis, p53 signaling, AGE-RAGE in diabetic complications, IL-17 cytokine, apoptosis, and viral infection. We further constructed the component-target-channel network diagram of Rhizoma Polygonati's mechanism of action by Cytoscape 3.7.2 software (Fig. 5 ). It showed that each active compound can act on multiple targets. There were 19 targets in 20 pathways, and 19 targets were responsive to 9 active ingredients.
Table 2.
KEGG enrichment analysis of targets.
| No. | Pathways | Gene count | No. | Pathways | Gene count |
|---|---|---|---|---|---|
| 1 | Pathways in cancer | 15 | 11 | Tuberculosis | 8 |
| 2 | Hepatitis B | 10 | 12 | AGE-RAGE signaling pathway in diabetic complications | 7 |
| 3 | Amyotrophic lateral sclerosis (ALS) | 8 | 13 | Chagas disease (American trypanosomiasis) | 7 |
| 4 | Measles | 9 | 14 | Human cytomegalovirus infection | 8 |
| 5 | Small cell lung cancer | 8 | 15 | p53 signaling pathway | 6 |
| 6 | Kaposi sarcoma-associated herpesvirus infection | 9 | 16 | Platinum drug resistance | 6 |
| 7 | Human immunodeficiency virus 1 infection | 9 | 17 | Leishmaniasis | 6 |
| 8 | Toxoplasmosis | 8 | 18 | Colorectal cancer | 6 |
| 9 | Apoptosis | 8 | 19 | Epstein-Barr virus infection | 7 |
| 10 | MAPK signaling pathway | 9 | 20 | IL-17 signaling pathway | 6 |
Fig. 5.
PPI network of Rhizoma Polygonati with pathway-target-compound analysis.
3.6. Molecular docking of the screened components of Rhizoma Polygonati and SARS-CoV-2 3CL hydrolase, ACE2, spike protein S1, SARS-CoV-2 RNA-dependent RNA polymerase
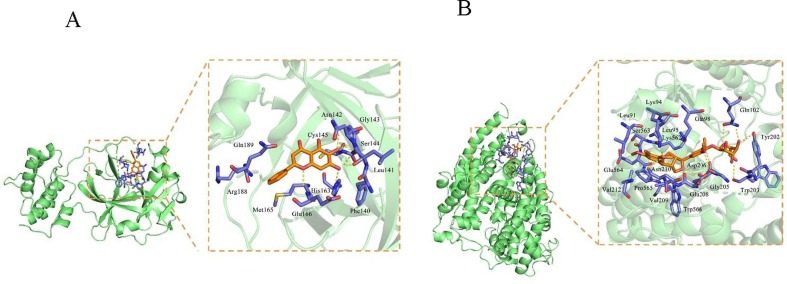
To perform molecular docking, 10 main compounds in Rhizoma Polygonati and main functional protein/drug targets of SARS-CoV-2 3CL hydrolase (PDBID: 6LU7), ACE2 (PDB ID: 1R42), Spike protein S1 (PDBID: 3BGF), RdRp (PDBID: 6M71) were selected. The active compounds of Rhizoma Polygonati were also compared to remdesivir and lopinavir which are current clinically recommended medicine. The molecular docking results showed that the 10 effective compounds had a docking score of less than −7.0 with 3CL hydrolase, ACE2, Spike protein S1, RdRp from SARS-CoV-2 virus, indicating they have strong binding activity (Table 3 ). Among them, the docking scores of diosgenin, (+)-Syringaresinol-O-beta-D-glucoside, beta-sitosterol, sitosterol, methylprotodioscin_qt, baicalein and ACE2 were significantly lower and suggetsing the stronger binding affinity than that of Remdesivir and Lopinavir. The docking results of methylprotodioscin_qt, baicalein, DFV and 3CL hydrolase are similar to the binding activity of Remdesivir. Therefore, these ingredients may be potential compounds in anti-COVID-19 in Rhizoma Polygonati. In Fig. 5, the docking results of diosgenin with the four proteins are displayed. As far as the binding mode between the compound diosgenin small molecule and the receptor protein 3CL is concerned, the amino acid residue Met276 forms a hydrogen bond interaction with the ligand small molecule, the amino acid residues of Arg131, Lys137, Asp289, Leu287, Leu286, Ala285, Gly275, Tyr239 form a hydrophobic interactions with the diosgenin (Fig. 6 A). For the binding mode between the small molecule of diosgenin and the receptor protein ACE2 (Fig. 6B), amino acid residues of Phe40, Asp350, Asp382, Ala348, His378, His401, Asn394, Arg393, Tyr385, Phe390, Trp69 form hydrophobic interactions with the small molecule diosgenin. Fig. 6C shows the binding mode between the small molecule of diosgenin ligand and Spike protein S1. Spike protein S1 amino acid residue Asn437 forms a hydrogen bond interaction with diosgenin, and amino acid residues Phe334, Lys333, Ile428, Thr431, Asn435, Tyr438, Ser336, Ala339 form a hydrophobic interaction with diosgenin. Fig. 6D shows the binding mode between the small molecule of diosgenin ligand and RdRp. The amino acid residue Lys267 and diosgenin form a hydrogen bond interaction, while the amino acid residues Pro461, Thr319, Val320, Phe321, Pro322, Trp268, Ile266, Tyr265, Ser255 and diosgenin form a hydrophobic interaction.
Table 3.
Molecular docking score.
| Molecule Names | Docking score (kcal/mol) |
|||
|---|---|---|---|---|
| ACE2 | 3CL | Spike protein S1 | RNA-dependent RNA polymerase | |
| Diosgenin | −9.2 | −8.2 | −7.9 | −9.1 |
| (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | −7.4 | −7.1 | −7.1 | −7 |
| 3′-Methoxydaidzein | −7.6 | −7.5 | −6.1 | −7.3 |
| 4′,5-Dihydroxyflavone | −7.2 | −7.1 | −6.4 | −7.3 |
| (+)-Syringaresinol-O-beta-D-glucoside | −9.5 | −7.1 | −6.8 | −7.7 |
| Beta-sitosterol | −8 | −7.5 | −6.3 | −7.5 |
| Sitosterol | −8.1 | −7.3 | −6.2 | −7.3 |
| Methylprotodioscin_qt | −8.1 | −7.6 | −6.4 | −7.9 |
| Baicalein | −7.9 | −7.8 | −6.5 | −7.3 |
| DFV | −7.5 | −7.6 | −7.1 | −7.3 |
| Lopinavir | −7.3 | −7.6 | −10.2 | −8.1 |
| Remdesivir | −7.3 | −6.8 | −8 | −6.2 |
Fig. 6.
Molecular docking of Rhizoma Polygonati compound diosgenin with targets of 3CL(A), ACE2(B), S1(C), RNA-dependent RNA polymerase (D).
In addition, we found binding mode between the small molecule of baicalein with 3CL. In details, amino acid residues Glu166, Ser144, Gly143, Cys145, Leu141, His163 form hydrogen bond interactions, amino acid residues Gln189, Arg188, Met165, Phe140, Asn142 form hydrophobic interactions (Fig. 7 A). For the binding mode between (+)-Syringaresinol-O-beta-D-glucoside small molecule and receptor protein ACE2, amino acid residues Glu564, Asn210, Lys94, Glu208, Asp206, Gly205, Trp203, Tyr202 and Gln102 form hydrogen bonding interactions with the small molecule, and amino acid residues Leu91, Lys94, Ser563, Leu95, Lys562, Val212, Pro565, Val209, Trp566 and Gln98 form hydrophobic interactions with the small molecules (Fig. 7B). Thus, based on our data, the Rhizoma Polygonati compounds have the great potential for treatment of COVID-19, which explains why the TCM plant products are recommended in the handbook of the First Affiliated Hospital, School of Medicine of Zhejiang University for emergent management of the disease.
Fig. 7.
Molecular docking of Rhizoma Polygonati compound baicalein with 3CL(A), and (+)-Syringaresinol-O-beta-D-glucoside small molecule with receptor protein ACE2 (B).
4. Discussion
Based on clinical application and recommendation evidence, we explored the potential compounds of the Rhizoma Polygonati in anti-COVID-19. Using the molecular docking we provided the mechanical insights of potential mechanisms of the druggable compounds.
It has been shown that the SARS virus (SARS-CoV) and SARS-CoV-2 could entry the host cells by viral Spike binding to the ACE2, a host cell receptor, resulting in the virus invading the human body and causing disease (Li et al., 2003, Ziegler et al., 2020). In addition, the SARS-CoV-2 enzyme, RNA-dependent RNA polymerase, is essential for synthesis of viral RNA for replication and life cycle of SARS-CoV-2 (Yin et al., 2020). TCM prescriptions for COVID-19, with complex of thousands of compounds, may target multiple signaling including above mentioned targets and host cell cytokine signaling. Thus, for precision TCM therapy, to clarify which compounds indeed play essential roles in different stages of COVID-19 would be much demand. In so short time to extract and purify the natural products is not applicable for urgent need of treatment. Therefore, using molecular docking we may speed up the drug design and screening for future experimental testing.
Rhizoma Polygonati grown in Mount Tai is one of the top grade quality herb among many kinds of species in the country by recent studies when compared to the minor trace element and polygonatum polysaccharide compounds because of its unique growth environment and local cooling climatic conditions (Zhang & Xia, 2007). Mount Tai-Rhizoma Polygonati, whose rhizome used as medicine, is one of Mount Tai's four famous Chinese medicines. Previous studies have shown that Mount Tai-Rhizoma Polygonati could significantly reduce the ears swelling caused by chemical xylene in mouse, and reduce the inflammatory reaction caused by tissue rupture in lung cancer mice (Zhang, Wei, Jia, & Zuo, 2013). Moreover, Rhizoma Polygonati could also eliminate cytokine storm of COVID-19 during treatment of lung inflammation by enhanced immune response in the mice (Zhao et al., 2018). Therefore, to explore the active ingredients and mechanism of different species of Rhizoma Polygonati based on their active compounds concentration in the treatment of pulmonary symptoms caused by the new coronavirus will help to precisely apply TCM for anti-virus. In a recent news report, Rhizoma Polygonati has been widely applied among more than 60% of anti-SARS-CoV-2 formula of TCM based on recommendation (https://k.sina.com.cn, xxxx). However, Rhizoma Polygonati potential compound need further be investigated by basic, translational research on specific targeting such as viral entry, replication, or host response. Moreover, clinical trials will also be needed to be warrant to develop Rhizoma Polygonati drugs.
In our study, diosgenin is among the top selective candidate to interact with drug targets. Diosgenin is identified as a precursor for some hormones, such as progesterone, cortisone, and also is used for oral contraceptive pills and starting materials of steroidal drugs (Marker and Krueger, 1940, Djerassi, 1992). Diosgenin is presented in medicinal plants such as Smilax and Heterosmilax species. In details, it can be purified in rhizomes of different species of Dioscorea, root extracts of Smilax China, extracts of Solanum nigrum zingiberensis berries, and extracts of Trigonella foenum graecum seeds (Jesus, Martins, Gallardo, & Silvestre, 2016). Diosgenin has pharmacological functions of antioxidant, anti-cancer, -Graves' disease, -diabetes, -bone loss, -obesity, -inflammatory, -infection (Jesus et al., 2016). Most importantly, it also exhibit anti-hepatitis C virus activity by inhibition of viral replication and downregulate protein and RNA levels (Wang et al., 2011). Thus, further experiment would warranty the potential of diosgenin in anti-COVID-19.
In addition, scientists have found polygonatum polysaccharide from Rhizoma Polygonati and compositions of sulphation Radix codonopsis polysaccharide have anti-new castle disease virus (NDV) effect, which makes the Rhizoma Polygonati promising in future emerging combating of new virus (https://patents.google.com, xxxx). Other herbal food-derived active compounds have been identified by molecular docking to target virus of COVID-19 (Yu et al., 2020). Thus, elucidating the mechanisms using molecular docking would provide a expedite screening, design and guidance for further experimental validation when new viral emergency happens.
Ethics statement
My research did not include any human subjects and animal experiments.
Data availability
All data is presented here.
CRediT authorship contribution statement
Chenglin Mu: Methodology, Validation, Performed bioinformatics analysis, Obtaining data, Data analysis, Writing - original draft. Yifan Sheng: Writing - original draft. Qian Wang: Visualization, Consulting. Amr Amin: Visualization, Conceptualization, Supervision. Xugang Li: Investigation, Supervision, Software, Writing - review & editing. Yingqiu Xie: Initiated the project, Management, Project design, Cell signaling analysis, Writing - original draft, Writing - review.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘Qian Wang works on Rhizoma Polygonati.’
Acknowledgments
Acknowledgement
Thank the Nazarbayev University Faculty-Development Competitive Research Grants Program (ID: 15798117; 110119FD4531) to Yingqiu Xie and United Arab Emirates University (UAEU)-Asian Universities Alliance (AUA) Fellowship program to Yingqiu Xie and Amr Amin. Thank the Taian Agricultural Poverty Alleviation project, Tai'an, Shandong Province. Thank Dr. Zhihong Liu from Sun Yat-sen University for providing fore-mentioned modeling and other technology for providing computation consulting.
Founding sources
Partially by Nazarbayev University Faculty-Development Competitive Research Grants Program (ID: 15798117; 110119FD4531); UAEU-AUA (2019) grant to Yingqiu Xie and Amr Amin; and partially by ZCHS fund # 31R174 for Amr Amin.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jff.2020.104149.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahmed S.S., Ramakrishnan V. Systems biological approach of molecular descriptors connectivity: Optimal descriptors for oral bioavailability prediction. PLoS ONE. 2012;7:e40654. doi: 10.1371/journal.pone.0040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Michielin O., Zoete V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Research. 2019;47:W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerassi C. Steroid research at Syntex: “The pill” and cortisone. Steroids. 1992;57:631–641. doi: 10.1016/0039-128x(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Goodsell D.S., Zardecki C., Di Costanzo L., et al. RCSB Protein Data Bank: Enabling biomedical research and drug discovery. Protein Science. 2020;29(1):52–65. doi: 10.1002/pro.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://k.sina.com.cn/article_2687386043_a02e41bb02000rw26.html?from=news&subch=onews.
- https://patents.google.com/patent/CN103520201B/en.
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Huang D., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jesus M., Martins A.P., Gallardo E., Silvestre S. Diosgenin: Recent highlights on pharmacology and analytical methodology. Journal of Analytical Methods in Chemistry. 2016;2016:4156293. doi: 10.1155/2016/4156293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Chen J., Cheng T., et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Research. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T.T., Lu Y., Yan S.K., Xiao X., Rong X.L., Guo J. Network pharmacology in research of Chinese medicine formula: Methodology, application and prospective. Chinese Journal of Integrative Medicine. 2020;26:72–80. doi: 10.1007/s11655-019-3064-0. [DOI] [PubMed] [Google Scholar]
- Marker R.E., Krueger J. Sterols. CXII. Sapogenins. XLI. The preparation of trillin and its conversion to progesterone. Journal of the American Chemical Society. 1940;62:3349–3350. [Google Scholar]
- New Coronavirus Pneumonia Collaboration Group (2020). Institute of Traditional Chinese Medicine and Clinical Medicine, Chinese Academy of Chinese Medical Sciences. Evidence-based TCM treatment recommendations for new coronavirus pneumonia (COVID-19). Chinese Medical Journal, 2020, 100 (2020-04-06).
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacological Research. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Current Protocols in Bioinformatics. 2016;54 doi: 10.1002/cpbi.5. 1.30.1–1.30.33. [DOI] [PubMed] [Google Scholar]
- Sun L., Dong S., Ge Y., Fonseca J.P., Robinson Z.T., Mysore K.S., et al. DiVenn: An interactive and integrated web-based visualization tool for comparing gene lists. Frontiers in Genetics. 2019;10:421. doi: 10.3389/fgene.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. H., Zhao, L. F., Wang, H. F., Wen, Y. T., Jiang, K. K., Mao, X. M., et al. (2019). GenCLiP 3: Mining human genes' functions and regulatory networks from PubMed based on co-occurrences and natural language processing. Bioinformatics (Oxford, England), btz807 (advance online publication). [DOI] [PubMed]
- Wang Z.F., Hu Y.Q., Wu Q.G., Zhang R. Virtual screening of potential anti-fatigue mechanism of Polygonati Rhizoma based on network pharmacology. Combinatorial Chemistry & High Throughput Screening. 2019;22(9):612–624. doi: 10.2174/1386207322666191106110615. [DOI] [PubMed] [Google Scholar]
- Wang Y.J., Pan K.L., Hsieh T.C., Chang T.Y., Lin W.H., Hsu J.T. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. Journal of Natural Products. 2011;74(4):580–584. doi: 10.1021/np100578u. [DOI] [PubMed] [Google Scholar]
- Wang Z., Sun Y., Qu R., Liu B., Fan Z., Tian J., et al. The mechanism of action of Maxing Shigan Decoction based on network pharmacology in the treatment of new coronavirus pneumonia (COVID-19) Chinese Herbal Medicine. 2020;51:1996–2003. [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Mao X., Cai T., Luo J., Wei L. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Research. 2006;34(Web Server issue):W720–W724. doi: 10.1093/nar/gkl167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang W., Huang C., Li Y., Yu H., Wang Y., et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Sciences. 2012;13:6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science (New York, N.Y.) 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.W., Wang L., Bao L.D. Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID-19) Based on molecular docking method. Journal of Functional Foods. 2020;71:104016. doi: 10.1016/j.jff.2020.104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wei C., Jia F., Zuo Li. Taishan Huangjing study on lung cancer and anti-inflammation caused by Uratan in mice. Journal of Taishan University. 2013;35(03):113–116. [Google Scholar]
- Zhang L., Xia Z. Analysis and determination of trace elements in Taishan Huangjing. China Medical Herald. 2007;26:105–106. [Google Scholar]
- Zhao P., Zhao C., Li X., Gao Q., Huang L., Xiao P., et al. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2018;214:274–291. doi: 10.1016/j.jep.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Zhejiang University School of Medicine. (2020). Chapter 12. TCM typing treatment improves curative effect. In: Handbook of COVID-19 prevention and treatment. Compiled according to clinical experience of The First Affiliated Hospital.
- Ziegler C., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is presented here.