Abstract
BACKGROUND
Knowledge of the circadian blood pressure (BP) variations in the acute hospital setting is very limited.
METHODS
This is a retrospective analysis of BP data for in-hospital patients stratified by age and sex. We used data collected with the help of a standardized electronic health record system between March 2014 and April 2018 on the adult general wards in 4 acute hospitals in Oxford, UK.
RESULTS
A total of 41,455 unique patient admissions with 1.7 million sets of vital-sign measurements have been included in the study. The typical 24-hour systolic BP profile (dipping pattern during sleep followed by a gradual increase during the day) was only seen in the younger age groups (up to 40–49 for men and 30–39 for women). For older age groups, there was a late nocturnal rise in systolic BP, the amplitude of which increased with age. The late nocturnal BP rise above the age of 50 was seen whether or not the patient was treated for or previously identified with hypertension.
CONCLUSION
Hospitalized patients’ circadian patterns of BP largely mirror those found in the community. High-quality hospital data may allow for the identification of patients at significant cardiovascular risk through either opportunistic screening or systematic screening.
Keywords: blood pressure, circadian rhythms, hospital measurements, hypertension
INTRODUCTION
Background
It is well known that in the community blood pressure (BP) is characterized by fluctuations occurring over different time scales.1 Rather than representing random phenomena, those changes are the result of interactions between environmental and behavioral factors and the cardiovascular regulation system.2 These give rise to long-term (clinic or visit-to-visit) variability,3 mid-term (day-to-day) variability, usually assessed by home monitoring,3 or short-term variability, known as circadian BP variation and assessed by 24-hour ambulatory monitoring.4 These fluctuations have prognostic importance. Recent analysis5 of data from a registry-based, multicenter, national cohort that included 63,910 adults recruited over a decade showed that measurements made over 24 hours with ambulatory BP monitoring (ABPM) were a stronger predictor of all-cause and cardiovascular mortality than clinic BP measurements.
Nighttime BP profiles can be classified into 3 patterns: dippers (subjects in whom BP falls at night), nondippers (subjects in whom BP does not fall at night), and risers or reverse dippers (subjects in whom BP rises at night).6,7 These categories have been observed from studies using ABPM,8,9 home BP monitoring,10,11 and a small-sample study in elderly hospitalized patients.12 The riser or reverse dipping pattern has been found to be more common in older patients.13–15 A systematic review by Taylor et al.4 found nighttime dipping was associated with a lower risk of cardiovascular events while rising BP at night compared with daytime values was associated with increased risk.
Measurements taken while in hospital have historically been recorded on paper charts. The advent of electronic vital-sign recording allows patterns of BP recorded in hospital to be analyzed at scale. The presence of multiple readings when patients are hospitalized may allow more reliable estimation of 24-hour BP profiles. Also, the stress associated with being in hospital may mimic laboratory studies suggesting that increased BP lability under stress itself predicts future cardiovascular events.16 Finally, being in hospital may present an opportunity to assess BP control while receiving prescribed medication.
Objectives
In this article, we determine the 24-hour BP profiles in the hospitalized adult population stratified by age and sex using a large database of 1.7 million sets of observations. We assess whether those 24-hour BP profiles are affected by the presence of a diagnosis and treatment for hypertension or affected by observations taken when patients are physiologically unstable.
METHODS
Study design
Our study is a retrospective analysis of BP data for in-hospital patients stratified by age and sex. Linked prescription data were available from the electronic patient record.
Participants
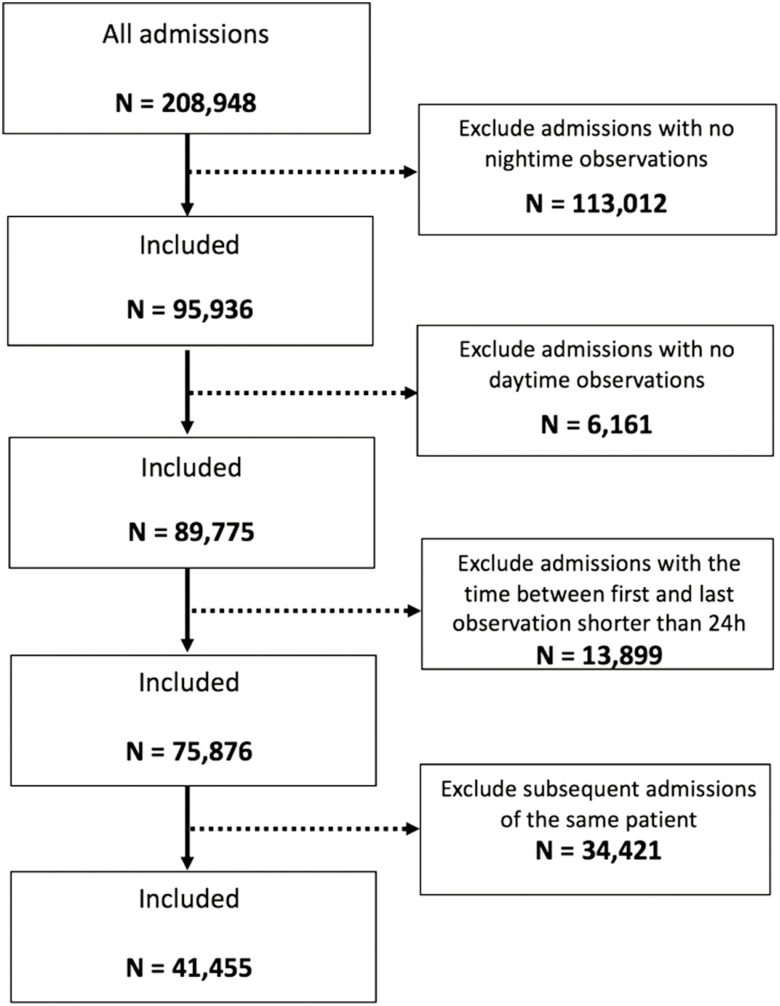
All patients aged 16 years and older admitted between March 2014 and April 2018 were eligible for the study, which was approved by the Oxfordshire Research Ethics Committee (reference: 16/SC/0264), with Confidential Advisory Group approval to process patient data without consent (reference: 16/CAG/0066). We included BP measured on all the adult wards (excluding the intensive care units) in 4 acute hospitals in the Oxford University Hospitals (OUH) NHS Foundation Trust. We included the first admission of each patient that had at least 3 observations, with at least one during the nighttime (defined as from midnight to 5:59 am) and at least one during the daytime (defined as from 10:00 am to 7:59 pm), with 2 observations at least 24 hours apart (Figure 1). The nighttime and daytime periods are defined in accordance with standard practice in the literature.7
Figure 1.
Inclusion/exclusion criteria flowchart.
Data sources
Patients had their vital-sign data, including heart rate, systolic and diastolic BP, collected using a standardized electronic health record system, System for Electronic Notification and Documentation (SEND), which records vital-sign values, calculates an Early Warning Score17 and enables the recognition of physiological deterioration on the ward.18 In the calculation of the Early Warning Score, each vital sign was assigned a weight from 0 to 3 depending on the degree of abnormality.
Nurses and health care assistants within OUH NHS Trust measure patients’ vital signs and receive regular training updates. The patient position in which BP is taken is optional. OUH NHS Trust policy recommends that observations should be undertaken a minimum of 12 hourly, with the observation frequency increasing to 4 hourly and then hourly depending on the degree of abnormality as defined by the Early Warning Score. All vital-sign equipment used within the study was purchased and maintained (including regular calibration) in accordance with the Oxford University Hospital Trust Medical Devices Management Policy. Other data (date of birth, sex, ICD-10 codes, and prescription information) were obtained from the Patient Administration System (PAS) in the hospitals’ electronic patient record, Cerner Millennium (North Kansas City, MO; https://www.cerner.com/).
Data analysis
Each participant’s BP and heart rate data were averaged by hour of the day. Individuals therefore contributed only averaged data for a maximum of 24 one-hour intervals regardless of their length of stay in hospital and number of measurements recorded. Supplementary Figures S1 and S2 illustrate typical systolic BP recordings taken during hospital stay. For the patient of Supplementary Figure S2, for example, 4 systolic BP measurements were taken between 6:00 am and 6:59 am (and plotted at hour 6:00 am in the figure) during the entire hospital stay. Subsequently, these were averaged (red line) and hourly values of averaged systolic BP constructed in a 24-hour plot (with some empty fields, the shaded regions in Supplementary Figures S1 and S2, for the 1-hour intervals for which no BP was recorded).
Mean population BP and heart rate stratified by age and sex were plotted for each hour of the day, providing summary 24-hour profiles by decade of age. To assess whether vital signs taken when patients were physiologically unstable affected our findings, we repeated this analysis by removing all observation sets, which contained a vital sign other than BP having a weight greater than 0.
We also investigated the effects of antihypertensive medication or a diagnosis of hypertension on the 24-hour BP profile. We again aggregated all the averaged data by hour of the day for each patient within 2 separate groups: (i) normotensive elderly group—patients above the age of 60 who did not have a prescription for any antihypertensive drug as listed in the British National Formulary19 (Supplementary Table S4) and who did not have an admission ICD-10 code for hypertension (Supplementary Table S5), (ii) hypertensive elderly group—patients above the age of 60 on at least one antihypertensive drug, and/or with an admission ICD-10 code for hypertension. We then compared the 24-hour BP profiles of the 2 groups, separated by sex. This analysis was restricted to patients over 60 years of age, as the hypertensive group was predominantly found within this age bracket. Finally, we explored whether differences in 24-hour profile exist between elective and emergency admissions.
The differences in BP between 2 time points were assessed using 2-sample t-test for difference of means.
RESULTS
The overall dataset included 41,455 admissions of unique patients with a total of 1,701,812 BP sets of vital-sign measurements (Figure 1).
Descriptive data
Table 1 shows patient demographics. The median length of time between the first and last BP measurement was 4.7 days, over which time a median of 27 BP measurements was taken. The frequency of admission by hour of the day is shown in Supplementary Figure S6. A more detailed description, including different age groups and hypertension categories, is also available (Supplementary Table S1).
Table 1.
Demographic descriptors
| Variable | All |
|---|---|
| Number, N (%) | 41,455 (100) |
| Sex, men, N (%) | 20,169 (49) |
| Age [years], mean (SD) | 64 (19) |
| LOM [days], median (IQR) | 4.7 (7.4) |
| Observations, median (IQR) | 27 (34) |
| Risk factor | |
| Charlson Comorbidity Index, median (IQR) | 3 (10) |
| In-hospital mortality, N (%) | 2,233 (5) |
| Theater admissions, N (%) | 20,480 (49) |
| Admission method | |
| Emergency, N (%) | 26,290 (63) |
| Elective, N (%) | 13,490 (33) |
| Other, N (%) | 1,675 (4) |
| Specialty | |
| Medical, N (%) | 18,113 (44) |
| Surgical, N (%) | 22,543 (54) |
| Other, N (%) | 792 (2) |
| Hypertension category | |
| Normotensives, N (%) | 19,312 (47) |
| Hypertensives, N (%) | 22,143 (53) |
LOM (length of measurements) denotes the time between the first and last blood pressure measurements taken during the hospital stay. IQR, interquartile range.
Patients 50–89 years of age accounted for nearly three-quarters of all admissions (70.1%). The number of men and women included in the study was similar (49% men and 51% women) with a median age of 63 years for men and 64 for women.
The frequency of BP measurements and the average value of systolic and diastolic BP, across the entire cohort, calculated for each 1-hour interval are shown in Table 2. The fewest measurements were taken between 3:00 am and 3:59 am (13,578), with the greatest number of measurements taken between 5:00 am and 5:59 am (34,158) before nursing hand-over and again soon after the nightshift starts, between 8:00 pm and 8.59 pm (32,467). The ratio between the maximum and the minimum numbers of measurements per hour was 2.5:1.
Table 2.
Frequency of measurements, systolic blood pressure values, and diastolic blood pressure values
| Hour | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | 17,663 | 17,364 | 15,524 | 13,578 | 22,488 | 34,158 | 29,171 | 20,332 | 21,260 | 27,509 | 29,961 | 25,414 |
| Mean SBP | 125 | 125 | 125 | 125 | 128 | 130 | 130 | 130 | 127 | 125 | 125 | 125 |
| SD SBP | 23 | 23 | 23 | 23 | 21 | 20 | 20 | 22 | 21 | 19 | 18 | 19 |
| Mean DBP | 67 | 67 | 67 | 67 | 69 | 70 | 70 | 70 | 69 | 68 | 68 | 69 |
| SD DBP | 13 | 13 | 14 | 14 | 12 | 11 | 11 | 13 | 13 | 11 | 11 | 12 |
| Hour | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | 22,558 | 23,702 | 26,832 | 30,151 | 29,598 | 27,069 | 24,829 | 29,012 | 32,467 | 28,211 | 22,311 | 19,127 |
| Mean SBP | 125 | 124 | 125 | 126 | 128 | 128 | 128 | 128 | 128 | 128 | 127 | 126 |
| SD SBP | 20 | 20 | 19 | 19 | 19 | 20 | 21 | 19 | 19 | 20 | 21 | 23 |
| Mean DBP | 68 | 67 | 68 | 69 | 69 | 69 | 69 | 69 | 69 | 69 | 68 | 67 |
| SD DBP | 12 | 12 | 11 | 11 | 11 | 12 | 12 | 11 | 11 | 11 | 12 | 13 |
SBP, systolic blood pressure (mm Hg); DBP, diastolic blood pressure (mm Hg).
Twenty-four-hour BP variation in hypertension and normotension
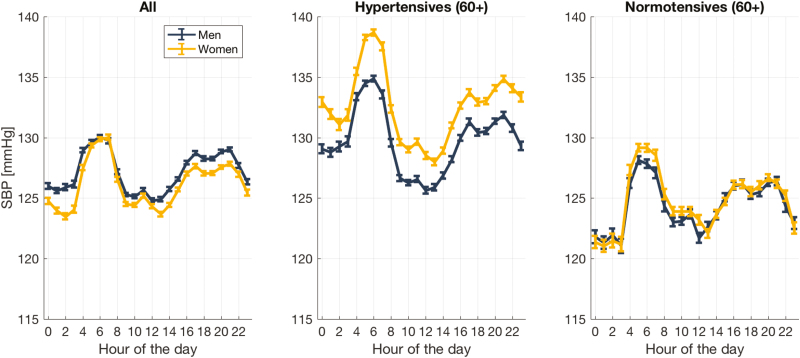
The 24-hour systolic BP variations for the overall hospitalized population (20,169 men and 21,286 women) is shown in Figure 2 (left). The general cohort experienced a late nocturnal rise in mean systolic BP between 2:00 am and 5:59 am for both men (3.8 mm Hg (95% confidence interval, 0.5)) and women (5.9 mm Hg (95% confidence interval, 0.6)) with P < 0.001 (Table 3).
Figure 2.
Twenty-four-hour (mean ± SE) systolic blood pressure profile for all admissible cohort (left); hypertensives above the age of 60 (center); and normotensives above the age of 60 for men and women.
Table 3.
Mean systolic blood pressure (mm Hg) change between 2:00 am and 5:59 am for different cohorts of patients
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Cohorts | SBP2:00 (95% CI) | SBP5:00 (95% CI) | Difference (95% CI) | SBP2:00 (95% CI) | SBP5:00 (95% CI) | Difference (95% CI) |
| All | 125.9 (0.3) | 129.7 (0.3) | 3.8 (0.5) | 123.5 (0.3) | 129.4 (0.3) | 5.9 (0.6) |
| Hypertensives (60+) | 129.3 (0.5) | 134.5 (0.4) | 5.2 (0.9) | 131.1 (0.5) | 138.3 (0.4) | 7.2 (0.9) |
| Normotensives (60+) | 122.0 (0.7) | 128.1 (0.6) | 6.1 (1.1) | 121.5 (0.7) | 129.2 (0.5) | 7.7 (1.1) |
| 20–39 years | 122.3 (0.7) | 121.0 (0.6) | −1.3 (1.3)* | 111.3 (0.6) | 112.2 (0.5) | 0.9 (1.2)** |
| 40–59 years | 124.2 (0.6) | 126.0 (0.5) | 1.8 (1.1) | 117.9 (0.6) | 121.5 (0.5) | 3.0 (1.1) |
| 60+ years | 127.3 (0.4) | 132.7 (0.3) | 5.4 (0.7) | 128.1 (0.4) | 135.4 (0.3) | 7.3 (0.7) |
SBP2:00, average systolic blood pressure (mm Hg) measured between 2:00 and 2:59 am; SBP5:00, average systolic blood pressure measured (mm Hg) between 5:00 and 5:59 am (mm Hg); difference, difference between mean systolic blood pressure (mm Hg), SBP2:00, and SBP5:00; CI, confidence intervals.
All differences are significant with P < 0.001 except *P < 0.15 and **P < 0.05.
The 24-hour systolic BP variations for hypertensive (9,102 men and 9,208 women) and normotensive (3,688 men and 4,328 women) patients of 60 years and older are shown in Figure 2 (center, right). Both hypertensive and normotensive cohorts experienced similar late nocturnal rises (between 2:00 am and 5:59 am) in mean systolic BP for both men and women (Table 3).
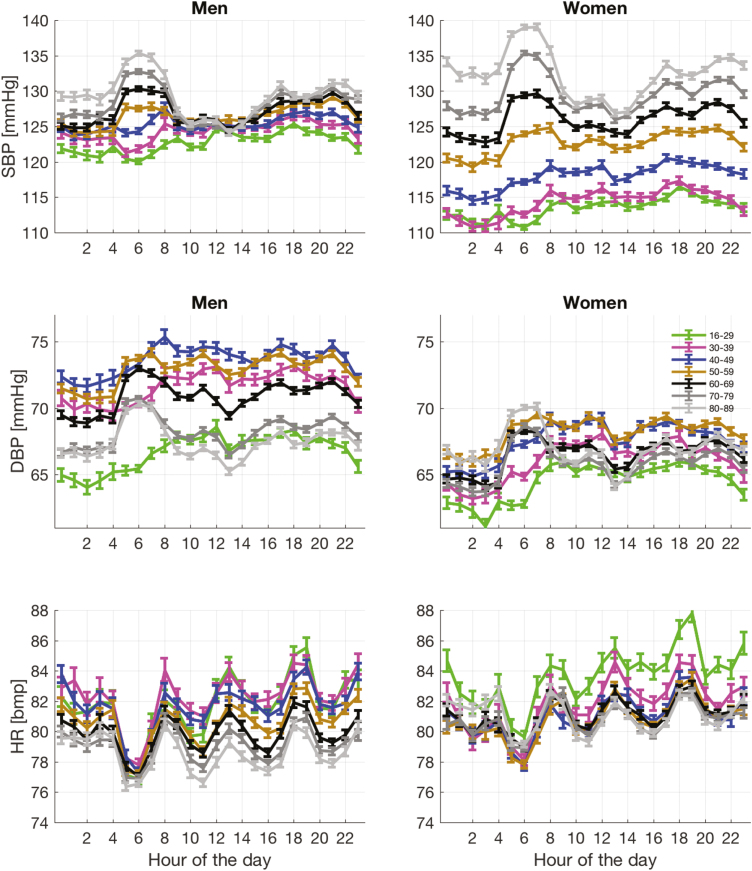
Twenty-four-hour BP variation for age categories
The 24-hour systolic BP variation for different age groups (Figures 3 and 4) shows a progressive change from the lowest recorded BP being at night in the younger age groups (dipping pattern) to the highest recorded BP being at night in the older age groups (rising pattern). The heart rate variation is shown in Figure 3; with all age groups, the heart rate decreases during the night, having its lowest value just before awakening.
Figure 3.
Twenty-four-hour (mean ± SE) systolic (top row) and diastolic (middle row) blood pressure profiles and twenty-four-hour (mean ± SE) heart rate variations stratified by age (18–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89 and 90+) for men and women.
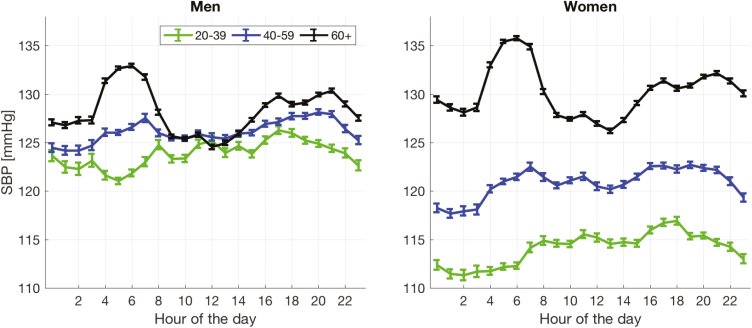
Figure 4.
Twenty-four-hour (mean ± SE) systolic blood pressure for men (left) and women (right) for three age groups 18–39, 40–59 and 60+.
Twenty-four-hour BP variation during physiological instability
The effect of physiological instability on the 24-hour systolic BP variations for hospitalized patients (Supplementary Figure S4) was assessed by constructing the 24-hour BP profile after removing all observation sets, which contained a vital sign other than BP having a weight greater than 0.
DISCUSSION
Little is known about BP characteristics and its circadian variation in the hospital setting. Using a large hospital database, comprised 1.7 million measurements collected between March 2014 and May 2018 in Oxford (UK), we have analyzed the 24-hour BP variation for men and women. The recording of BP in the hospital setting over a number of days and nights allowed us to compute 24-hour BP profiles. We excluded patients who stayed in hospital less than 24 hours, those with fewer than 3 measurements, and who did not have either daytime measurements or nighttime measurements.
In-hospital variations of mean systolic and mean diastolic BP with age were similar to those observed in the outpatient populations20,21—see also Supplementary Table S2 and Supplementary Figure S3. The 24-hour BP patterns in the overall population are also similar to those described in outpatient populations using ABPM.6,22
The nocturnal dipping pattern was not observed with in-hospital patients from late middle age onwards. Instead, it was replaced by a late nocturnal rise in BP above the age of 60, such that the peak nighttime systolic BP was greater than the peak daytime systolic BP, with the mean nighttime systolic BP also greater than the daytime systolic BP (Supplementary Table S3). The effect remained in patients with and without hypertension. To the best of our knowledge, these circadian patterns in BP in hospitalized patients have not been described. However, the patterns seem to agree with previous ABPM studies in the community.6,11
ABPM studies are normally synchronized to waking.22 Our analysis may also be serendipitously synchronized with waking, as the most common time to measure BP in the hospitals was between 5:00 am and 5:59 am. The second most common time to measure BP was between 8:00 pm and 8:59 pm, suggesting nighttime rest may also be somewhat synchronized. These external hospital-imposed rhythms may contribute to the alignment of the circadian rhythms of patients, allowing the 24-hour BP curves for subpopulations stratified by age to show very clear patterns.
The European Society of Hypertension Position Paper on ABPM states that patients with a nocturnal rising pattern have poor cardiovascular prognosis.23 Taylor et al.,4 in their systematic review and meta-analysis of multiple ABPM studies, also reported that rising BP at night compared with the daytime is associated with increased risk of cardiovascular events. Cuspidi et al.24 in their recent review highlighted that although the literature on the clinical and prognostic implications of nocturnal rising, or reverse dipping, is very limited, it is a powerful marker of poor cardiovascular prognosis, after adjusting for traditional risk factors. The concept of chronotherapy, administering antihypertensive drugs to lower BP over 24 hours, while maintaining nocturnal dipping25 may help ameliorate these risks.
Comparison with studies investigating changes in BP is hampered by differences in definition. Nocturnal hypertension is normally characterized by a simple threshold (mean systolic BP during the night greater than 120 mm Hg).9 Dipping or nondipping is assessed somewhat arbitrarily: if the mean nighttime BP is lower than the mean daytime BP by 10% or more, then the BP variation is said to follow a dipping pattern.7 The definition of the nighttime and daytime periods over which BP values are averaged to obtain the mean values varies extensively. For example, Taylor et al. report 13 different definitions of nighttime and daytime periods. We chose our ranges to be comparable to those used in most studies.7 The traditional assessment of dipping or nondipping tends to disguise the late nocturnal BP rise, which was the main characteristic of systolic BP data observed for in-hospital patients above the age of 60, as the lower BP found between midnight and 1:59 am balances the 4:00 am–5:59 am rise in the computation of mean nighttime BP.
Our study has important limitations. First, it is a retrospective study using measurements made on the wards by nursing staff as part of their regular observations of in-hospital patients. Patients who have longer hospital stays will therefore contribute more data to a hospital database of vital-sign observations. We compensated for this by generating one 24-hour BP profile per patient, regardless of their length of hospital stay. Second, the question of selection bias also arises. The patients whose BP is of most concern to the nursing staff will have their BP taken most frequently, possibly more often at night. However, patients may also get observations at night as a result of their admission time and the hospital protocol. As a result, BP was frequently recorded across the 24-hour period.
Several factors suggest that our findings may be robust to bias in sampling. In general, BP is measured more frequently in hospital to detect hypotension rather than the nocturnal hypertension described here for older patients. The late nocturnal BP rise for these patients is not accompanied by increased heart rates (see lowest plots in Figure 3), as would be expected if the presence of readings in the time period were disproportionately caused by being less well. The expected nocturnal dip in BP remains in younger patients. Removing observations taken when the patient was otherwise physiologically unstable had little effect on our findings (Supplementary Figure S4). Finally, the 24-hour BP patterns for elective and emergency patients also showed similar characteristics (Supplementary Figure S5).
It is not known whether the higher nighttime BP levels are a result of a nurse waking a patient up to fit a cuff around their arm, in comparison to it being inflated automatically as with ABPM. There may be a mechanistic link to the taking of vital-sign observations. Regular interruptions during the night may cause sleep deprivation, especially in elderly patients, which will lead to increased sympathetic activity.26 The renin–angiotensin–aldosterone system is normally activated in the early morning before arousal as a result of sympathetic neuronal activation leading to increased BP, and this may be the mechanism underlying the observed late nocturnal BP rise in the elderly in-hospital patient population.27 However, if the rise in nighttime BP was solely the result of being woken up, the phenomenon would affect all patients, regardless of their age. That is not the case, because the dipping pattern is clearly maintained below the age of 50 for men and below the age of 40 for women. In addition, the disturbance caused by the recording of the vital-sign observations would also be expected to increase the heart rate. Our results show that heart rate decreases at night for all age groups, and so it can be argued that the arousal at the time of the vital-sign recording is unlikely to be the direct cause of the nocturnal rise in BP in elderly patients.
Future work will explore the effects of different admission times and of different primary reasons for admission. The association with comorbidities also requires investigation. Outside hospital, increased nighttime systolic BP is known to be a significant risk factor for cardiovascular events.6 Understanding whether in-hospital patterns encode increased cardiovascular risk and/or persist on discharge are the next steps required to place these observations in context.
SUPPLEMENTARY MATERIAL
Supplementary material is available at American Journal of Hypertension online.
FUNDING
The research described in this paper is supported by the NIHR Biomedical Research Centre, Oxford, and by an EPSRC grant (EP/N024966/1—Intelligent Wearable Sensors for Predictive Patient Monitoring).
DISCLOSURE
A.M. is funded by an EPSRC grant (EP/N024966/1—Intelligent Wearable Sensors for Predictive Patient Monitoring) and undertakes consultancy work for Sensyne Health. P.W. works part-time for Sensyne Health and has share options in Sensyne Health. L.T. is a nonexecutive Director of Sensyne Health and holds share options in the company. R.J.M. has received BP monitors for research purposes from Omron and travel expenses and honoraria for speaking from the Japanese Society of Hypertension and the American Society of Nephrology.
ACKNOWLEDGMENTS
The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute for Health Research, or the Department of Health. This research would not have been possible without the help of Dr. Marco Pimentel (University of Oxford), who was instrumental in compiling the anonymized patient data. A.M. thank Dr. Mauro Santos (University of Oxford) for preprocessing the early version of the database.
REFERENCES
- 1. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 2013; 10:143–155. [DOI] [PubMed] [Google Scholar]
- 3. Kim KI, Nikzad N, Quer G, Wineinger NE, Vegreville M, Normand A, Schmidt N, Topol EJ, Steinhubl S. Real world home blood pressure variability in over 56,000 individuals with nearly 17 Million measurements. Am J Hypertens 2018; 31:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A. Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis. PLoS One 2015; 10:e0126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018; 378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 6. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension 2018; 71:997–1009. [DOI] [PubMed] [Google Scholar]
- 7. Staessen JA, Bieniaszewski L, O’Brien E, Gosse P, Hayashi H, Imai Y, Kawasaki T, Otsuka K, Palatini P, Thijs L, Fagard R. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc” Working Group. Hypertension 1997; 29:30–39. [DOI] [PubMed] [Google Scholar]
- 8. Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, O’Brien E, Roush GC; ABC-H Investigators Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) meta-analysis. Hypertension 2016; 67:693–700. [DOI] [PubMed] [Google Scholar]
- 9. Cuspidi C, Sala C, Valerio C, Negri F, Mancia G. Nocturnal hypertension and organ damage in dippers and nondippers. Am J Hypertens 2012; 25:869–875. [DOI] [PubMed] [Google Scholar]
- 10. Kario K, Hamasaki H. Nocturnal blood pressure surge behind morning surge in obstructive sleep apnea syndrome: another phenotype of systemic hemodynamic atherothrombotic syndrome. J Clin Hypertens (Greenwich) 2015; 17:682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida T, Kuwabara M, Hoshide S, Kario K. Recurrence of stroke caused by nocturnal hypoxia-induced blood pressure surge in a young adult male with severe obstructive sleep apnea syndrome. J Am Soc Hypertens 2016; 10:201–204. [DOI] [PubMed] [Google Scholar]
- 12. Salvo F, Lonati C, Berardi M, Errani AR, Muzzulini CL, Morganti A. Nocturnal blood pressure dipping is abolished in old-elderly hospitalized patients. High Blood Press Cardiovasc Prev 2017; 24:413–417. [DOI] [PubMed] [Google Scholar]
- 13. Su D, Guo Q, Gao Y, Han J, Yan B, Peng L, Song A, Zhou F, Wang G. The relationship between red blood cell distribution width and blood pressure abnormal dipping in patients with essential hypertension: a cross-sectional study. BMJ Open 2016; 6:e010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Satoh H, Hisamichi S. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens 1997; 10:1201–1207. [DOI] [PubMed] [Google Scholar]
- 15. Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA; African American Study of Kidney Disease and Hypertension Collaborative Research Group Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53:20–27. [DOI] [PubMed] [Google Scholar]
- 16. Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010; 55:1026–1032. [DOI] [PubMed] [Google Scholar]
- 17. Tarassenko L, Clifton DA, Pinsky MR, Hravnak MT, Woods JR, Watkinson PJ. Centile-based early warning scores derived from statistical distributions of vital signs. Resuscitation 2011; 82:1013–1018. [DOI] [PubMed] [Google Scholar]
- 18. Wong D, Bonnici T, Knight J, Morgan L, Coombes P, Watkinson P. SEND: a system for electronic notification and documentation of vital sign observations. BMC Med Inform Decis Mak 2015; 15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joint Formulary Committee. British National Formulary (BNF) No. 72. Pharmaceutical Press: London, UK, 2016, 1424 p. [Google Scholar]
- 20. Wilkins K, Campbell NR, Joffres MR, McAlister FA, Nichol M, Quach S, Johansen HL, Tremblay MS. Blood pressure in Canadian adults. Health Rep 2010; 21:37–46. [PubMed] [Google Scholar]
- 21. Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Rep 2011; 35:1–22, 24. [PubMed] [Google Scholar]
- 22. Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev 2007; 59:904–922. [DOI] [PubMed] [Google Scholar]
- 23. O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 24. Cuspidi C, Sala C, Tadic M, Gherbesi E, De Giorgi A, Grassi G, Mancia G. Clinical and prognostic significance of a reverse dipping pattern on ambulatory monitoring: an updated review. J Clin Hypertens (Greenwich) 2017; 19:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011; 57:3–10. [DOI] [PubMed] [Google Scholar]
- 26. Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens 1999; 12:63–68. [DOI] [PubMed] [Google Scholar]
- 27. Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev 2012; 16:151–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.