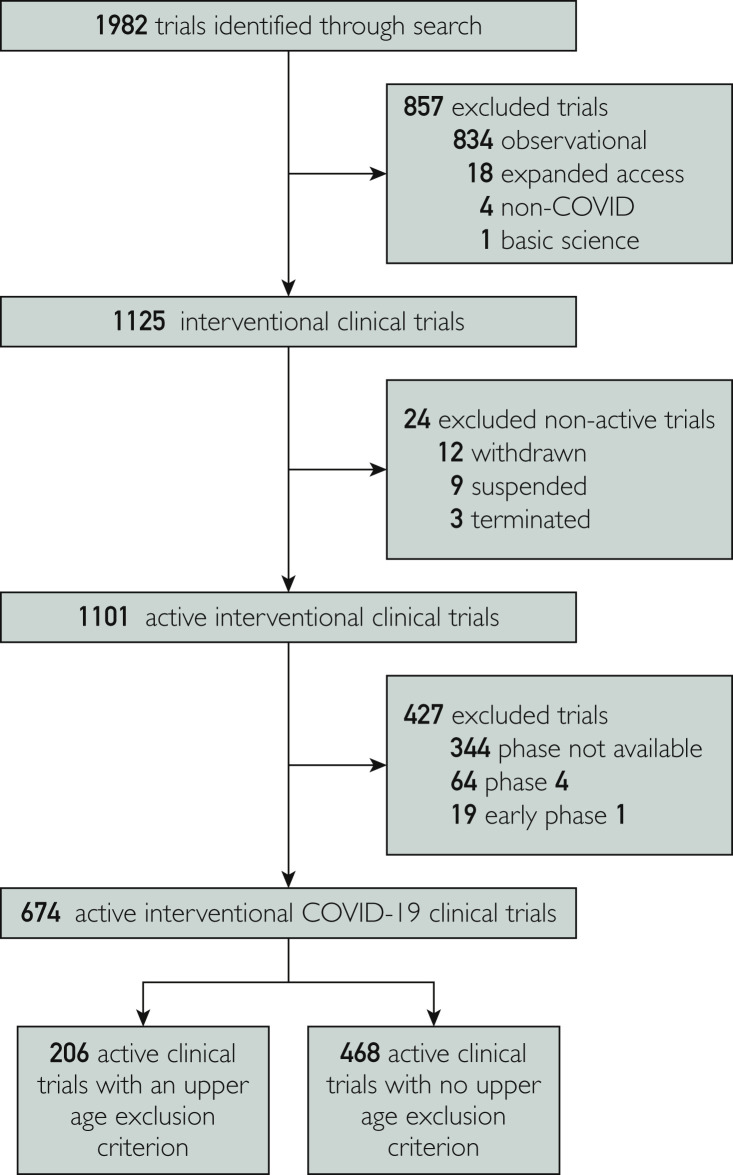
To the Editor:
The coronavirus disease 2019 (COVID-19) pandemic has resulted in the infection of millions around the world.1 , 2 The majority of COVID-19 hospitalizations and related deaths have been reported in older patients.1 , 2 As such, it is crucial for COVID-19–related trials to enroll representative patients, and to be inclusive of older patients to generate valid and generalizable results. Here, we analyze the age inclusion/exclusion criteria of current COVID-19 trials, and the enrolled participants' ages among reported trials. We performed a data query of the ClinicalTrials.gov registry for trials regarding COVID-19 on June 8, 2020 (Figure ).3 We identified trials with an upper age exclusion criterion. We also identified trials with reported results, and analyzed the age of included patients.
Figure.
Flowchart of screening, eligibility, and inclusion of coronavirus disease 2019 (COVID-19) clinical trials.
We identified 674 COVID-19 interventional trials; 206 trials (30.6%) had an upper age exclusion criterion. The median upper age exclusion was 75 years (interquartile range [IQR], 65 to 85 years). Thirteen trials (1.9%) had reported results, enrolling a total of 9014 patients. Three of 13 (23.1%) reported trials had an upper age exclusion criterion in their protocol (median exclusion age: 65 years). The median age of enrolled patients across all reported studies was 58 years (IQR, 38 to 63 years). The median age was 51 years (IQR, 39 to 62 years) among experimental arms (13 studies), and 59 years (IQR, 40 to 65 years) among control arms (seven studies). Lastly, three trials assessed a preventive intervention and had a patient median age of 36 years; the other 10 trials assessed therapeutic interventions and had a median age of 60 years.
Among identified COVID-19 trials, more than 30% include an upper age limit for patient inclusion. The exclusion of older patients from clinical trials is sometimes justified because of concerns of polypharmacy, comorbidities, consent limitations, and more, in order to preserve patient safety. Nevertheless, recent epidemiologic data on COVID-19 have shown that COVID-19 hospitalization is more common among older patients, especially patients older than 65 years.2 Not only may exclusionary eligibility criteria hinder efficient accrual of trials, but also exclusion of older patients from clinical trials dramatically increases the risk of nonrepresentative trial populations compared with real-world counterparts. Coronavirus disease 2019 mortality rates seem to be particularly high among old patients, with approximately 70% of COVID-related deaths occurring in patients older than 70 years.4 This age-related disproportionate mortality of COVID-19 highlights the imperative of trials to be inclusive of older patients. In excluding older patients, COVID-19 trials might generate results with questionable applicability to those with the greatest need. Among trials with reported results, the median age of enrolled patients was younger than 60 years, despite only a minority of reported trials using age-restrictive eligibility criteria. The accrual of younger patients to COVID-19 trials, irrespective of age-restrictive enrollment criteria, limits the generalizability of results, particularly among an emerging disease with disproportionate morbidity and mortality among older patients.1 , 2
Given the ongoing efforts to rapidly complete trials, our data underscore an opportunity for course-correction as COVID-19 trials continue to accrue. In addition to removing restrictive eligibility criteria, trialists should consider trials specifically designed for older patients, specification of anticipated age distribution for accrued patients a priori, and standardizing screening procedures to better capture older patients who may not otherwise be considered for trials.5 We urge trialists to be inclusive of older patients in order to generate clinically relevant evidence.
Footnotes
Potential Competing Interests: Dr Taniguchi is supported by funding from NIH under award R01CA227517-01A1, Cancer Prevention & Research Institute of Texas (CPRIT) grant RR140012, V Foundation (V2015-22), the Kimmel Foundation, Sabin Family Foundation Fellowship, and the McNair Foundation. All other authors report no financial disclosures or conflicts of interests related to this work.
References
- 1.Calgary O. Provisional COVID-19 Death Counts by Sex, Age, and State | Data | Centers for Disease Control and Prevention. Data. CDC.gov. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku/data
- 2.CDC. COVID View Key Updates for Week 26. Centers for Disease Control and Prevention. Published July 3, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
- 3.Kouzy R., Jaoude J.A., Garcia C.J.G., Alam M.B.E., Taniguchi C.M., Ludmir E.B. Characteristics of the multiplicity of randomized clinical trials for coronavirus disease 2019 launched during the pandemic. JAMA Netw Open. 2020;3(7):e2015100. doi: 10.1001/jamanetworkopen.2020.15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Freedman R.A., Dockter T.J., Lafky J.M. Promoting accrual of older patients with cancer to clinical trials: an alliance for clinical trials in oncology member survey (A171602) Oncologist. 2018;23(9):1016–1023. doi: 10.1634/theoncologist.2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]