Primary aldosteronism (PA) is largely subtyped into unilateral and bilateral forms. Unilateral PA is commonly attributed to an aldosterone-producing adenoma (APA), for which surgical removal of the host adrenal gland has been the treatment of choice. Many studies have demonstrated that cross-sectional imaging is unreliable in identifying an APA, primarily because co-existing sizable nonfunctional adrenal nodules are common, while APAs are often small. As such, expert guidelines recommend adrenal vein sampling (AVS) in all patients with PA older than 35 years in whom surgery is considered.1
The development of highly-specific monoclonal antibodies against human aldosterone synthase (CYP11B2)2 has revealed a wider spectrum of PA pathologies than initially perceived based on H & E histology. While CYP11B2 immunostaining remains primarily a research tool, most clinical pathology laboratories are only able to identify morphologically distinct cortical adenomas or cortical hyperplasia (irrespective of functionality in both cases). The implementation of CYP11B2 immunostaining has debunked the traditional PA subtyping, by demonstrating that the adrenal glands of patients with PA might harbor multiple APAs, micronodules, aldosterone-producing cell clusters (APCC), focal or continuous hyperplasia of the zona glomerulosa, in any combinations. A classical unilateral CYP11B2-positive APA might be neighbored by APCC or satellite micronodules within the same adrenal.3 While in the overwhelming majority of cases the contralateral adrenal is not available for study, it is highly likely that similar APCC are present in the contralateral adrenal. To what extent small CYP11B2-positive areas outside of APAs contribute to the autonomous aldosterone excess in PA is difficult to evaluate.
CYP11B2-guided DNA sequencing studies have recently identified aldosterone-driver somatic mutations in 94% of APA, but also in 15 of 57 APCC adjacent to APA.3 Affected genes associated with APA include KCNJ5 (encoding inwardly rectifying potassium channel GIRK4), ATP1A1 (encoding the alpha 1 subunit of Na+/K+-ATPase), ATP2B3 (encoding a Ca2+-ATPase), CACNA1D (encoding subunit of L-type voltage-gated calcium channel CaV1.3), CACN1H (encoding subunit of T-type voltage-gated calcium channel CaV3.2) and CTNNB1 (encoding β-catenin). Data regarding the pathogenesis of bilateral hyperaldosteronism (BHA), previously called idiopathic hyperaldosteronism (IHA) or bilateral adrenal hyperplasia (BHA) have been limited by the lack of tissue availability, as most such patients are treated medically. In a study of 15 adrenals removed from patients with BHA, true hyperplasia of the zona glomerulosa was observed in only 4 glands.4 Of 99 APCC from these BHA adrenals, 57 were found to harbor mutations in CACNA1D.4 Intriguingly, despite being acquired, and not inherited events, such APA-associated mutations have distinct racial and sex distributions, pointing to other predisposing factors, shared among specific populations. Taken together, these data suggest that, in many cases, multiple, yet asynchronous processes are likely to occur within both adrenal glands.
AVS is used in many referral centers across the globe, however, standard protocols and criteria for data interpretation are lacking.1 In view of the complex spectrum of PA histopathologies discussed above, it is not surprising that establishing if one or both adrenal glands contribute to PA through AVS is not a simple task. What AVS can accomplish, in most cases, is to determine if one of the two adrenal glands overtakes the other in producing aldosterone. What is the minimum lateralization index that indicates a clinically meaningful difference between the two adrenal glands in regard to aldosterone production to warrant unilateral adrenalectomy, however, remains elusive. In part, this is due to limited postoperative follow up data available. Moreover, in selected cases, unilateral adrenalectomy might provide clinical and biochemical benefit even to patients with BHA, by attenuating the burden of aldosterone excess.5
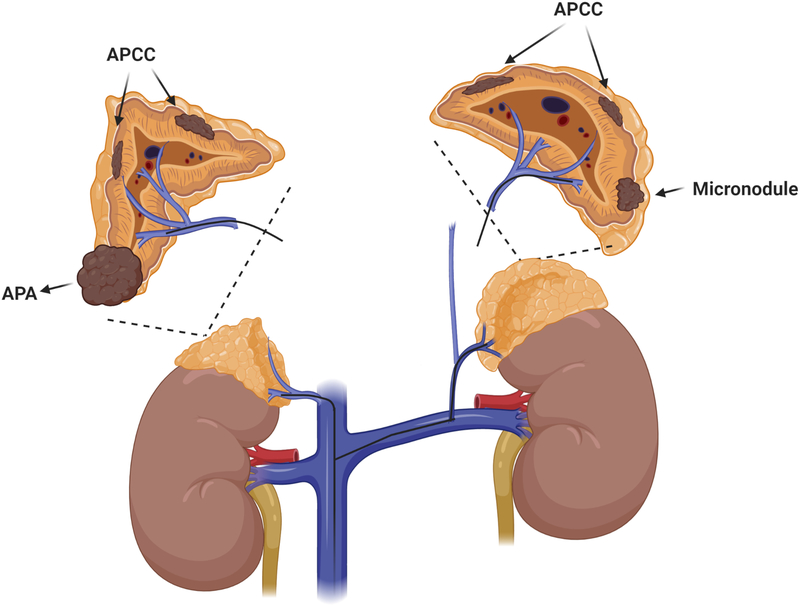
In the current issue of the journal, Kitamoto and colleagues 6 report their experience with serum sampling from segmental adrenal veins during AVS as a tool to accurately identify the intra-adrenal source(s) of aldosterone excess (Fig. 1). Such an approach could guide partial adrenalectomy, a strategy particularly useful if distinct sources of PA are found in both adrenal glands. Using a threshold of more than 1400 ng/dl cosyntropin-stimulated aldosterone in the segmental adrenal vein (threshold derived from 39 pathologically-proven APA cases supposedly without concomitant co-secretion of cortisol), segmental AVS was suggestive of APAs in 25/158 (16%) of patients with bilateral disease, who were subsequently directed to surgery. Although pathology was consistent with APAs in 24/25 patients, and only one case showed micronodules, complete biochemical success was achieved in only 28% of these patients, as compared to 98% of those who had unilateral results. These data suggest that more diffuse or multifocal PA involvement of the adrenal glands was present than what was apparent from segmental AVS.
Figure 1.
Hypothetical case of primary aldosteronism undergoing segmental adrenal vein sampling (sAVS). Based on the criterion proposed by Kitamoto et al6 of plasma aldosterone >1400 ng/dl, sAVS would localize the aldosterone producing adenoma (APA) in the right inferior adrenal pole. Multiple additional sources of autonomous aldosterone production might be present in both adrenal glands, such as aldosterone-producing cell clusters (APCC) or micronodules, which would remain undiagnosed with sAVS and could cause residual PA following partial adrenalectomy.
Compared to central AVS performed under the same conditions, segmental AVS identified 11 more unilateral PA cases within those with successful cannulation (4% of cases reclassified). Aldosterone concentrations measured in the adrenal veins are routinely normalized by cortisol, to account for variable dilution with mixed venous blood. The presence of autonomous cortisol production from one adrenal leads to ACTH suppression and consequent suppression of cortisol in the contralateral adrenal, artificially elevating the corrected aldosterone/cortisol ratio from that gland. While the authors do not report how many such cases confounded the results of central AVS, mild autonomous cortisol excess is not uncommon patients with primary aldosteronism7 It remains to be determined if segmental AVS could potentially be useful in such cases, particularly by comparing segments outside of a cortisol-producing nodule.
It is important to note that in the study of Kitamoto et al,6 segmental AVS was conducted only after cosyntropin stimulation, and after baseline and cosyntropin-stimulated samples were obtained by conventional AVS. Compared to central AVS data prior to cosyntropin stimulation, segmental AVS diagnosed fewer patients with unilateral PA. Similar discrepancies were noted between baseline and cosyntropin-stimulated central AVS results, in agreement with other published reports. The use of cosyntropin during AVS has been a major point of debate among experts in the field.1 While cosyntropin stimulation greatly enhances the rates of successful cannulation and circumvents hormonal fluctuations related to the stress of the procedure, cosyntropin could negate an aldosterone gradient between the two adrenal glands by stimulating contralateral aldosterone synthesis. Nevertheless, APAs, particularly those harboring ATPase mutations, can enhance their relative aldosterone synthesis following cosyntropin stimulation.8 If PA cases that lateralize only in the absence of cosyntropin are truly unilateral, remains difficult to demonstrate in the absence of bilateral adrenalectomy, which is not an ethical approach. Considering, however, that such cases tend to have lower aldosterone concentrations and less pronounced contralateral suppression as compared to patients who display consistent unilateral AVS results,8 the presence of asymmetrical bilateral PA is plausible. Unfortunately, the role of segmental AVS under basal conditions was not assessed in the study by Kitamoto et al.6 Expectedly, such a procedure is laborious and lengthy, increasing the risk of hormonal fluctuations during the procedure.
What segmental AVS was shown to achieve in the study by Kitamoto et al,6 was to correctly guide partial adrenalectomy in 68/120 patients with unilateral disease, all followed by complete biochemical success. Previous attempts of partial adrenalectomy targeting anatomically obvious nodularity on the adrenal gland with aldosterone lateralization on AVS have been often futile, for the same reasons that explain the high rate of discordance between cross sectional imaging and AVS data, as discussed above. It should be noted that, in this more recent study, partial adrenalectomy was only offered to patients who had a corresponding CT nodule in the tributary area indicated by segmental AVS. These tumors were also larger and were associated with higher aldosterone concentrations than cases treated with total unilateral adrenalectomy. Such tumors commonly harbor KCNJ5 mutations, which account for the vast majority of APAs in East Asians, including Japanese PA patients. If segmental AVS-guided partial adrenalectomy would be as successful in non-Asian populations, that display a wider variety of underlying aldosterone-driver mutations in APAs, remains to be determined.
CYP11B2 staining identifies cells within the zona glomerulosa where aldosterone is synthesized. In young individuals CYP11B2 expression is relatively continuous throughout the zona glomerulosa, while with aging it becomes fragmented into discrete clusters (APCC).2 The main regulator of aldosterone biosynthesis is the renin-angiotensin system, which is suppressed by a high sodium diet combined with autonomous hypersecretion of aldosterone. Consequently, the physiologic reserve of aldosterone production, both basal and after ACTH-stimulation, should be very low, but that is not always the case. The aldosterone threshold of 1400 ng/dl from the Kitamoto et al report,6 which was derived from 39 patients with classical unilateral APA, does not exclude co-existing APCC, many of which have aldosterone-driving gene mutations3 and are thus likely to also produce some aldosterone autonomously (Fig. 1). Even under stringent AVS criteria for aldosterone excess lateralization, in a considerable percentage of patients PA is not cured but improved. The biochemical criteria of normalization of plasma potassium and a renin/aldosterone ratio that is “normal” does not certify that the contralateral adrenal is normal.9 Dynamic tests in patients with improved, but not cured presumably unilateral PA following ipsilateral adrenalectomy retain an abnormal fludrocortisone suppression test10 The weight of the evidence is that many patients classified by current criteria as unilateral hyperaldosteronism, have, in fact, multifocal asymmetric disease, and removal of the adrenal producing the bulk of aldosterone results in improvement, but not cure of PA. If segmental AVS, coupled with advanced microsurgery techniques, will ever transform clinical practice for PA remains to be further investigated.
Acknowledgments
Sources of funding:
This work was supported by grants: R01 HL144847, from the National Heart, Lung and Blood Institute, 1U54GM115428 from the National Institute of General Medical Sciences, and BX004681 from the Department of Veteran Affairs awarded to C.E.GS.; and grants 1K08DK109116 from the National Institute of Diabetes and Digestive and Kidney Diseases and DDCF_2019087 from the Doris Duke Charitable Foundation, awarded to AFT.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, Widimsky J, Naruse M, Deinum J, Schultzekool L, Kocjan T, Negro A, Rossi E, Kline G, Tanabe A, Satoh F, Rump LC, Vonend O, Willenberg HS, Fuller P, Yang J, Nian Chee NY, Magill SB, Shafigullina Z, Quinkler M, Oliveras A, Chang CC, Wu VC, Somloova Z, Maiolino G, Barbiero G, Battistel M, Lenzini L, Quaia E, Pessina AC and Rossi GP. Subtyping of Primary Aldosteronism in the AVIS-2 Study: Assessment of Selectivity and Lateralization. The Journal of clinical endocrinology and metabolism. 2020;105. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H and Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Molecular and cellular endocrinology. 2014;383:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sousa K, Boulkroun S, Baron S, Nanba K, Wack M, Rainey WE, Rocha A, Giscos-Douriez I, Meatchi T, Amar L, Travers S, Fernandes-Rosa FL and Zennaro MC. Genetic, Cellular and Molecular Heterogeneity in Adrenals with Aldosterone Producing Adenoma. Hypertension. 2020;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omata K, Satoh F, Morimoto R, Ito S, Yamazaki Y, Nakamura Y, Anand SK, Guo Z, Stowasser M, Sasano H, Tomlins SA and Rainey WE. Cellular and Genetic Causes of Idiopathic Hyperaldosteronism. Hypertension. 2018;72:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukor N, Gordon RD, Ku YK, Jones M and Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. The Journal of clinical endocrinology and metabolism. 2009;94:2437–45. [DOI] [PubMed] [Google Scholar]
- 6.Kitamoto T, Omura M, Takiguchi T, Tsurutani Y, Kubo H, Yamazaki Y, Sasano H, Saito J and Nishikawa T. The precise mapping of intra-adrenal aldosterone activities provides a novel surgical strategy for primary aldosteronism. Hypertension. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O’Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CHL, Deeks JJ, Walch AK, Beuschlein F and Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wannachalee T, Zhao L, Nanba K, Nanba AT, Shields JJ, Rainey WE, Auchus RJ and Turcu AF. Three Discrete Patterns of Primary Aldosteronism Lateralization in Response to Cosyntropin During Adrenal Vein Sampling. The Journal of clinical endocrinology and metabolism. 2019;104:5867–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr., Gomez-Sanchez CE, Funder JW, Reincke M and Primary Aldosteronism Surgery Outcome i. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. The lancet Diabetes & endocrinology. 2017;5:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford JC, Taylor WL, Stowasser M and Gordon RD. Success of surgery for primary aldosteronism judged by residual autonomous aldosterone production. World journal of surgery. 1998;22:1243–1245. [DOI] [PubMed] [Google Scholar]