Abstract
The excess of orbital detection of smectite deposits compared to carbonate deposits on the martian surface presents an enigma because smectite and carbonate formations are both favored alteration products of basalt under neutral to alkaline conditions. We propose that Mars experienced acidic events caused by sulfuric acid (H2SO4) that permitted phyllosilicate, but inhibited carbonate, formation. To experimentally verify this hypothesis, we report the first synthesis of smectite from Mars-analogue glass-rich basalt simulant (66 wt% glass, 32 wt% olivine, 2 wt% chromite) in the presence of H2SO4 under hydrothermal conditions (~200 °C). Smectites were analyzed by X-ray diffraction, Mossbauer spectroscopy, visible and near-infrared reflectance spectroscopy and electron microprobe to characterize mineralogy and chemical composition. Solution chemistry was determined by Inductively Coupled Plasma Mass Spectrometry. Basalt simulant suspensions in 11–42 mM H2SO4 were acidic with pH ≤ 2 at the beginning of incubation and varied from acidic (pH 1.8) to mildly alkaline (pH 8.4) at the end of incubation. Alteration of glass phase during reaction of the basalt simulant with H2SO4 led to formation of the dioctahedral smectite at final pH ~3 and trioctahedral smectite saponite at final pH ~4 and higher. Anhydrite and hematite formed in the final pH range from 1.8 to 8.4 while natroalunite was detected at pH 1.8. Hematite was precipitated as a result of oxidative dissolution of olivine present in Adirondack basalt simulant. Formation of secondary phases, including smectite, resulted in release of variable amounts of Si, Mg, Na and Ca while solubilization of Al and Fe was low. Comparison of mineralogical and solution chemistry data indicated that the type of smectite (i.e., dioctahedral vs trioctahedral) was likely controlled by Mg leaching from altering basalt and substantial Mg loss created favorable conditions for formation of dioctahedral smectite. We present a model for global-scale smectite formation on Mars via acid-sulfate conditions created by the volcanic outgassing of SO2 in the Noachian and early Hesperian.
1. INTRODUCTION
Two global eras have been proposed to explain the observed mineralogy on Mars (Bibring et al., 2006). Abundant phyllosilicates dominated by the smectite group (nontronite, montmorillonite and saponite) were formed in the first era under water-rich neutral to alkaline conditions during the Noachian. Formation of sulfate-bearing phases occurred in the second era under acidic conditions likely caused by sulfuric acid during the Hesperian (Poulet et al., 2005; Bibring et al., 2006; Murchie et al., 2009; Bishop et al., 2013). However, such simplified pH-based division of aqueous history conflicts with some mineralogical observations. Large carbonate deposits together with phyllosilicates would be a characteristic of a Noachian Mars dominated by abundant liquid water, neutral/alkaline pH conditions, and a CO2-rich atmosphere (Fairén et al., 2004), but mineralogical observations have detected only isolated carbonate deposits (Ehlmann et al., 2008; Milliken et al., 2009; Morris et al., 2010; Wray et al., 2016). The absence of widespread carbonate deposits could result from carbonate deposition on early Mars followed by its decomposition in acidic environments during Hesperian epoch (Fairén et al., 2004; Bibring et al., 2006; Chevrier et al., 2007). Alternatively, abundant carbonates may never have formed on early Mars because of short-term stability of liquid water and/or lack of dense CO2 atmosphere during Noachian epoch (Bibring et al., 2006; Chevrier et al., 2007; Niles et al., 2013; Edwards and Ehlmann, 2015; Zolotov and Mironenko, 2016). Acidic events could also explain the apparent lack of abundant carbonate/phyllosilicate associations, because carbonate minerals do not form at acidic pH<6 conditions (Fairén et al., 2004; Fairén, 2013; Peretyazhko et al., 2016; Zolotov and Mironenko, 2016). The carbonate/smectite mineralogical observation might indicate that early Mars was not exclusively neutral-to-alkaline in pH but experienced local and perhaps widespread acidic events. As a result, smectite formation on Mars could occur not only under commonly expected neutral/alkaline conditions but also in acidic environments.
In terrestrial environments smectites formed under acidic conditions have been reported in acidic saline lakes, seafloor hydrothermal vents and fumarolic areas (Haymon and Kastner, 1986; Story et al., 2010; Hynek et al., 2013). Limited laboratory observations have revealed smectite formation through hydrothermal basalt alteration in acidic pH 3–6 environments (Berger et al., 1987; Ghiara et al., 1993; Abdelouas et al., 1997; Dehouck et al., 2014). We have demonstated formation of saponite through alteration of Mars-analogue glass-rich basalt simulant in hydrothermal oxic and anoxic systems buffered by acetic acid (200 °C, pHrt ~4 pH measured at room temperature, (Peretyazhko et al., 2016)).
The source of acidity on early Mars remains a subject for discussion. Two major acidity sources have been proposed for early Mars: (1) Fe(II) oxidative hydrolysis (Tosca et al., 2008; Hurowitz et al., 2010), and (2) volcanic release of sulfur dioxide, SO2 (Zolotov and Mironenko, 2007; Berger et al., 2009; Gaillard and Scaillet, 2009; Righter et al., 2009; Gaillard et al., 2013; Zolotov and Mironenko, 2016). We have previously hypothesized that sulfuric acid (H2SO4) produced by volcanic SO2 degassing on early Mars was the major source of acidity for the alteration of basaltic materials and subsequent formation of smectite (Peretyazhko et al., 2016). Our hypothesis was supported by modeling and mineralogical observations. For instance, recent detection of smectite minerals co-existing with sulfates might indicate that basalt weathering and smectite formation occurred under acidic conditions caused by sulfuric acid (Farrand et al., 2009; Wray et al., 2011; Cavanagh et al., 2015; Flahaut et al., 2015; Rampe et al., 2017).
Thermodynamic modeling of basalt interaction with H2SO4-rich solutions revealed formation of Al-rich phyllosilicates (kaolinite, montmorillonite) under mildly acidic conditions followed by Mg/Fe smectites at higher pH (Zolotov and Mironenko, 2007; Zolotov and Mironenko, 2016) and modelling of Mars-like aqueous systems predicted coexistence of smectite and sulfate minerals under mildly acidic pH 4–6 conditions (Fairén, 2013).
Formation of smectite through basalt alteration under Mars-relevant acid sulfate conditions has not been experimentally studied and the effect of pH and the nature of forming phyllosilicate minerals remains unknown. The objective of this work was to investigate formation of smectite through hydrothermal alteration of Mars-analogue basalt in the presence of sulfuric acid of variable concentrations, to assess acidity sources and to determine the extent to which H2SO4 is the source of acidity on early Mars.
2. MATERIALS AND METHODS
2.1. Synthesis and characterization of Adirondack basalt simulant
Synthetic basalt simulant of composition similar to that for Adirondack class rocks analyzed by the Mars Exploration Rover Spirit at Gusev Crater (McSween et al., 2006) was used in smectite formation studies (hereafter, denoted as Adirondack basalt simulant). The detailed synthesis procedure of the Adirondack basalt simulant is reported by Peretyazhko et al., (2016). Briefly, a powdered mixture of reagent-grade oxides and carbonates was melted at 1400 °C in an Au-Pt alloy crucible for 3d under the oxygen fugacity IW+1 (IW = iron-wustite buffer) and quenched in water. The glassy product was then crushed, ground and sieved to <53 μm particle diameter for characterization and smectite formation experiments. X-ray diffraction analysis revealed that Adirondack simulant contained 66 wt% X-ray amorphous glass, 32 wt% olivine and 2 wt% chromite quench crystals within the glass matrix (Peretyazhko et al., 2016). Composition of the glass phase, olivine and chromite are summarized in Table EA-1.
2.2. Hydrothermal smectite formation experiments and characterization
Adirondack basalt simulant suspensions of 17 g/l (water to rock ratio = 60) were prepared by mixing 250 mg simulant with 15 ml of solutions having variable H2SO4 concentrations (11.0 ± 0.1 mM, 13.6 ± 0.1 mM, 16.3 ± 0.1 mM, 21.9 ± 0.1 mM, 30.6 ± 0.3 mM and 42.5 ± 0.2 mM). Initial sulfuric acid concentrations were measured as total sulfate by ion chromatography as described below. Duplicate samples were placed in batch reactors (Teflon lined 23 ml Parr acid digestion vessel) and incubated in an oven at 200 °C for 14d. The temperature of 200 °C was chosen because hydrothermal conditions are favorable for smectite formation promoting breaking of chemical bonds and rapid basalt alteration (Berger et al., 2014). However, smectite formation through alteration of basalts has been shown to occur at lower temperatures (Seyfried et al., 1978; McMurtry and Yeh, 1981; Kloprogge et al., 1999; Hynek et al., 2013). Sample preparation and hydrothermal incubation were performed under ambient atmosphere (oxidizing conditions). Our previous study (Peretyazhko et al., 2016) demonstrated that smectite formed through alteration of Adirondack basalt simulant under oxidizing and reducing acidic hydrothermal conditions and that oxidizing conditions only affected Fe oxidation state in smectite.
Suspension pH was measured immediately after mixing Adirondack basalt simulant with H2SO4 (pH0d) and after opening the reactors at the end of 14d incubation (pH14d) with AccupHast pH electrode using Orion Star A329 meter. Both measurements were performed at room temperature (RT) of ~25 °C. After pH measurement, solution was collected in a syringe and passed through a 0.2 μm syringe filter (PVDF, Fisherbrand). An aliquot of each filtered solution was acidified with ultra-pure HNO3 (Fisher) for dissolved Ca, Mg, Na, Si, Al and Fe analysis by Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Element XR Thermo Scientific) with argon as the carrier gas. The remaining solution was analyzed for sulfate by ion chromatography using Dionex ICS-2000 equipped with Dionex IonPac AS18 column (4 × 250 mm), Dionex EGC III KOH eluent and a suppressed conductivity detector Dionex AERS 500 4 mm with a 20 μm injection volume.
Separated solids were dried in an oven at 40 °C for ~1h prior to analysis. X-ray diffraction (XRD) patterns were recorded using a Panalytical X’Pert Pro with Co Ka radiation. Samples were analyzed at 45 kV/40 mA with a 0.02° 2θ step for 1 min. Programmable divergent and antiscatter slits were set to 1/4° with manual 1/2° antiscatter slit. The instrument was operated under ambient conditions and calibrated with novaculite (quartz) standard (Gemdugout, State College, PA). Standard powder mounts and low background silicon holders were used for analyses of bulk samples and clay size fractions, respectively. Clay size fractions were separated by ultrasonic dispersion (Kachanoski et al., 1988). Smectite-containing suspensions were ultrasonically treated with a probe-type sonicator (Soniprep 150) for 10s. The suspension was then allowed to sediment by gravity for ~1hr and supernatant containing the clay size fraction was collected. The procedure was repeated until supernatant solutions became clear and the collected clay fraction was separated by centrifugation. The clay fraction slurry was spread over the low background holder with a pipette and dried at RT prior to analysis. Clay fractions were treated with glycerol and KCl in order to confirm formation of smectite (Whittig and Allardice, 1986).
Visible and near-infrared reflectance (VNIR) spectra (0.35–2.5 μm) were measured with an Analytical Spectral Devices FieldSpec3 fiber-optic based spectrometer with a reflectance probe attachment (internal light source). Spectra were collected in absolute reflectance mode using a Spectralon standard. Continuum removed spectra were obtained using ENVI software (ENvironment for Visualizing Images, Harris Geospatial Solutions).
Mössbauer analyses were performed at RT under ambient conditions using MIMOS-II instruments that are the laboratory equivalent of the instruments onboard the Mars Exploration Rovers [SPESI, Inc., (Klingelhoefer et al., 2003)]. Velocity calibration was carried out with the program MERView (Agresti et al., 2007) using the MIMOS-II differential signal and the spectrum for metallic Fe foil acquired at RT. Mössbauer parameters [center shift (CS) with respect to metallic Fe foil at RT, quadrupole splitting (QS), magnetic hyperfine field strength (Bhf), and subspectral areas (A)] were obtained with a least-squares fitting procedure using MERFit (Agresti and Gerakines, 2009). Reported values for A were converted to % Fe using the f-factor ratio, fFe(III)/fFe(II) = 1.21 (Morris et al., 1995). Uncertainty for CS, QS, and full width at half maximum (FWHM) was ± 0.02 mm/s and uncertainty for A was ± 2% absolute. The following constraints were used in the least squares fitting procedure. (1) For each doublet, the component peaks were constrained to have equal widths and areas. (2) Because of the low observed intensity of hematite sextets, values for CS and QS were fixed to literature values (e.g.,(Morris et al., 1985)), peak areas were constrained to 3 : x : 1 : 1 : x : 3, where x is variable and FWHM decreased symmetrically toward zero velocity with skewed Lorentzian lineshapes. Four representative samples were analyzed by Mossbauer spectroscopy covering the whole range of the final pH14d values.
Electron microprobe analyses of polished sections of smectite-containing Adirondack basalt simulant were analyzed for elemental composition using a CAMECA SX-100 electron microprobe (Figure EA-1). Backscattered electron imaging and analyses were collected at an accelerating voltage of 15 kV and probe current of 20 nA with a spot size of 1 μm. Element concentrations were calibrated using University of Tokyo Oxides standards. All measurements were made under 20s peak counting and 10s background counting. Because of high water content in smectite, total elemental content was lower than 100% in all analyzed samples. Data with totals ≥74 wt% were used for smectite formula calculation. Residual sulfur and phosphorous were considered as impurities and subtracted from the totals in formula calculations. All Si atoms were assigned to tetrahedral sites and the remaining tetrahedral sites were filled with Al (Al = 8-Si). The remaining Al atoms, Mg, Fe and minor Ti, Ni, Cr and Mn were assigned to the octahedral sites, while Na, Ca and K were assigned to interlayer sites (Bain and Smith, 1987).
2.3. Calculations of sulfur degassing on Mars
Crust production model and the amount of sulfur degassing from magma were used to determine the release of SO2 during Noachian/Early Hesperian epoch on Mars and then to obtain the total amount of acidity (total amount of protons, H+) produced from the oxidation of SO2 to H2SO4.
First, a crust production model described by Hirschmann and Withers (2008) was used to calculate crust accumulation from early Noachian (~4.5 Ga) to late Amazonian (0 Ga). The model is based on assumptions that crust formation is proportional to the heat flow and that total crust production during 4.5 Ga is 654·106 km3 as estimated by (Greeley and Schneid, 1991). The model is described by the equation:
| (1) |
where variables are V (crust production) and t (time) and constants are a = 9.277·105 km3, b = 2.385·105 km3, c = 6·10−3 Ma−1, d = 3.98·10−4 Ma−1 (Hirschmann and Withers, 2008). The calculated total crust production is shown in Figure EA-2. The amount of sulfur released from magma was then calculated as
| (2) |
where ρ is an average basalt density of 2800 kg/m3 and Smagma is the amount of sulfur degassed from magma of 2400 mg/kg (Righter et al., 2009).
3. RESULTS
3.1. pH before and after incubation
Different initial H2SO4 concentrations led to variable degrees of basalt neutralization after 14d incubation. The average initial pH for all experiments was acidic (pH0d ≤ 2), and the final pH14d varied over a wide range including acidic (pH14d 1.8 and 2.2), moderately acidic (pH14d 3.1 and 3.7), neutral (pH14d 7.2) and slightly alkaline (pH14d 8.4) (Figure 1).
Figure 1.
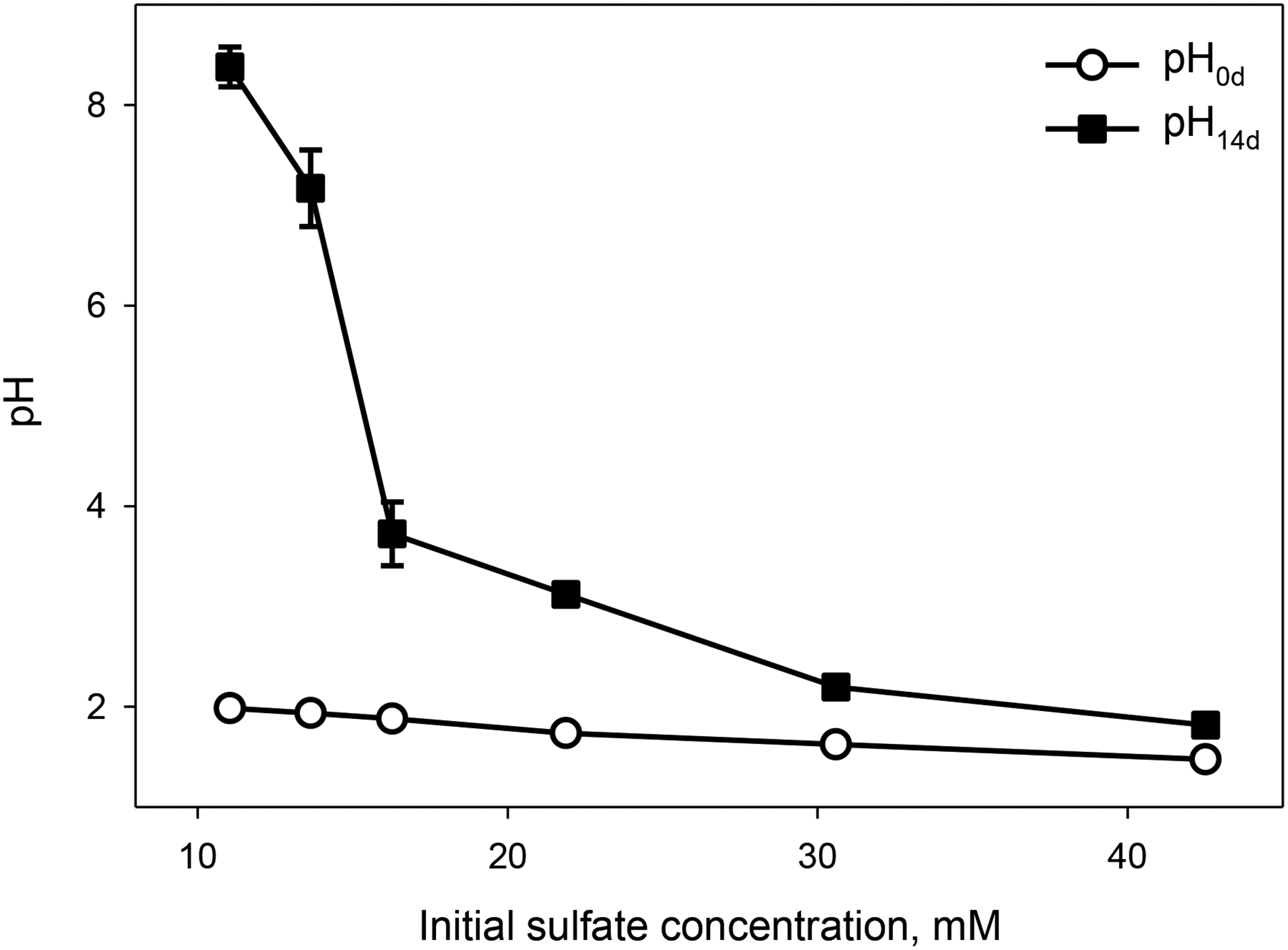
Initial (pH0d) and final (pH14d) pH in Adirondack basalt simulant suspensions as a function of initial sulfate concentrations.
3.2. Mineralogical characterization
3.2.1. X-ray diffraction
Powder XRD analyses of reaction products showed increasing phyllosilicate formation at pH14d ≥3 on the basis of increasing intensity of the 001 diffraction peak at ~15 Å while no phyllosilicate formation occurred at pH14d 1.8 and 2.2 (Figure 2a, Table 1). After separation of the clay size fraction, the as smectite based on expansion of the 001 peak to 17.4–18 Å after glycerol treatment, its collapse to 12.phyllosilicate was identified 8 Å at room temperature upon KCl addition, and its additional collapse to 10.1 Å after heating at 550 °C (Figure EA-3). The positions of 02l and 060 d-spacings allowed distinguishing dioctahedral from trioctahedral smectite (Moore and Reynolds, 1997; Vaniman et al., 2014). The 02l and 060 d-spacings revealed that trioctahedral smectite formed at pH14d 3.7, 7.2 and 8.4 and the peak positions (Figure 2b, Table 2) were within the range reported for saponite (02l: 4.52–4.64 Å and 060: 1.53–1.56 Å (Treiman et al., 2014; Chemtob et al., 2015; Peretyazhko et al., 2016)). The 02l and 060 d-spacings at 4.48 Å and 1.50 Å, respectively, indicated formation of dioctahedral smectite at pH14d 3.1 (Figure 2b, Table 2). The peak positions were in agreement with d-spacing reported for montmorillonite (02l: 4.47 Å and 060: 1.492–1.504 Å, (Moore and Reynolds, 1997; Vaniman et al., 2014)). However, formation of montmorillonite could not be confirmed with VNIR and electron microprobe analyses (sections 3.2.3 and 3.2.4) and therefore phyllosilicate formed in the pH14d 3.1 sample is referred as dioctahedral smectite. Formation of smectite in all samples was accompanied by decrease in intensity of the broad basaltic glass peak between 20° and 40° 2θ with respect to the unaltered material (Figure 2a) suggesting glass as the smectite progenitor.
Figure 2.
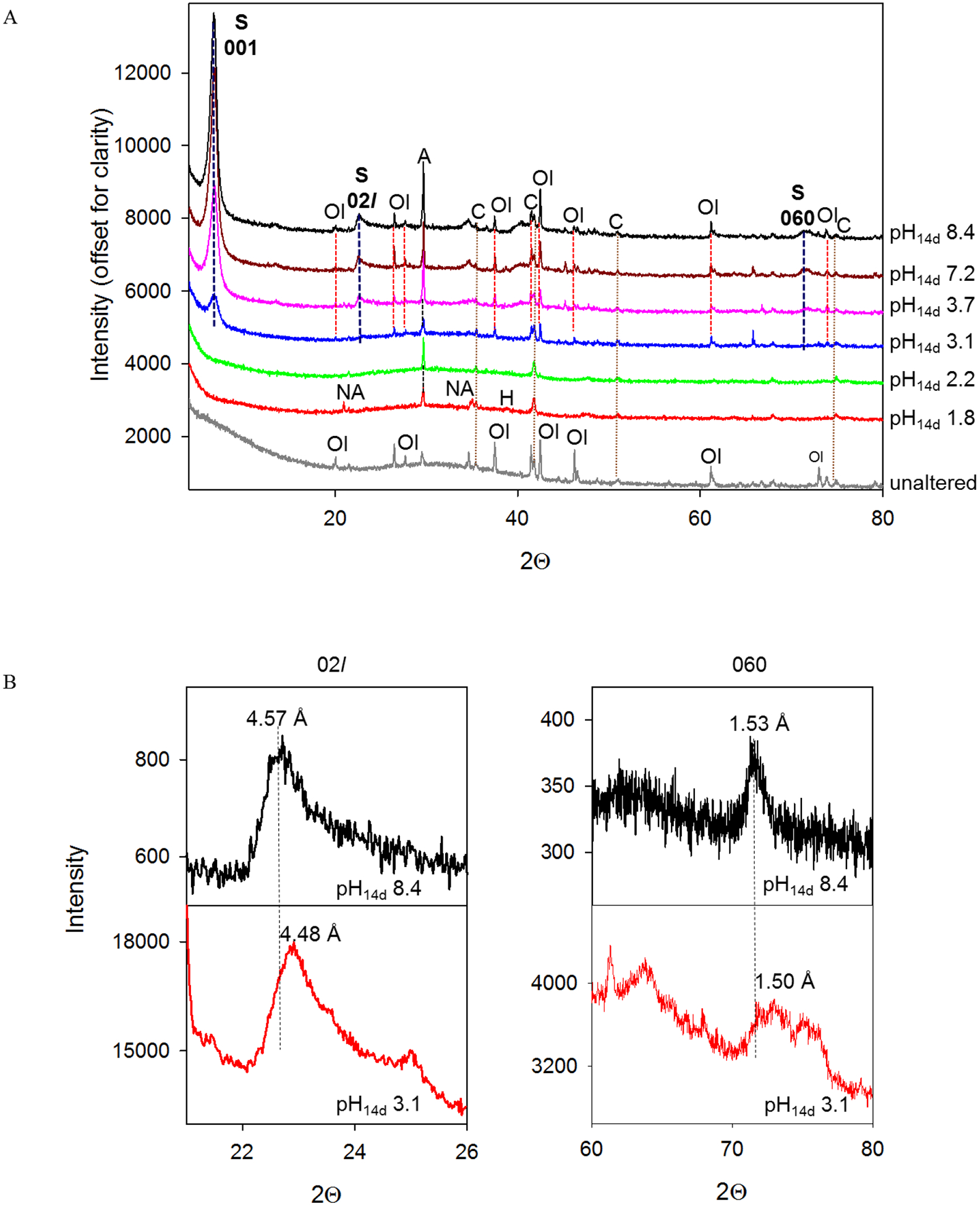
(a) XRD for unaltered and 14d-incubated Adirondack basalt simulant samples with different pH14d. S = smectite (001, 02l and 060 diffraction peaks are marked next to or above the peaks), A = anhydrite, Ol = olivine, C = chromite, NA = natroalunite, H = hematite; (b) 02l and 060 diffraction bands for clay fractions separated from the pH14d 8.4 and 3.1 samples. The bands were analyzed with a 0.02° 2θ step for 1 min and 0.02° 2θ step for 17 min in the pH14d 8.4 and 3.1 clay fractions, respectively.
Table 1.
Phases determined in the 14d-incubated Adirondack basalt simulants by XRD and Mössbauer spectroscopy. Olivine and chromite are unaltered phases from the original basalt simulant.
| pH14d | Crystalline phases |
|---|---|
| 1.81 ± 0.01 | anhydrite, natroalunite, hematitea, chromite |
| 2.19 ± 0.01 | anhydrite, chromite |
| 3.12 ± 0.03 | dioctahedral smectite, anhydrite, hematite, olivine, chromite |
| 3.72 ± 0.34 | saponite, anhydrite, hematite, olivine, chromite |
| 7.17 ± 0.38 | saponite, anhydrite, olivine, chromite |
| 8.38 ± 0.20 | saponite, anhydrite, hematite, olivine, chromite |
Hematite was detected by both XRD and Mossbauer in the pH14d 1.8 sample and by Mössbauer spectroscopy alone in the other samples. Hematite was present within the pH14d range from 1.8 to 8.4 and likely precipitated in the pH14d 2.2 and 7.2 samples not analyzed by Mössbauer spectroscopy.
Table 2.
Smectite characterization data obtained by XRD and VNIR.
| pH14d | Smectite | XRD d-spacing, Å |
VNIR combination band position, μm |
|||||
|---|---|---|---|---|---|---|---|---|
| 02l | 060 | Al(Fe(III), Fe(II), Mg)OHb | (Mg, Fe(II), Fe(III))3OHb | (Mg, Fe(II), Fe(III))3OHc | ||||
| 3.12 ± 0.03 | dioctahedral smectite | 4.48 | 1.50 | nda | nd | nd | ||
| 3.72 ± 0.34 | saponite | 4.55 | 1.53 | 2.239 | 2.319 | nd | ||
| 7.17 ± 0.38 | saponite | 4.58 | 1.53 | 2.246 | 2.319 | 2.403 | ||
| 8.38 ± 0.20 | saponite | 4.57 | 1.53 | 2.243 | 2.300 | 2.408 | ||
nd-not detected;
Combination stretching and bending vibration (Neumann et al., 2011; Treiman et al., 2014);
Combination stretching and translation vibration (Frost et al., 2002; Chemtob et al., 2015).
Anhydrite (CaSO4) was found to precipitate at all values of pH14d and natroalunite (NaAl3(SO4)2(OH)6) was detected only at pH14d 1.8 (Figure 2a, Table 1). A weak diffraction peak of hematite (α-Fe2O3) was observed in the pH14d 1.8 sample (Figure 2a, Table 1), and the presence of hematite was confirmed by Mossbauer spectroscopy (Section 3.2.2). Hematite was not detected in any other samples by XRD, but Mossbauer analysis showed that hematite was present in the pH14d range from 1.8 to 8.4 (Section 3.2.2). Chromite, present in the unaltered material, did not dissolve during incubation while olivine completely dissolved in the pH14d 1.8 and 2.2 samples as evident from the disappearance of its sharp diffraction peaks (Figure 2a, Table 1).
3.2.2. Mössbauer spectroscopy
Mössbauer analysis of unaltered Adirondack basalt simulant showed that Fe was associated with olivine and glass phases, 19 ± 2 and 81 ± 2% respectively and was mostly Fe(II) (Table EA-2, (Peretyazhko et al., 2016)).
Analysis of altered Adirondack basalt simulant revealed formation of smectite through alteration of the glass phase. The decrease in the concentration of total Fe associated with the glass phase (39 ± 2% pH14d 3.1, 43 ± 2% pH14d 3.7 and 54 ± 2% pH14d 8.4, Figure 3a–c, Table EA-2) compared to the unaltered simulant, was equal within error to amount of total Fe associated with the smectite formed after 14d incubation (38 ± 2% pH14d 3.1, 41 ± 2% pH14d 3.7 and 56 ± 2% pH14d 8.4, Figure 3a–c, Table EA-2). Smectite spectra were fit with one Fe(II) doublet (CS = 1.04 −1.09 mm/s and QS = 2.42 −2.66 mm/s) and one Fe(III) doublet (CS = 0.41 −0.48 mm/s and QS = 0.54 – 0.70 mm/s QS, Table EA-2) and assigned to Fe(II) and Fe(III) in octahedral coordination (Treiman et al., 2014). The smectite was oxidized with Fe(III)/ΣFe equal to 0.61, 0.73 and 0.82 at pH14d 3.1, 3.7 and 8.4, respectively.
Figure 3.
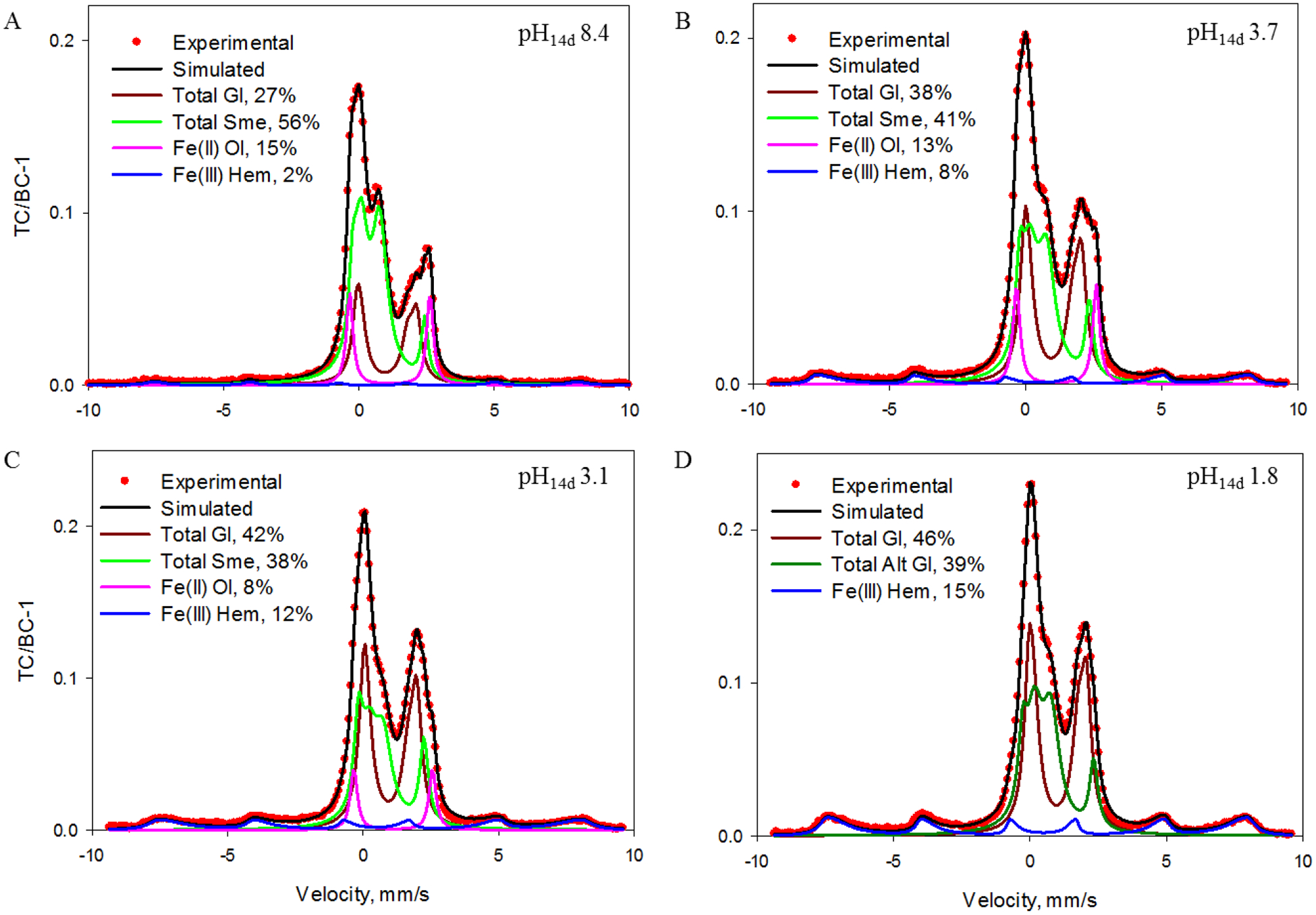
Mössbauer spectra and fit subspectra for 14d-incubated (a) pH14d 8.4, (b) pH14d 3.7, (c) pH14d 3.1 and (d) pH14d 1.8 Adirondack basalt simulant collected at RT [subspectral areas in % are for Ol = olivine; Total Gl = total glass, a sum of 2D1 and 2D2 doublets (Table EA-2), Total Sme = total smectite, and Total Alt Gl = total altered glass, a sum of 3D2 and 2D4 doublets (Table EA-2)]. TC = total counts, BC = baseline counts.
The most acidic pH14d 1.8 sample was also characterized by Fe(II) and Fe(III) doublets with parameters similar to those assigned to smectite in the other samples (Fe(II) doublet: CS = 1.03 mm/s and QS = 2.60 mm/s and Fe(III) doublet: CS = 0.46 mm/s and QS = 0.62 mm/s, Table EA-2). However, smectite did not form in this sample within XRD detection limits (Table 1). The Fe might be associated with an altered glass layer which forms during first stage of acidic basalt alteration (Berger et al., 1987; Oelkers, 2001) and/or with an incipient phyllosilicate with domains too small to coherently scatter X-rays.
The concentration of total Fe associated with olivine progressively decreased with decreasing pH14d, and no Fe in olivine was detected in the pH14d 1.8 sample as shown in Table EA-2. Olivine dissolution by acid-sulfate solutions could contribute Fe to smectite precipitation. However, the Mössbauer evidence is that Fe(II) from olivine dissolution was oxidized and precipitated as hematite, because the decrease in concentration of Fe(II) associated with olivine was associated with a concomitant increase in the concentration of Fe(III) associated with hematite (Figure 3, Table EA-2). Iron content in hematite (15 ± 2% pH14d 1.8, 12 ± 2% pH14d 3.1, 8 ± 2% pH14d 3.7 and 2 ± 2% pH14d 8.4, Figure 3, Table EA-2), was equal within an error of fitting to decrease in olivine Fe content with respect to the unaltered Adirondack basalt simulant (19 ± 2% pH14d 1.8, 11 ± 3% pH14d 3.1, 6 ± 3% pH14d 3.7 and 4 ± 3% pH14d 8.4, Figure 3, Table EA-2). The hematite peaks were broad and skewed toward zero velocity, implying poor crystallinity (small particle diameter) and potentially Al substitution for Fe(III).
3.2.3. Smectite composition and structural formula
The structural formula calculated from electron microprobe analysis of smectite-containing particles (Table EA-3, Figure EA-1) showed that smectite contained Si and Al in tetrahedral layers, Al, Mg, Fe with traces of Ti, Mn, Ni and Cr in octahedral layers, and Na, Ca and K occupied interlayer sites. Given the high uncertainties in the measured data due to high water content in smectite, the results of electron microprobe analysis did not allow to accurately quantify smectite composition. Electron microprobe analysis indicated that smectite enriched in Fe and Mg formed at pH14d 3.7, 7.2 and 8.4 while smectite equally enriched in Al, Mg and Fe formed at pH14d 3.1 (Table EA-3). The average total number of octahedral cations in a unit cell was close to 6 in the pH14d 3.7, 7.2 and 8.4 samples (Table EA-3) and was consistent with trioctahedral saponite. The total number of octahedral cations was close to 5 in the pH14d 3.1 sample (Table EA-3) which might indicate formation of smectite containing dioctahedral and trioctahedral domains.
3.2.4. Visible and near-infrared spectroscopy
VNIR spectral features between ~1.2 and 2.5 μm (Figure 4, Table 2) for saponite formed at pH14d 3.7, 7.2, and 8.4 were assigned to OH in H2O and M-OH (~1.4 μm), to interlayer and adsorbed H2O (~1.9 μm), and to trioctahedral M3-OH and dioctahedral M2-OH functional groups (2.2–2.5 μm), where M is any combination of Al, Mg, Fe(II) and Fe(III) as octahedral cations. The band centered between 2.30 and 2.32 μm was assigned to (Mg, Fe(II), Fe(III))3OH combination stretching and bending vibration (Neumann et al., 2011; Treiman et al., 2014). (Mg, Fe(II), Fe(III))3OH band center position was shifted to shorter wavelength in the pH14d 8.4 sample with respect to the pH14d 3.7 and 7.2 samples (Figure 4b, Table 2). The band position shift could be due to increase in degree of Fe oxidation in saponite from Fe(III)/ΣFe = 0.73 at pH14d 3.7 to Fe(III)/ΣFe = 0.82 at pH14d 8.4, (Table EA-2, (Peretyazhko et al., 2016)). The position variability for the ~2.31 μm band might also result from heterogeneous distributions of cations on octahedral sites [e.g., (Fe(III)2Mg)-OH versus (Fe(III)Mg2)-OH]. Saponite observed at pH14d 7.2 and 8.4 had a weaker band near 2.40 μm band commonly observed in Fe/Mg smectites (e.g., Carter et al., 2015; Chemtob et al., 2015). The exact band assignment is uncertain but might be due to (Mg, Fe(II), Fe(III))3OH combination stretching and translation vibration (Frost et al., 2002; Chemtob et al., 2015). The spectral feature near 2.24 μm was assigned to Al(Fe(III), Fe(II), Mg)OH (Madejová et al., 2011; Chemtob et al., 2015) and indicated the presence of octahedral Al, possibly present as dioctahedral domains as previously observed in synthetic saponite (Chemtob et al., 2015; Peretyazhko et al., 2016). Spectral properties of saponite synthesized at pH14d 3.7, 7.2 and 8.4 were similar to the properties reported for smectite on Mars by VNIR spectrometers. The bands near 2.30 and 2.40 μm has been reported in Fe/Mg-smectite identified on Mars while the band at 2.24 μm is present in about 10% of Fe/Mg-phyllosilicate spectra (Carter et al., 2013), including nontronite (Bishop et al., 2013) but not saponite.
Figure 4.
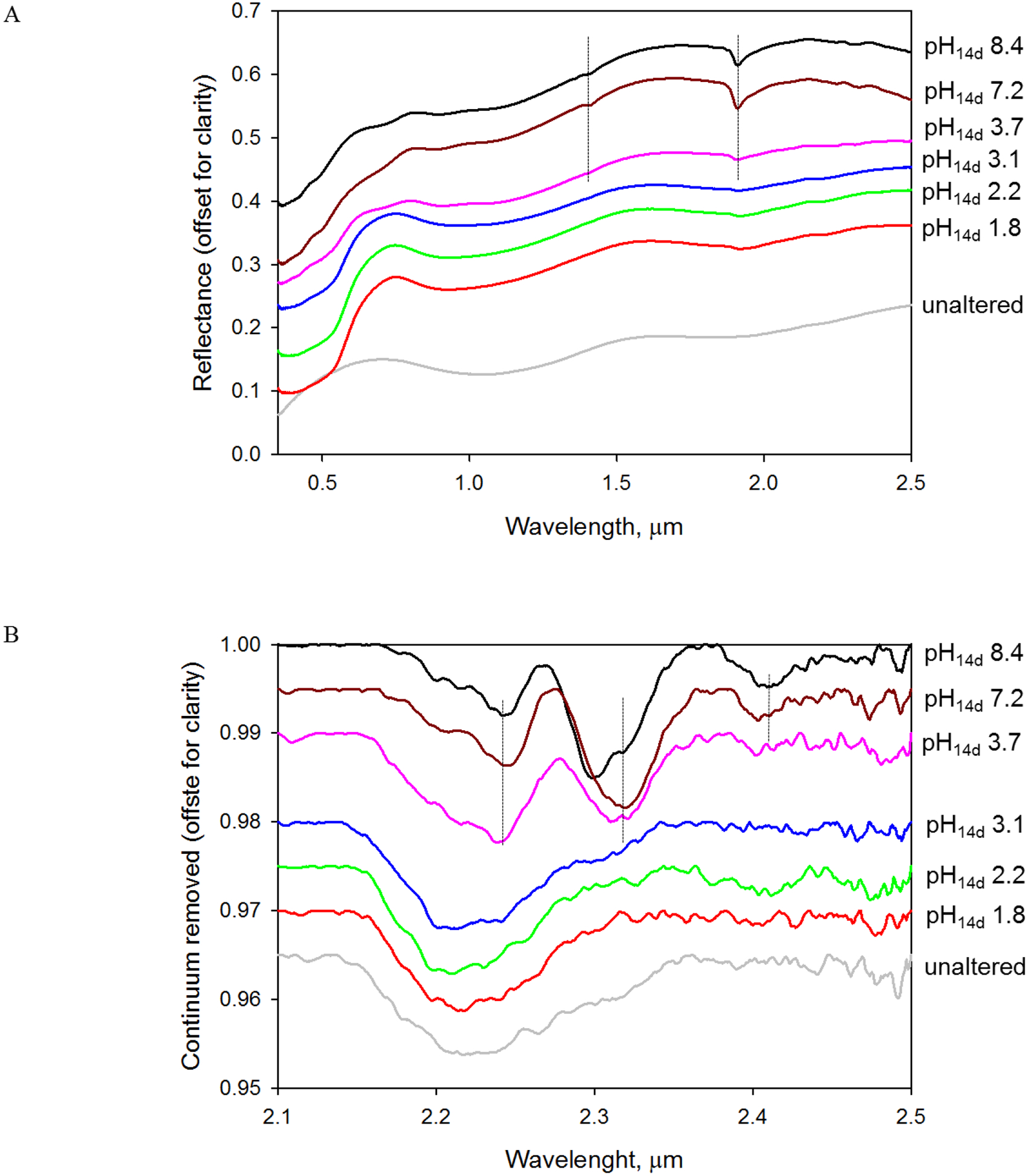
(a) VNIR reflectance spectra of unaltered and 14d-incubated Adirondack basalt simulant samples with different pH14d. Bands at 1.4 and 1.9 μm in the saponite-containing pH14d 8.4, 7.2 and 3.7 samples are marked with dotted lines. (b) 2–2.5 μm range of continuum removed reflectance spectra. Positions of M3-OH and M2-OH combination bands in the saponite- containing samples are marked with dotted lines (M is any combination of Al, Mg, Fe(II) and Fe(III) as octahedral cations). Characteristic Al2OH band of montmorillonite at 2.21 μm in the pH14d 3.1 sample was not detected.
The spectrum of the pH14d 3.1 sample containing dioctahedral smectite was similar to the spectra of the untreated Adirondack basalt simulant and pH14d 1.8 and 2.2 samples (Figure 4b). All these samples had a broad band centered on 2.21–2.22 μm likely resulting from vibration of Si-OH associated with the unaltered glass phase. The diagnostic band of montmorillonite at 2.20 μm was not distinguishable from the basalt band because of relatively low smectite abundance at pH14d 3.1 (indicative by low intensity 001 diffraction peak in the sample, Figure 2a).
Hematite presence was evident from the ferric absorption edge between ~0.50 um and ~0.75 um (Figure 4a) corresponding to the onset of strong absorption and a relative reflectivity maximum for hematite (Morris et al., 1985). The VNIR manifestation of the hematite was most noticeable for the pH14d 1.8 sample which had the highest hematite content (15% of total Fe, Table EA-2)
3.3. Characterization of aqueous phase
Mineralogical transformations of Adirondack basalt simulant to smectite, anhydrite, hematite and natroalunite (Table 1) resulted in release of variable proportions of Si, Mg, Na and Ca. Complete dissolution of olivine in the pH14d 1.8 and 2.2 samples and subsequent release of Mg and Si into solution (Figure 5a) were lower than the expected Si and Mg concentrations if all olivine was dissolved (32 ± 3 mM Si and 42 ± 4 mM Mg based on the total SiO2 and MgO contents in olivine, Table EA-1). The results suggest that Si and Mg dissolved from olivine were partially incorporated into an altered glass phase under acidic conditions (Berger et al., 1987).
Figure 5.
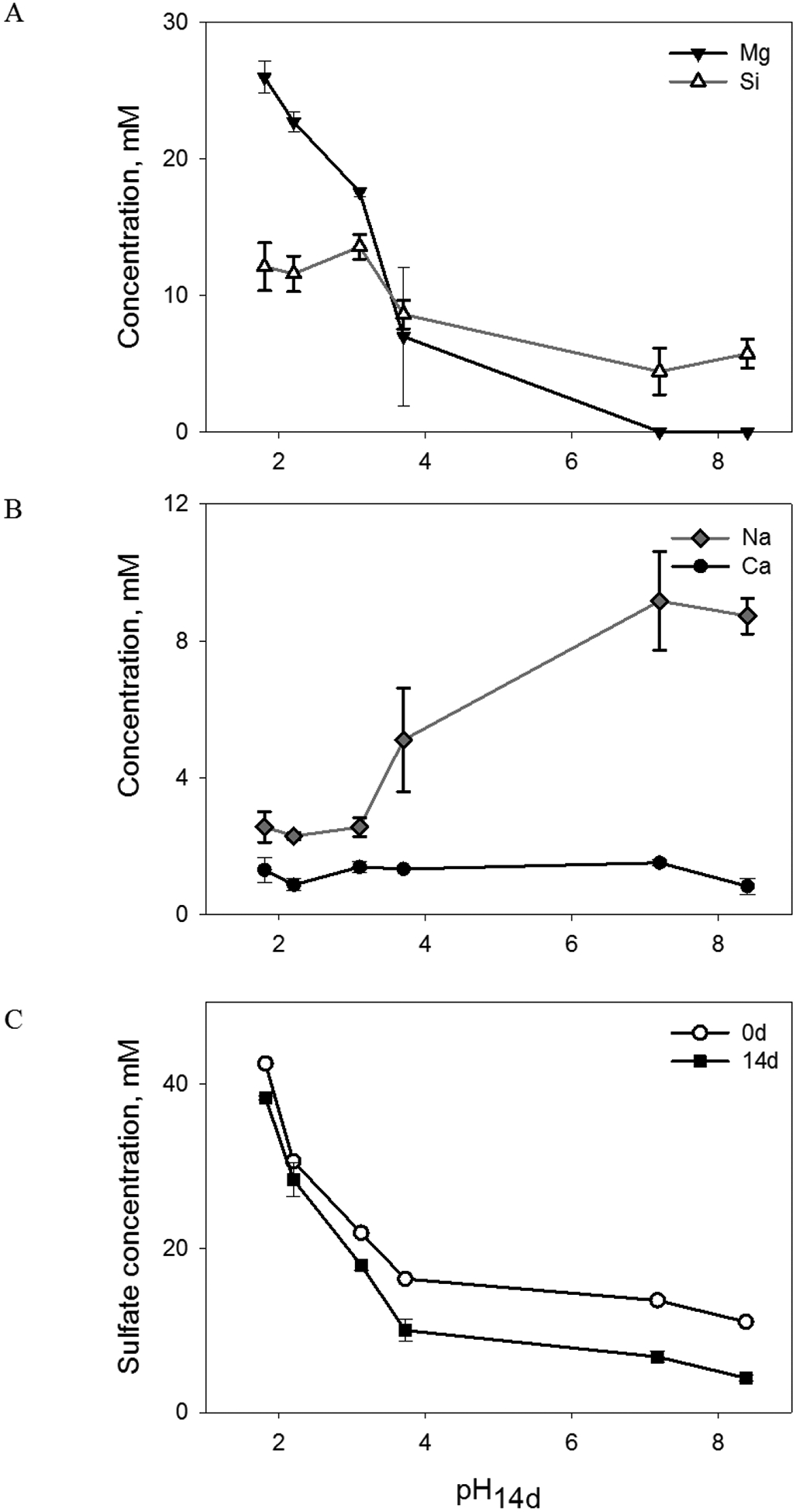
Concentrations of dissolved (a) Mg and Si, (b) Na and Ca and (c) sulfate in Adirondack basalt simulant suspensions as a function of pH14d.
Smectite formation in the pH14d range from 3.1 to 8.4 (Figure 2a) led to decrease in Si and Mg dissolution (Figure 5a) where the Mg dissolution likely controlled the nature of the forming smectite mineral (Meunier, 2005). Dioctahedral smectite was only detected in the pH14d 3.1 sample in which nearly 50% of the total Mg was leached into solution (calculations are based on dissolved Mg concentrations in Figure 5a and total MgO in the glass phase, Table EA-1) while formation of trioctahedral saponite (pH14d 3.7, 7.2 and 8.4) was accompanied by lower or no Mg release into solution.
Dissolved Na was affected by precipitation of natroalunite and smectite formation. Dissolved Na concentration was low and did not change in the pH14d range from 1.8 to 3.1 then increased as pH shifted from acidic to slightly alkaline (Figure 5b). Low release of Na from basaltic glass at pH14d 1.8–3.1 could result from precipitation of natroalunite, although the presence of this phase was only confirmed at pH14d 1.8 (Table 1). Dissolved Na increased in the pH14d range from 3.1 to 8.4 (Figure 5b) due to Na loss from glass phase during alteration of Adirondack basalt simulant into smectite (Peretyazhko et al., 2016). Similar to Na, Ca was expected to increase in solution with increasing basalt alteration as pH rose (Berger et al., 1987). However, dissolved Ca concentration did not change (Figure 5b), and such non-pH-dependent Ca release, together with the observed decrease in sulfate concentration (Figure 5c), resulted from anhydrite precipitation (Figure 2a, Table 1) which is favorable at elevated temperatures (Bishop et al., 2014).
Solution concentrations of Al and Fe were low for all samples resulting from their incorporation into secondary phases (Figure EA-4, Tables 1 and 2). Dissolved Al did not exceed 0.04 mM (Figure EA-4a) as a result of precipitation of natroalunite at pH14d 1.8, low release from glass alteration and incorporation into smectite at higher pH values (Tables 2 and EA-3) and potentially in hematite. Dissolved Fe was <0.15 mM (Figure EA-4b) in all samples due to precipitation of hematite accompanying olivine dissolution (Figure 3), low release from altering glass phase and smectite formation (Tables 2 and EA-3).
4. DISCUSSION
The formation of smectite in the Noachian and early Hesperian has been attributed to aqueous alteration of basaltic materials under neutral to alkaline pH aqueous conditions (Bibring et al., 2006; Chevrier et al., 2007; Ehlmann et al., 2009; Bishop et al., 2013; Bristow et al., 2015; Flahaut et al., 2015; Jain and Chauhan, 2015). However, the results of our experiments show that formation of smectite from Mars-analogue basalt simulant is possible in much more acidic environments. We found that gradual acid neutralization during acid-sulfate alteration of the basalt simulant under hydrothermal conditions led to smectite formation at pH14d ≥ 3. The nature of the forming smectite mineral varied with the solution final pH. Dioctahedral smectite equally enriched in Al, Fe and Mg formed at pH14d ~3 followed by saponite enriched in Fe and Mg at pH14d close to 4 or higher.
Our experimental results demonstrate that smectite formation through basalt alteration on Mars was not limited to neutral/alkaline pH conditions but could partially occur under acidic conditions once solution pH was close to 3 or higher. Sources of acidity on early Mars and the persistence of acidic aqueous environments have been controversial. The basaltic crust should provide enormous acid neutralizing potential (Niles and Michalski, 2009) suggesting that aqueous systems on Mars should be largely alkaline. However, as noted above, absence of large scale carbonate deposits shows an evidence for acidic conditions even in heavily altered regions of the martian crust (Fairén et al., 2004). The principal acidity sources proposed for early Mars are Fe(II) oxidative hydrolysis (Tosca et al., 2008; Hurowitz et al., 2010) and SO2 degassing (Berger et al., 2009; Gaillard and Scaillet, 2009; Righter et al., 2009; Gaillard et al., 2013). We argue here that SO2 degassing was the major source of acidity on early Mars generating H2SO4 sufficient for acidic phyllosilicate formation through basalt alteration and Fe(II) oxidative hydrolysis (i.e., Fe(III) hydrolysis) was likely not the source of wide-spread acidity on Mars.
4.1. Acidity sources on early Mars: Fe(II) oxidative hydrolysis
Iron(III) hydrolysis was first suggested as the source of acidity at Meridiani Planum (Hurowitz et al., 2010) and was further generalized as a possible widespread acidity source on early Mars (e.g., (Ehlmann et al., 2011; Bowen et al., 2012; Horgan and Bell III, 2012; McLennan, 2012; Marlow et al., 2014; Kaplan et al., 2016)). The Fe(II) oxidative hydrolysis model proposed that upwelling of anoxic Fe(II)-containing groundwater generated from basalt dissolution under anoxic circum-neutral pH conditions could lead to surface oxidation of Fe(II) to Fe(III) followed by Fe(III) hydrolysis, release of H+ and precipitation of Fe(III) secondary minerals (Hurowitz et al., 2010). The latter include nanophase iron(III) (hydr)oxides (interpreted as schwertmannite (FeO(OH)0.75(SO4)0.125) (Hurowitz et al., 2010)), jarosite ((K,Na)Fe3(SO4)2(OH)6) and hematite (α-Fe2O3) detected by Mossbauer spectroscopy at Meridiani Planum (Morris et al., 2006). The reactions below summarize the mechanism proposed for acidity generation following the sequence developed by Hurowitz et al., (2010) with the exception that eq. 3 is included for basalt dissolution (eq. 3 written for olivine Fe(II)-end member fayalite (Fe2SiO4)):
| (3a) |
| (3b) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
While groundwater interaction with basalt was suggested to be a source of dissolved Fe(II), basalt dissolution (eq. 3) was not included into Fe(II) oxidative hydrolysis model (Hurowitz et al., 2010). As a result, the model predicted that sufficient amount of H+ was produced through Fe(III) hydrolysis to develop acidic conditions (Hurowitz et al., 2010). If basalt dissolution reactions are added to the model, then no acidity would be generated because Fe(II) release is accompanied by OH− release (e.g., eq. 3b, Text EA-1). While our calculations only account for Fe, the release of Mg, Ca, and Na from the basalts will also be accompanied by release of OH− which will contribute to generation of additional alkalinity.
The OH− generated from dissolution of basalt (eq. 3) could be neutralized by H+ produced through oxidative dissolution of Fe(II) sulfides present in basalt. However, the produced H+ will not be sufficient to neutralize all released OH− on the basis of current estimates of low abundances of Fe(II) sulfides in martian basalts (1600 mg/kg S in shergottite sulfides or 0.16 wt%, (Righter et al., 2009)). Alternatively, the OH− released from basalt together with Fe(II), Mg, Ca and Na could be neutralized if the anoxic alkaline solutions encounter anoxic acidic solutions. If all released OH− is neutralized, then Fe(II) oxidation followed by hydrolysis under oxic conditions will lead to development of acidic conditions according to the Hurowitz et al., (2010) model (eqs. 4–8). However, such scenario will require presence of acidic conditions, for instance H2SO4, before Fe(III) hydrolysis occurred.
4.2. Acidity sources on early Mars: SO2 degassing and H2SO4 formation
Magmatic sulfur degassing was the dominant source for sulfates deposited on the surface of Mars (Righter et al., 2009), but was SO2 release on early Mars able to generate the amount of H2SO4 sufficient for acidic phyllosilicate formation through alteration of basalt?
The calculated amount of sulfur release (eq. 2) during Noachian/early Hesperian epoch was 2.5 × 1021 g [~4.5 Ga – ~3.4 Ga; an intermediate value of 3.4 Ga was used for early Hesperian/Late Hesperian boundary which is around 3.2 Ga – 3.6 Ga (Hartmann, 2005); Figure 6]. If all sulfur was released as SO2 and converted to H2SO4 then around 1.6×1020 moles of H+ was produced during dissociation of all acid in water. Formation of sulfuric acid might occur through disproportionation of SO2 to H2SO4 and elemental S and S could be further transformed into H2SO4 in the presence of oxidizing agent and water (Habashi and Bauer, 1966; Halevy et al., 2007). It should be noted, however, an assumption that all degassed sulfur was transformed into sulfuric acid likely overestimates the amount of produced acidity but our calculations allows to potentially evaluate an upper limit of acidity that could have been generated on early Mars.
Figure 6.
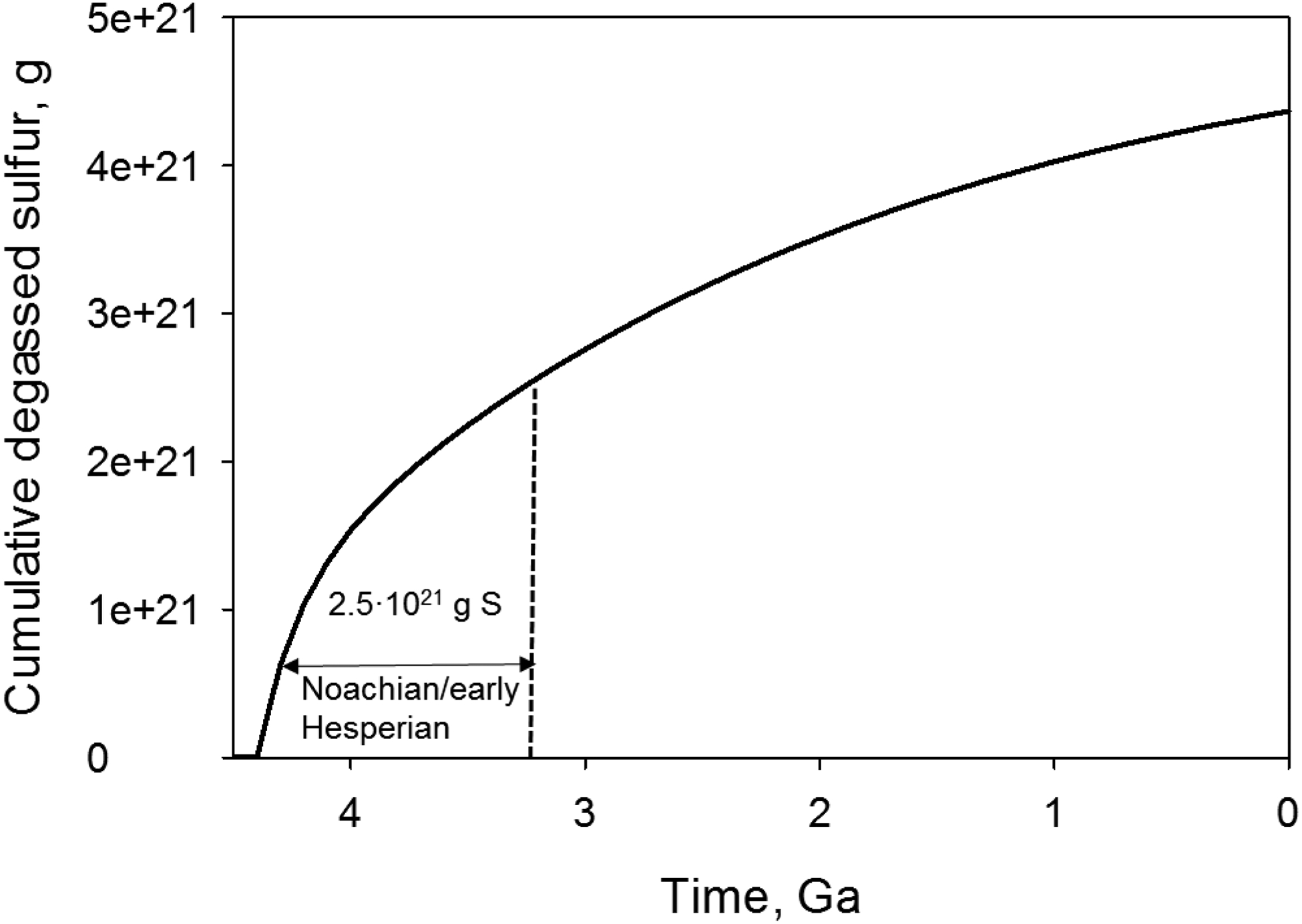
Sulfur degassed on Mars from 4.5 Ga to present calculated using the crust production model (eq. 2, Hirschmann and Withers 2008) and amount of sulfur degassed from 1 kg magma (2400 mg/kg, Righter et al., 2009). The calculated total degassed sulfur from 4.5 Ga to present was 4.4·1021 g and within the range of estimates of total sulfur release on Mars: 2.2·1022 g (McLennan, 2012), 5.4·1021 g (Gaillard and Scaillet, 2009), 1.7·1020 g (Craddock and Greeley, 2009) and 4.5·1019 g (Righter et al., 2009). Dashed line shows a boundary between early and late Hesperian.
The protons generated from sulfuric acid are neutralized by Ca, Mg and Na in basalt via proton exchange leading first to formation of an altered hydrated silica layer enriched in Fe and Al (Berger et al., 1987; Peretyazhko et al., 2016) by schematic reactions:
| (9) |
| (10) |
| (11) |
Crystallization of phyllosilicate from an altered layer does not substantially change pH as shown in the previous studies of smectite formation through basalt alteration (Ghiara et al., 1993; De La Fuente et al., 2002; Peretyazhko et al., 2016). Iron was not included into calculations because overall process including dissolution of olivine followed by Fe(II) oxidation, Fe(III) hydrolysis and hematite precipitation (Table 1) does not produce or consume H+ (Text EA-1).
Approximately 9.4 molH+/kgbasalt H+ is required for complete neutralization of Na, Ca and Mg in basalt of Adirondack composition (Text EA-2). The amount of H+ generated from volcanic outgassing of SO2 (1.6×1020 moles) during the Noachian and Hesperian would be sufficient for neutralization of Ca, Mg and Na along with phyllosilicate formation in a 42 m thick basalt layer over the entire planet (Text EA-3). Given that formation of smectite requires Mg to be present in the altering basalt (Kloprogge et al., 1999), partial H+ neutralization by cations would be a more realistic scenario on Mars. Noachian-aged terrains such as Mawrth Vallis have been estimated to contain 20–65% smectite (Poulet et al., 2008). Assuming that the smectite abundance in Mawrth Vallis is representative of H+ neutralization and, consequently, degree of basalt alteration in ancient martian terrains, the calculated average global depth of basaltic alteration would be between 60 m (65% smectite content) to 200 m (20% smectite content) on the entire planet under the constraint that alteration of 42 m thick basalt leads to 100% neutralization of Na, Ca and Mg. The calculated depth range is consistent with phyllosilicate layer thickness of a few hundreds of meters estimated in Noachian-aged terrains (Loizeau et al., 2012). Our acidity calculations and experimental results, therefore, provide evidence that SO2 degassing would result in production of large amounts of H+ and gradual neutralization of sulfuric acid during basalt alteration could be an important mechanism of smectite formation on early Mars.
5. SIGNIFICANCE AND CONCLUSIONS
Our experiments demonstrated formation of smectite through hydrothermal acid-sulfate alteration of Mars-analogue glass-rich basalt (66 wt% glass, 32 wt% olivine and 2 wt% chromite). Basalt simulant suspensions were acidic with initial pH0d ≤ 2 while final pH14d varied from acidic to mildly alkaline (pH14d 1.8–8.4) at the end of 14d incubation at 200 °C. Alteration of the glass phase of basalt simulant resulted in formation of dioctahedral smectite as pH14d reached ~3 followed by trioctahedral saponite at pH14d close to 4 or higher. Reaction of Adirondack basalt simulant with sulfuric acid also led to formation of anhydrite at all pH14d values while natroalunite was detected at pH14d 1.8. Hematite precipitated as a result of partial or complete oxidative dissolution of olivine in the pH14d range from 1.8 to 8.4.
Acidity on early Mars can be accounted for by volcanic outgassing of SO2 and formation of sulfuric acid. Acid-sulfate conditions, suitable enough to form smectite and prevent carbonate formation were likely sustained by low water/rock ratio and short duration of water-rock interaction (Berger et al., 2009; Berger et al., 2014). Acidic smectite formation areas on early Mars might be constrained to near-surface hydrothermal areas with magmatic outgassing resulted from volcanic or impact activity (Solomon et al., 2005; Hynek et al., 2013). In these systems only small portions of crust are exposed to acidity generated by H2SO4 formed from degassed SO2. Short-term aquatic systems analogous to ephemeral acid saline lakes in Western Australia might also allow development of acidic conditions (Story et al., 2010; Bowen et al., 2012). Although there may also be acidity generated by the Fe(II) oxidative hydrolysis in local environments, our calculations indicate that this mechanism is not a plausible large-scale acidity source because of the alkalinity that is generated during basalt dissolution.
Formation of phyllosilicate by sulfuric acid alteration of basaltic materials on Mars would likely be accompanied by precipitation of sulfate minerals including anhydrite. Although anhydrite cannot be detected by the same remote sensing measurements used to detect phyllosilicates because of the absence of diagnostic H2O spectral features in the VNIR, anhydrite might coprecipitate with phyllosilicate in deposits formed under hydrothermal sulfuric acid environments (>60 °C (Bishop et al., 2014)). Acid-sulfate smectite formation might also occur in younger terrains. Phyllosilicates (e.g., smectite) co-existing with anhydrite in Gale crater may have formed along with the anhydrite under acid sulfate conditions (Rampe et al., 2017). The hypothesis is supported by occurrence of jarosite in these mudstones in Gale crater indicative of acidic environments (Rampe et al., 2017). Fe/Mg-rich phyllosilicates interbedded with sulfate minerals in Meridiani Planum have been proposed to form prior to sulfates in moderate pH, sulfate-free aqueous environments followed by sulfate precipitation from acidic surface and groundwater (Flahaut et al., 2015). Our findings demonstrate that multiple events are not required to form interbedded phyllosilicates and sulfates deposits. Acid-sulfate alteration can explain martian mineralogical observations including mixed phyllosilicate/sulfate deposits and lack of abundant carbonate deposits.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Z. Peng for performing ICP-MS analysis. We thank Dr. Cuardos, Dr. Zolotov and two anonymous reviewers for valuable suggestions and comments that help to improve the quality of the manuscript. We thank the Associate Editor Dr. Catalano for handling the manuscript.This work was supported by NASA’s Mars Fundamental Research Program grant # 11-MFRP11-0090.
REFERENCES
- Abdelouas A, Crovisier J-L, Lutze W, Grambow B, Dran J-C and Müller R (1997) Surface layers on a borosilicate nuclear waste glass corroded in MgCh solution. J. Nucl. Mater 240, 100–111. [Google Scholar]
- Agresti DG, Dyar MD and Schaefer MW (2007) Velocity scales for Mars Mössbauer data. NASSAU2006, 67–74. [Google Scholar]
- Agresti DG and Gerakines PA (2009) Simultaneous fitting of Mars Mössbauer data. ICAME 2007, 1347–1354. [Google Scholar]
- Bain DC and Smith BFL (1987) Chemical analysis, In: A handbook of determinative methods in clay mineralogy (ed. Wilson MJ). Chapman and Hall, New York, pp. 248–275. [Google Scholar]
- Berger G, Meunier A and Beaufort D (2014) Clay mineral formation on Mars: Chemical constraints and possible contribution of basalt out-gassing. Planet. Space Sci 95, 25–32. [Google Scholar]
- Berger G, Schott J and Loubet M (1987) Fundamental processes controlling the first stage of alteration of a basalt glass by seawater: an experimental study between 200 and 320 C. Earth. Planet. Sci. Lett 84, 431–445. [Google Scholar]
- Berger G, Toplis MJ, Treguier E, d’Uston C and Pinet P (2009) Evidence in favor of small amounts of ephemeral and transient water during alteration at Meridiani Planum, Mars. Am. Mineral 94, 1279–1282. [Google Scholar]
- Bibring J-P, Langevin Y, Mustard JF, Poulet F, Arvidson R, Gendrin A, Gondet B, Mangold N, Pinet P and Forget F (2006) Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 312, 400–404. [DOI] [PubMed] [Google Scholar]
- Bishop JL, Lane MD, Dyar MD, King SJ, Brown AJ and Swayze GA (2014) What lurks in the martian rocks and soil? Investigations of sulfates, phosphates, and perchlorates. Spectral properties of Ca-sulfates: gypsum, bassanite, and anhydrite. Am. Mineral 99, 2105–2115. [Google Scholar]
- Bishop JL, Loizeau D, McKeown NK, Saper L, Dyar MD, Des Marais DJ, Parente M and Murchie SL (2013) What the ancient phyllosilicates at Mawrth Vallis can tell us about possible habitability on early Mars. Planet. Space Sci 86, 130–149. [Google Scholar]
- Bowen BB, Benison KC and Story S (2012) Early diagenesis by modern acid brines in Western Australia and implications for the history of sedimentary modification on Mars Mars Sedimentology, SEPM Special Publication; 102, 229–252. [Google Scholar]
- Bristow TF, Bish DL, Vaniman DT, Morris RV, Blake DF, Grotzinger JP, Rampe EB, Crisp JA, Achilles CN and Ming DW (2015) The origin and implications of clay minerals from Yellowknife Bay, Gale crater, Mars. Am. Mineral 100, 824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J, Poulet F, Bibring JP, Mangold N and Murchie S (2013) Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 118, 831–858. [Google Scholar]
- Cavanagh PD, Bish DL, Blake DF, Vaniman DT, Morris RV, Ming DW, Rampe EB, Achilles CN, Chipera SJ, Treiman AH, Downs RT and Morrison SM (2015) Confidence Hills mineralogy and CheMin results from base of Mt. Sharp, Pahrump Hills, Gale Crater, Mars LPSC [Google Scholar]
- Chemtob SM, Nickerson RD, Morris RV, Agresti DG and Catalano JG (2015) Synthesis and structural characterization of ferrous trioctahedral smectites: Implications for clay mineral genesis and detectability on Mars. J.Geophys. Res. Planets 120, 1119–1140 [Google Scholar]
- Chevrier V, Poulet F and Bibring J-P (2007) Early geochemical environment of Mars as determined from thermodynamics of phyllosilicates. Nature 448, 60–63. [DOI] [PubMed] [Google Scholar]
- Craddock RA and Greeley R (2009) Minimum estimates of the amount and timing of gases released into the martian atmosphere from volcanic eruptions. Icarus 204, 512–526. [Google Scholar]
- De La Fuente S, Cuadros J and Linares J (2002) Early stages of volcanic tuff alteration in hydrothermal experiments: formation of mixed-layer illite-smectite. Clays Clay Miner. 50, 578–590. [Google Scholar]
- Dehouck E, Gaudin A, Mangold N, Lajaunie L, Dauzeres A, Grauby O and Le Menn E (2014) Weathering of olivine under CO2 atmosphere: a martian perspective. Geochim. Cosmochim. Acta 135, 170–189. [Google Scholar]
- Edwards CS and Ehlmann BL (2015) Carbon sequestration on Mars. Geo 43, 863–866. [Google Scholar]
- Ehlmann BL, Mustard JF, Murchie SL, Bibring J-P, Meunier A, Fraeman AA and Langevin Y (2011) Subsurface water and clay mineral formation during the early history of Mars. Nature 479, 53–60. [DOI] [PubMed] [Google Scholar]
- Ehlmann BL, Mustard JF, Murchie SL, Poulet F, Bishop JL, Brown AJ, Calvin WM, Clark RN, Des Marais DJ and Milliken RE (2008) Orbital identification of carbonate-bearing rocks on Mars. Science 322, 1828–1832. [DOI] [PubMed] [Google Scholar]
- Ehlmann BL, Mustard JF, Swayze GA, Clark RN, Bishop JL, Poulet F, Des Marais DJ, Roach LH, Milliken RE and Wray JJ (2009) Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. Planets 114. [Google Scholar]
- Fairén AG (2013) Coeval synthesis of cold aqueous mineralogies on Mars, In: Mars: evolution, geology and exploration (ed. Fairén AG). Nova Science Publisher. [Google Scholar]
- Fairén AG, Fernández-Remolar D, Dohm JM, Baker VR and Amils R (2004) Inhibition of carbonate synthesis in acidic oceans on early Mars. Nature 431, 423–426. [DOI] [PubMed] [Google Scholar]
- Farrand WH, Glotch TD, Rice JW, Hurowitz JA and Swayze GA (2009) Discovery of jarosite within the Mawrth Vallis region of Mars: Implications for the geologic history of the region. Icarus 204, 478–488. [Google Scholar]
- Flahaut J, Carter J, Poulet F, Bibring J-P, van Westrenen W, Davies G and Murchie S (2015) Embedded clays and sulfates in Meridiani Planum, Mars. Icarus 248, 269–288. [Google Scholar]
- Frost RL, Kloprogge JT and Ding Z (2002) Near-infrared spectroscopic study of nontronites and ferruginous smectite. Spectrochim. Acta 58, 1657–1668. [DOI] [PubMed] [Google Scholar]
- Gaillard F, Michalski J, Berger G, McLennan SM and Scaillet B (2013) Geochemical reservoirs and timing of sulfur cycling on Mars. Space Sci. Rev 174, 251–300. [Google Scholar]
- Gaillard F and Scaillet B (2009) The sulfur content of volcanic gases on Mars. Earth. Planet. Sci. Lett 279, 34–43. [Google Scholar]
- Ghiara M, Franco E, Petti C, Stanzione D and Valentino G (1993) Hydrothermal interaction between basaltic glass, deionized water and seawater. Chem. Geol 104, 125–138. [Google Scholar]
- Greeley R and Schneid B (1991) Magma generation on Mars- Amounts, rates, and comparisons with earth, moon, and Venus. Science 254, 996–998. [DOI] [PubMed] [Google Scholar]
- Habashi F and Bauer E (1966) Aqueous oxidation of elemental sulfur. Ind. Eng. Chem. Fundam 5, 469–471. [Google Scholar]
- Halevy I, Zuber MT and Schrag DP (2007) A sulfur dioxide climate feedback on early Mars. Science 318, 1903–1907. [DOI] [PubMed] [Google Scholar]
- Hartmann WK (2005) Martian cratering 8: Isochron refinement and the chronology of Mars. Icarus 174, 294–320. [Google Scholar]
- Haymon RM and Kastner M (1986) The formation of high temperature clay minerals from basalt alteration during hydrothermal discharge on the East Pacific Rise axis at 21 N. Geochim. Cosmochim. Acta 50, 1933–1939. [Google Scholar]
- Hirschmann MM and Withers AC (2008) Ventilation of CO2 from a reduced mantle and consequences for the early Martian greenhouse. Earth. Planet. Sci. Lett 270, 147–155. [Google Scholar]
- Horgan B and Bell III JF (2012) Widespread weathered glass on the surface of Mars. Geology 40, 391–394. [Google Scholar]
- Hurowitz JA, Fischer WW, Tosca NJ and Milliken RE (2010) Origin of acidic surface waters and the evolution of atmospheric chemistry on early Mars. Nat. Geosci 3, 323–326. [Google Scholar]
- Hynek BM, McCollom TM, Marcucci EC, Brugman K and Rogers KL (2013) Assessment of environmental controls on acid-sulfate alteration at active volcanoes in Nicaragua: Applications to relic hydrothermal systems on Mars. J. Geophys. Res. Planets 118, 2083–2104. [Google Scholar]
- Jain N and Chauhan P (2015) Study of phyllosilicates and carbonates from the Capri Chasma region of Valles Marineris on Mars based on Mars Reconnaissance Orbiter-Compact Reconnaissance Imaging Spectrometer for Mars (MRO-CRISM) observations. Icarus 250, 7–17. [Google Scholar]
- Kachanoski R, Voroney R and Gregorich E (1988) Ultrasonic dispersion of aggregates: distribution of organic matter in size fractions. Can. J. Soil. Sci 68, 395–403. [Google Scholar]
- Kaplan HH, Milliken RE, Fernandez-Remolar D, Amils R, Robertson K and Knoll AH (2016) Orbital evidence for clay and acidic sulfate assemblages on Mars based on mineralogical analogs from Rio Tinto, Spain. Icarus 275, 45–64. [Google Scholar]
- Klingelhoefer G, Morris RV, Bernhardt B, Rodionov D, De Souza P, Squyres S, Foh J, Kankeleit E, Bonnes U and Gellert R (2003) Athena MIMOS II Mossbauer spectrometer investigation. J. Geophys. Res. Planets 108. [Google Scholar]
- Kloprogge JT, Komarneni S and Amonette JE (1999) Synthesis of smectite clay minerals: a critical review. Clays Clay Miner 47, 529–554. [Google Scholar]
- Loizeau D, Werner S, Mangold N, Bibring J-P and Vago J (2012) Chronology of deposition and alteration in the Mawrth Vallis region, Mars. Planet. Space Sci 72, 31–43. [Google Scholar]
- Madejová J, Balan E and Petit S (2011) Application of vibrational spectroscopy to the characterization of phyllosilicates and other industrial minerals. Advances in the Characterization of Industrial Minerals: Notes in Mineralogy 9, 171–226. [Google Scholar]
- Marlow JJ, LaRowe DE, Ehlmann BL, Amend JP and Orphan VJ (2014) The potential for biologically catalyzed anaerobic methane oxidation on ancient Mars. Astrobiology 14, 292–307. [DOI] [PubMed] [Google Scholar]
- McLennan SM (2012) Geochemistry of sedimentary processes on Mars. Sediment. Geol.Mars 102, 119–138. [Google Scholar]
- McMurtry GM and Yeh H-W (1981) Hydrothermal clay mineral formation of East Pacific Rise and Bauer Basin sediments. Chem. Geol 32, 189–205. [Google Scholar]
- McSween HY, Wyatt MB, Gellert R, Bell J, Morris RV, Herkenhoff KE, Crumpler LS, Milam KA, Stockstill KR and Tornabene LL (2006) Characterization and petrologic interpretation of olivine-rich basalts at Gusev Crater, Mars. J. Geophys. Res. Planets 111. [Google Scholar]
- Meunier A (2005) Clays. Springer Science & Business Media. [Google Scholar]
- Milliken R, Fischer W and Hurowitz J (2009) Missing salts on early Mars. Geophys. Res. Lett 36. [Google Scholar]
- Moore DM and Reynolds RCJ (1997) X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, New York. [Google Scholar]
- Morris RV, Golden D, Bell JF and Lauer H (1995) Hematite, pyroxene, and phyllosilicates on Mars: Implications from oxidized impact melt rocks from Manicouagan Crater, Quebec, Canada. J. Geophys. Res. Planets 100, 5319–5328. [Google Scholar]
- Morris RV, Klingelhoefer G, Schröder C, Rodionov DS, Yen A, Ming DW, De Souza P, Wdowiak T, Fleischer I and Gellert R (2006) Mossbauer mineralogy of rock, soil, and dust at Meridiani Planum, Mars: Opportunity’s journey across sulfate-rich outcrop, basaltic sand and dust, and hematite lag deposits. J. Geophys. Res. Planets 111. [Google Scholar]
- Morris RV, Lauer HV, Lawson CA, Gibson EK, Nace GA and Stewart C (1985) Spectral and other physicochemical properties of submicron powders of hematite (α-Fe2O3), maghemite (γ-Fe2O3), magnetite (Fe3O4), goethite (α-FeOOH), and lepidocrocite (γ-FeOOH). J. Geophys. Res. Solid Earth 90, 3126–3144. [DOI] [PubMed] [Google Scholar]
- Morris RV, Ruff SW, Gellert R, Ming DW, Arvidson RE, Clark BC, Golden D, Siebach K, Klingelhöfer G and Schröder C (2010) Identification of carbonate-rich outcrops on Mars by the Spirit rover. Science 329, 421–424. [DOI] [PubMed] [Google Scholar]
- Murchie SL, Mustard JF, Ehlmann BL, Milliken RE, Bishop JL, McKeown NK, Noe Dobrea EZ, Seelos FP, Buczkowski DL and Wiseman SM (2009) A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J. Geophys. Res. Planets 114. [Google Scholar]
- Neumann A, Petit S and Hofstetter TB (2011) Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy. Geochim. Cosmochim. Acta 75, 2336–2355. [Google Scholar]
- Niles PB, Catling DC, Berger G, Chassefiere E, Ehlmann BL, Michalski JR, Morris R, Ruff SW and Sutter B (2013) Geochemistry of carbonates on Mars: implications for climate history and nature of aqueous environments. Space Scie. Rev 174, 301–328. [Google Scholar]
- Niles PB and Michalski J (2009) Meridiani Planum sediments on Mars formed through weathering in massive ice deposits. Nat. Geosci 2, 215–220. [Google Scholar]
- Oelkers EH (2001) General kinetic description of multioxide silicate mineral and glass dissolution. Geochim. Cosmochim. Acta 65, 3703–3719. [Google Scholar]
- Peretyazhko T, Sutter B, Morris R, Agresti D, Le L and Ming D (2016) Fe/Mg smectite formation under acidic conditions on early Mars. Geochim. Cosmochim. Acta 173, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet F, Bibring J-P, Mustard J, Gendrin A, Mangold N, Langevin Y, Arvidson R, Gondet B, Gomez C and Berthé M (2005) Phyllosilicates on Mars and implications for early Martian climate. Nature 438, 623–627. [DOI] [PubMed] [Google Scholar]
- Poulet F, Mangold N, Loizeau D, Bibring J-P, Langevin Y, Michalski J and Gondet B (2008) Abundance of minerals in the phyllosilicate-rich units on Mars. Astron.Astrophys 487, L41–L44. [Google Scholar]
- Rampe EB, Ming DW, Blake DF, Bristow TF, Chipera SJ, Grotzinger JP, Morris RV, Morrison SM, Vaniman DT, Yen AS and et al. (2017) Mineralogy of an ancient lacustrine mudstone succession from the Murray formation, Gale crater, Mars. Earth. Planet. Sci. Lett 471, 172–185. [Google Scholar]
- Righter K, Pando K and Danielson L (2009) Experimental evidence for sulfur-rich martian magmas: Implications for volcanism and surficial sulfur sources. Earth. Planet. Sci. Lett 288, 235–243. [Google Scholar]
- Seyfried W, Shanks W and Dibble W (1978) Clay mineral formation in DSDP Leg 34 basalt. Earth. Planet. Sci. Lett 41, 265–276. [Google Scholar]
- Solomon SC, Aharonson O, Aurnou JM, Banerdt WB, Carr MH, Dombard AJ, Frey HV, Golombek MP, Hauck SA and Head JW (2005) New perspectives on ancient Mars. Science 307, 1214–1220. [DOI] [PubMed] [Google Scholar]
- Story S, Bowen BB, Benison KC and Schulze DG (2010) Authigenic phyllosilicates in modern acid saline lake sediments and implications for Mars. J. Geophys. Res. Planets 115. [Google Scholar]
- Tosca NJ, McLennan SM, Dyar MD, Sklute EC and Michel FM (2008) Fe oxidation processes at Meridiani Planum and implications for secondary Fe mineralogy on Mars. J. Geophys. Res. Planets 113. [Google Scholar]
- Treiman AH, Morris RV, Agresti DG, Graff TG, Achilles CN, Rampe EB, Bristow TF, Blake DF, Vaniman DT and Bish DL (2014) Ferrian saponite from the Santa Monica Mountains (California, USA, Earth): Characterization as an analog for clay minerals on Mars with application to Yellowknife Bay in Gale Crater. Am. Mineral 99, 2234–2250. [Google Scholar]
- Vaniman D, Bish D, Ming D, Bristow T, Morris R, Blake D, Chipera S, Morrison S, Treiman A and Rampe E (2014) Mineralogy of a mudstone at Yellowknife Bay, Gale crater, Mars. Science 343, 1243480. [DOI] [PubMed] [Google Scholar]
- Whittig LD and Allardice WR (1986) X-ray diffraction techniques, In: Methods of soil analysis. Part 1 Physical and mineralogical methods (ed. Klute A). American Society of Agronomy, Madison, pp. 331–363. [Google Scholar]
- Wray J, Milliken R, Dundas C, Swayze G, Andrews-Hanna J, Baldridge A, Chojnacki M, Bishop J, Ehlmann B and Murchie SL (2011) Columbus crater and other possible groundwater-fed paleolakes of Terra Sirenum, Mars. J. Geophys. Res. Planets 116. [Google Scholar]
- Wray JJ, Murchie SL, Bishop JL, Ehlmann BL, Milliken RE, Wilhelm MB, Seelos KD and Chojnacki M (2016) Orbital evidence for more widespread carbonate-bearing rocks on Mars. J. Geophys. Res. Planets 121, 652–677. [Google Scholar]
- Zolotov MY and Mironenko MV (2007) Timing of acid weathering on Mars: A kinetic-thermodynamic assessment. J. Geophys. Res. Planets 112. [Google Scholar]
- Zolotov MY and Mironenko MV (2016) Chemical models for martian weathering profiles: Insights into formation of layered phyllosilicate and sulfate deposits. Icarus 275, 203–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


