Abstract
The present study examines outcomes in patients with stage IV rectal cancer receiving some form of local therapy. That local therapy was either surgery alone or chemoradiation followed by surgery. The authors’ analysis showed a benefit to the addition of chemoradiation to surgery, even in the metastatic setting highlighting the need for multidisciplinary management in this patient population.
Background
With advances in systemic therapies, the role of primary tumor resection may be of increased importance in patients with metastatic rectal cancer. The role of combining pelvic radiotherapy with surgical resection in the metastatic setting is unknown. We utilized the National Cancer Database to examine outcomes in patients with metastatic rectal adenocarcinoma with primary tumor resection with and without pelvic radiotherapy.
Materials and Methods
We queried the National Cancer Database from 2004 to 2014 for patients with stage IV rectal adenocarcinoma receiving chemotherapy. We identified 4051 patients in that group that had primary tumor resection. Patients were then stratified by receipt of pelvic radiotherapy (yes = 1882; no = 2169) Univariable and multivariable analyses identified characteristics predictive of overall survival. Propensity-adjusted Cox proportional hazard ratios for survival were used to account for indication bias.
Results
The median patient age was 63 years (range, 18–90 years) with a median follow-up of 32.3 months (range, 3.02–151.29 months). There were proportionately more patients with T3/T4 disease or N1 disease in the surgery plus radiotherapy arm. The median survival was 46.3 months versus 35.3 months in favor of addition of radiotherapy (P < .001). The 2- and 5-year overall survival was 68.4% and 24.8% for surgical resection alone compared with 77.2% and 39.6% for surgery + radiotherapy. On propensity-adjusted multivariable analysis, radiotherapy was associated with a statistically significant reduction in risk of death (hazard ratio, 0.722; 95% confidence interval, 0.0665–0.784).
Conclusion
This analysis indicates that in patients with metastatic rectal adenocarcinoma receiving chemotherapy, pelvic radiotherapy in addition to primary tumor resection may be of significant benefit.
Keywords: Local therapy, Radiation therapy, Rectal cancer, Stage IV
Introduction
Approximately 15% to 20% of newly diagnosed patients with rectal cancer present with metastatic disease.1 Classically, chemotherapy and expectant palliative management of the primary rectal tumor were the standard of care. However, in our modern era of effective systemic and liver-directed therapies, metastatic disease can often be controlled for an extended period of time with a median survival of 19.5 months.2 Given this extended survival, surgical management of the rectal primary tumor may be of increased utility.
A Surveillance, Epidemiology, and End Results (SEER) analysis from 1988 to 2000 indicated a 10-month median survival advantage with primary rectal tumor resection in the metastatic setting.3 However, controversy exists as this study was performed in an era prior to modern systemic therapy, which may obviate the need for primary tumor management. Furthermore, it did not indicate whether radiotherapy prior to resection would be of benefit as it is in management of locally advanced disease. Accordingly, primary rectal tumor management in the metastatic setting can include a variety of different approaches, including observation and surgery, with or without pelvic radiotherapy. A meta-analysis by Lee et al indicates that neoadjuvant radiotherapy followed by surgery may be the best approach in the metastatic setting; however, their study is limited by retrospective data, inconsistent account of complications, and publication bias.4
Given the paucity of evidence to support or refute neoadjuvant radiotherapy prior to surgical resection in metastatic rectal adenocarcinoma, we utilized the National Cancer Database (NCDB) dataset to characterize survival outcomes after surgical primary tumor management with and without radiotherapy in the modern chemotherapy era.
Materials and Methods
Data Source
We utilized the population-based data set of the NCDB to perform an institutional review board-exempt retrospective review of de-identified patient data. The NCDB is a national cancer database maintained by the American Cancer Society and the American College of Surgeons, pooling data from Commission on Cancer-accredited hospitals. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Patient Selection
Data for patients diagnosed with stage IV rectal cancer was obtained from 2004 to 2014. Patients were excluded for having non-adenocarcinoma histology or American Joint Committee on Cancer (AJCC) seventh edition stage I, II, III, or not recorded stage. We then excluded patients who did not receive chemotherapy or received single-agent chemotherapy in order to represent a metastatic population receiving standard multi-agent chemotherapy, such as FOLFOX (5-fluoruricil, leucovorin, and oxaliplatin). Additionally, we excluded patients starting chemotherapy > 90 days from diagnosis, as delay in management would, in theory, be associated with worse outcome. This resulted in 15,643 patients with metastatic rectal adenocarcinoma receiving definitive chemotherapy.
We then did a preliminary analysis of surgical management of the primary tumor, which yielded 4051 patients with surgical resection and 7875 patients without surgical resection. In this analysis, 3717 patients were excluded for missing data pertaining to surgical resection and/or follow-up.
We then excluded patients with follow-up < 3 months or non-recorded follow-up and those without surgical resection or unrecorded primary tumor management. Additionally, we excluded non-standard radiotherapy doses using cutoffs of < 20 Gy and > 70 Gy.
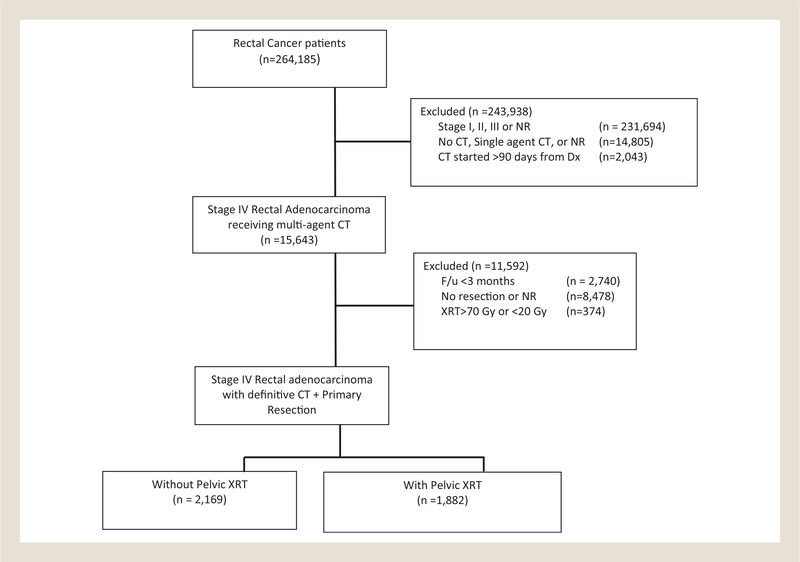
This resulted in a homogenous population of 4051 patients who had definitive chemotherapy for metastatic rectal adenocarcinoma and surgical management of their primary tumor. Patients were then stratified by receipt of pelvic radiotherapy (n = 1882) or no pelvic radiotherapy (n = 2169). A CONsolidated Standards Of Reporting Trials (CONSORT) diagram is provided to detail the selection criteria (Figure 1).
Figure 1. CONsolidated Standards of Reporting Trials (CONSORT) Diagram Showing Patient Selection.
Abbreviations: CT = chemotherapy; Dx = date of diagnosis; NR = not recorded/unknown; XRT = pelvic radiotherapy.
Comorbidity was quantified via Charlson/Deyo comorbidity index, and stage was defined by AJCC seventh edition clinical staging. Income data in the patients’ residence census tract were provided as quartiles. Population classification was based on typology published by the United States Department of Agriculture Economic Research Service, facility type was assigned according to Commission on Cancer accreditation category, and insurance status was reported on the admission page. To account for limited (oligometastatic disease) versus extensive metastatic burden, patients were stratified based on metastasis confined to 1 organ site versus multiple organ sites.
Statistics
Statistical analyses were performed with SPSS Version 20.0 (Chicago, IL). Summary statistics were reported for discrete variables, and χ2 tests were used to compare socioeconomic, clinical, and treatment characteristics between groups. Bivariate logistic regression models were used to evaluate the association between independent variables of interest. Overall survival was calculated from the date of diagnosis to the date of last contact or death using Kaplan-Meier curves to present the cumulative probability of survival and logrank statistics to assess statistical significance between groups. Univariable survival analysis was performed for all demographic, tumor, and treatment characteristics, and statistically significant factors were then entered in a hierarchical fashion using forward selection of the covariates’ likelihood ratios for multivariable analysis. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) are reported, and α = 0.05 was used to indicate statistical significance.
Propensity score analysis was used to account for indication bias caused by lack of randomization.5,6 The propensity models included observable variables significantly associated with pelvic radiation on multivariable logistic regression within the radiotherapy and non-radiotherapy patients. Subsequently, we constructed a Cox proportional hazards model adjusting for propensity score, and considering baseline differences in covariates in the model separately.7 To strengthen the assumption of balance between groups, the propensity-adjusted score was validated by stratification into propensity score-based quintiles, which demonstrated that standardized difference between the treatment groups was less than 0.10.
Results
Patient Characteristics
The median patient age was 63 years (range, 18–90 years). The median follow-up was 32.3 months (range, 3.02–151.29 months). Baseline patient and treatment characteristics for patients are outlined in Table 1. There were disproportionately less patients > 65 years old (18.9% vs. 26.6%) as well as with Charlson-Deyo Comorbidity index ≥ 1 (15.4% vs. 17.9%) in the surgery plus radiotherapy arm. There were proportionately more patients with T3/T4 disease (69.6% vs. 46.5%) or N1 disease (41.5% vs. 27.3%) in the surgery plus radiotherapy arm. There was a similar distribution of gender, insurance status, income, rural/urban location, academic/community care, and tumor grade among the radiotherapy and non-radiotherapy groups. Metastatic burden was confined to 1 organ in 40.5% of patients as a surrogate for oligometastatic disease. This was equally distributed between the radiotherapy and non-radiotherapy groups (odds ratio, 0.92; 95% CI, 0.81–1.04). The pattern of metastases was categorized in 2344 (57.9%) of 4051 patients. Seventy-eight percent of patients had liver metastases, 22% had lung metastases, 3% had bone metastases, and 0.5% had brain metastases.
Table 1.
Baseline Patient and Treatment Characteristics
| No XRT, n (%) | XRT, n (%) | OR | 95% CI | P Value | |
|---|---|---|---|---|---|
| Total patients | 2169 | 1882 | |||
| Gender | |||||
| Male | 1352 (62.3) | 1150 (61.1) | 1 | Reference | |
| Female | 817 (37.7) | 732 (38.9) | 1.05 | 0.93–1.20 | .423 |
| Age, y | |||||
| ≤ 65 | 1591 (73.4) | 1527 (81.1) | 1 | Reference | |
| > 65 | 578 (26.6) | 355 (18.9) | 0.64 | 0.55–0.7 | < .0001 |
| Insurance | |||||
| Insured | 2067 (95.3) | 1780 (94.6) | 1 | Reference | |
| Uninsured | 102 (4.7) | 102 (5.4) | 1.16 | 0.88–1.54 | .298 |
| Income, $ | |||||
| < 48,000 | 877 (40.4) | 733 (38.9) | 1 | Reference | |
| ≥ 48,000 | 1292 (59.6) | 1149 (61.1) | 1.06 | 0.94–1.21 | .335 |
| Facility | |||||
| Community | 1377 (63.5) | 1196 (63.6) | 1 | Reference | |
| Academic | 792 (36.5) | 686 (36.4) | 1.00 | 0.88–1.13 | .966 |
| Population | |||||
| Metro/urban | 2049 (94.5) | 1760 (93.5) | 1 | Reference | |
| Rural | 120 (5.5) | 122 (6.5) | 1.18 | 0.91–1.54 | .204 |
| Comorbidity score | |||||
| 0 | 1781 (82.1) | 1592 (84.6) | 1 | Reference | |
| ≥ 1 | 388 (17.9) | 290 (15.4) | 0.84 | 0.71–0.99 | .035 |
| Clinical T stage | |||||
| T1/T2 | 233 (10.7) | 155 (8.2) | 1 | Reference | |
| T3/T4 | 1009 (46.5) | 1309 (69.6) | 1.95 | 1.57–2.43 | < .0001 |
| Unknown | 927 (42.7) | 418 (22.2) | 0.68 | 0.54–0.86 | .001 |
| Clinical N stage | |||||
| N0 | 659 (30.4) | 498 (26.5) | 1 | Reference | |
| N1 | 593 (27.3) | 782 (41.5) | 1.75 | 1.49–2.04 | < .0001 |
| N2 | 336 (15.5) | 294 (15.6) | 1.16 | 0.95–1.41 | .141 |
| Unknown | 581 (26.8) | 308 (16.4) | 0.70 | 0.59–0.84 | .0001 |
| Grade | |||||
| 1–2 | 1777 (81.9) | 1581 (84.0) | 1.0 | Reference | |
| 3 | 392 (18.1) | 301 (16.0) | 0.86 | 0.73–1.02 | .080 |
| Metastatic burden | |||||
| 1 organ involved | 858 (39.6) | 783 (41.6) | 1 | Reference | |
| Multiple organs involved | 1311 (60.4) | 1099 (58.4) | 0.92 | 0.81–1.04 | .186 |
Abbreviations: CI = confidence interval; OR = odds ratio; XRT = pelvic radiotherapy.
Radiotherapy Details
Of 4051 patients receiving definitive surgical management of their primary tumor, 53.5% received surgical resection without radiotherapy and 46.5% had pelvic radiotherapy in addition to surgical resection. The median composite total dose was 50.4 Gy (range, 20–76.44 Gy). Short-course radiotherapy (25 Gy in 5 fractions) was delivered in 139 (7.4%) patients. Of 1882 patients receiving pelvic radiotherapy, 1606 (85.3%) received radiotherapy prior to surgical resection at a median of 92 days prior to resection.
Survival Analysis
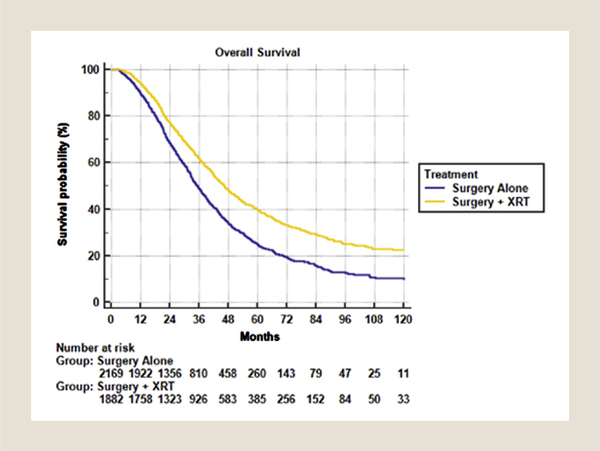
The median survival was 46.3 months versus 35.3 months in favor of addition of radiotherapy (P < .001). The 2-, 5-, and 10-year overall survival was 68.4%, 24.8%, and 9.5% for surgical resection alone compared with 77.2%, 39.6%, and 22.3% for surgery + radiotherapy (Figure 2). On multivariable analysis, radiotherapy was associated with a statistically significant reduction in risk of death (HR, 0.718; 95% CI, 0.661–0.780). This benefit was upheld on propensity-matched analysis (HR, 0.722; 95% CI, 0.0665–0.784).
Figure 2.
Overall Survival for Surgery Alone Versus Pelvic Radiotherapy + Surgery in the Metastatic Setting With Chemotherapy
Multivariable Cox regression revealed age > 65 years, income < $48,000, grade 3 disease, and N2 disease to correlate with decreased survival, whereas treatment in the academic setting and having a single metastatic site correlated with increased survival (Table 2). Multivariable Cox regression including propensity score revealed maintained survival benefit for radiotherapy, treatment in an academic setting, and having a metastatic disease to a single organ. Income < $48,000 held as the only negative predictor of survival.
Table 2.
Multivariable Cox Proportional Hazards Models for Overall Survival
| Without Propensity Match | With Propensity Match | |||
|---|---|---|---|---|
| Hazard of Death (95% CI) | P Value | Hazard of Death (95% CI) | P Value | |
| Pelvic radiotherapy | ||||
| Surgery alone | Reference | |||
| Surgery + XRT | 0.72 (0.66–0.78) | < .001 | 0.72 (0.67–0.78) | < .001 |
| Gender | ||||
| Male | Reference | |||
| Female | 1.35 (1.23–1.48) | < .001 | ||
| Age, y | ||||
| ≤ 65 | Reference | |||
| > 65 | 1.35 (1.23–1.48) | < .001 | ||
| Insurance | ||||
| Insured | Reference | |||
| Uninsured | 1.07 (0.90–1.28) | .445 | 1.08 (0.90–1.28) | .430 |
| Income, $ | ||||
| < 48,000 | Reference | |||
| ≥ 48,000 | 1.11 (1.03–1.21) | .008 | 1.10 (1.01–1.19) | .022 |
| Facility | ||||
| Community | Reference | |||
| Academic | 0.80 (0.73–0.87) | < .001 | 0.78 (0.72–0.85) | < .001 |
| Population | ||||
| Metro/urban | Reference | |||
| Rural | 1.08 (0.90–1.28) | .445 | 1.13 (0.96–1.33) | .159 |
| Comorbidity score | ||||
| 0 | Reference | |||
| ≥ 1 | 0.94 (0.84–1.04) | .216 | 0.95 (0.85–1.05) | .316 |
| Clinical T stage | ||||
| T1/T2 | Reference | |||
| T3/T4 | 0.80 (0.88–1.18) | .803 | ||
| Unknown | 0.92 (0.96–1.30) | .14 | ||
| Clinical N stage | ||||
| N0 | Reference | |||
| N1 | 0.93 (0.84–1.03) | .803 | ||
| N2 | 1.36 (1.20–1.54) | .14 | ||
| Unknown | 1.18 (1.05–1.32) | .007 | ||
| Grade | ||||
| 1–2 | Reference | |||
| 3 | 1.51 (1.37–1.67) | < .001 | ||
| Metastatic burden | ||||
| 1 organ involved | Reference | |||
| Multiple organs involved | 0.74 (0.67–0.80) | < .001 | 0.73 (0.67–0.79) | < .001 |
Abbreviations: CI = confidence interval; XRT = pelvic radiotherapy.
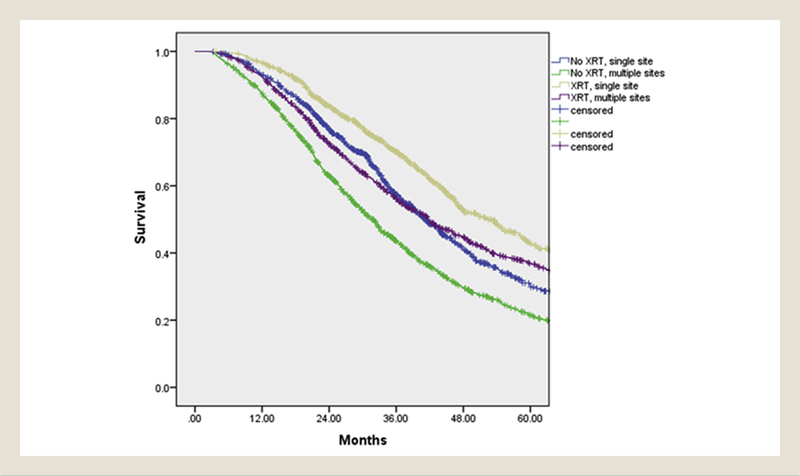
In patients with disease confined to 1 organ, the median 2- and 5-year survival was 40.9 months (range, 38.1–43.8 months), 76.7%, and 30.3% for surgery without pelvic radiotherapy and 52.2 months (range, 47.2–57.3 months), 83.7%, and 42.7% for surgery and pelvic radiotherapy (P < .001). This benefit was also seen in patients with multi-organ metastatic disease, with a median 2- and 5-year survival of 31.8 months (range, 30.1–33.6 months), 63%, and 21.5% for surgery without pelvic radiotherapy and 41.8 months (range, 38.5–45.1 months), 72.6%, and 36.5% for surgery and pelvic radiotherapy (P < .001). (Figure 3)
Figure 3. Survival for Pelvic Radiotherapy + Surgery Versus Surgery Alone in the Oligometastatic and Extensive Metastatic Settings.
Abbreviation: XRT = pelvic radiotherapy.
Discussion
Advances in systemic therapy for metastatic rectal adenocarcinoma have dramatically improved survival over the past decade.8,9 Across many tumor types, when metastatic disease is present at diagnosis, the primary tumor is often managed expectantly and for palliation. However, in metastatic rectal adenocarcinoma, definitive management of the primary tumor has been shown to improve outcomes with the advent of effective systemic therapies such as FOLFOX, FOLFIRI (leucovorin, 5-fluoruricil, and irinotecan), bevacizumab, and cetuximab.4,10,11 This is especially true in patients with oligometastatic disease amenable to locally ablative therapies such as resection, radiofrequency ablation, or stereotactic body radiation therapy.12–14 We are now in an era where select patients with limited disease burden may experience durable long-term survival, making the precise management of their primary rectal tumor of upmost importance. This notion is supported by our preliminary analysis showing a large survival benefit in favor or primary tumor management over chemotherapy alone, as well as in the American College of Radiology Appropriateness Criteria recommendations for rectal cancer with metastatic disease at presentation.1
Although there is considerable debate over in which patients primary tumor resection provides benefit over chemotherapy alone,4,15,16 there is little, if any, evidence supporting/refuting the role of pelvic radiotherapy in addition to surgery as it is conventionally used in the locally advanced setting. There are numerous retrospective series quantifying the survival and palliative benefits of primary tumor resection in the metastatic setting; however, many, if not all, are confounded by the inclusion of all colon sites and are not exclusive to rectal cancer, where radiotherapy plays an important role in aiding with surgical resection.17 Additionally, details of including pelvic radiotherapy as it pertains to rectal tumor resection are often missing in these analyses. Accordingly, we sought to quantify this outcome specific to a rectal primary site in a sample with both limited, oligometastatic, and extensive metastatic burden.
In our attempt to account for oligometastatic patients, the NCDB only records organs involved and not a numerical or size depiction of metastatic disease. In addition, resection of sites of metastatic disease is not characterized in the NCDB either. We therefore analyzed patients with 1 organ involved as a surrogate for an “oligometastatic” state. In our sample, 78% of patients had liver metastasis, and presumably the majority of single-organ disease was confined to the liver. However, unobservable confounding variables such as bulk of disease and number of metastasis is not accounted for. Contrary to this, in the 59.5% of patients in this sample with multi-organ disease, a proportion of patients may have had limited disease burden despite multiple organs involved. Regardless, our study showed a significant benefit for the addition of pelvic radiotherapy across both limited and extensive metastatic disease (Figure 3).
The association with longer survival for all patients with pelvic radiation was pronounced even though patients receiving radiotherapy had more extensive T stage, N stage, and more comorbidity compared with patients managed with surgery alone (P < .05). On multivariable analysis, both grade 3 disease and more advanced N stage demonstrated significant correlation with survival for the addition of pelvic radiotherapy to surgical resection. Concordantly, a retrospective series by Aslam et al also indicated a benefit to primary tumor management with higher nodal stage on multivariate analysis.18 Thus, a subset of patients with significant nodal burden not amenable to complete resection is a strong indication for pelvic radiotherapy in the metastatic setting.
Primary rectal tumor management in the metastatic setting has multiple benefits that may lead to increased survival. The first of which is, most obviously, treating all disease sites in the oligometastatic state, which is now becoming standard practice. Additionally, bulky primary rectal tumors are problematic and cause symptoms of obstruction and bleeding in 10% to 25% of cases.19–23 Primary management with radiotherapy and resection has an established history for symptom palliation in the metastatic setting, but also may provide a benefit in preventing symptomatic obstruction, sepsis, hemorrhage, and anemia, which may complicate and delay life-saving chemotherapy. This concept has been shown with the addition of bevacizumab, as the de novo primary tumor has been postulated to be at risk for increased bleeding complications when bevacizumab is given.24–26 Thus, primary tumor radiotherapy and surgery may allow for uninterrupted effective systemic therapy in certain instances. Of course, the benefit of pelvic radiotherapy and resection must be weighed against the risk of perioperative complications, as these would be counterproductive in delaying chemotherapy. However, postoperative complications in patients with metastatic colorectal cancer tend to be minimal in the modern era with laparoscopic techniques,27,28 and perioperative mortality in the metastatic setting is less then 2%.29 Furthermore, planned elective rather than emergent surgery resection may lower perioperative mortality and contribute to improved outcomes.15,30
Although the distinct survival benefit with the addition of pelvic radiotherapy to primary tumor resection in the metastatic setting is hypothesis-generating, it must be considered with the selection bias inherent to any large retrospective study. The numbers supplied by the NCDB allow investigators to explore associations that are otherwise difficult to unveil owing to a limited sample size. Nevertheless, unobserved confounding variables limit the interpretation of observational data, regardless of attempts to mitigate bias with multivariable analysis and propensity matching. Additionally, clinical treatment response, salvage therapies, specific chemotherapeutic agents, and number of cycles administered are not included in the data, which may have otherwise affected the interpretation of results.
Conclusion
Patients with metastatic rectal adenocarcinoma have extended survival in the era of modern effective systemic therapy. Therefore, select patients, such as those with response to chemotherapy, may benefit from aggressive local primary tumor management. Our study indicates that, in the setting of double-agent chemotherapy, pelvic radiotherapy in addition to surgery benefits not only oligometastatic patients, but also those with more extensive disease burden. Prospective investigation of the management of the rectal primary tumor with chemotherapy, pelvic radiotherapy, and surgical resection in the setting of responsive metastatic disease are warranted.
Clinical Practice Points.
It is well known that there are select patients with stage IV rectal cancer that may benefit from treatment of their primary tumor. The results presented here show a potential benefit to local therapy (both chemotherapy and radiation in addition to surgery alone) in this patient population.
Keeping in mind that selection bias is present in NCDB studies, the results can likely be extrapolated to fit, young patients with low metastatic burden.
Based on this analysis, there appears to be a benefit to the addition of radiation therapy to surgery, and IT should be considered in appropriate patients.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Herman J, Messersmith W, Suh WW, et al. ACR Appropriateness Criteria: rectal cancer-metastatic disease at presentation. Curr Probl Cancer 2010; 34:201–10. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22:23–30. [DOI] [PubMed] [Google Scholar]
- 3.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of Surveillance, Epidemiology, and End Results data, 1988 to 2000. Ann Surg Oncol 2005; 12:637–45. [DOI] [PubMed] [Google Scholar]
- 4.Lee K-C, Ou Y-C, Hu W-H, Liu C-C, Chen H-H. Meta-analysis of outcomes of patients with stage IV colorectal cancer managed with chemotherapy/radiochemotherapy with and without primary tumor resection. Onco Targets Ther 2016; 9:7059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–81. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci 1988; 2:567. [Google Scholar]
- 7.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffee P, Osipov A, Tan C, Tuli R, Hendifar A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol 2015; 6:185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loree JM, Kopetz S. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol 2017; 9:551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J Am Coll Surg 2003; 196:722–8. [DOI] [PubMed] [Google Scholar]
- 11.Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg 2000; 135:530–5. [DOI] [PubMed] [Google Scholar]
- 12.Clark ME, Smith RR. Liver-directed therapies in metastatic colorectal cancer. J Gastrointest Oncol 2014; 5:374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly CM, Kemeny NE. Liver-directed therapy in metastatic colorectal cancer. Expert Rev Anticancer Ther 2017; 17:745–58. [DOI] [PubMed] [Google Scholar]
- 14.Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol 2014; 20:4220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage iv colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010; 34:797–807. [DOI] [PubMed] [Google Scholar]
- 16.Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev 2012; 8:CD008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauer R, Becker H, Hohenberger W, et al. , German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–40. [DOI] [PubMed] [Google Scholar]
- 18.Aslam MI, Kelkar A, Sharpe D, Jameson JS. Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg 2010;8:305–13. [DOI] [PubMed] [Google Scholar]
- 19.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 1999; 6:651–7. [DOI] [PubMed] [Google Scholar]
- 20.Tebbutt NC, Norman AR, Cunningham D, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut 2003; 52:568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Nonoperative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg 2001; 88:1352–6. [DOI] [PubMed] [Google Scholar]
- 22.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009; 27:3379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP Trial C-10. J Clin Oncol 2012; 30:3223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008; 26:5326–34. [DOI] [PubMed] [Google Scholar]
- 25.Kozloff M, Yood MU, Berlin J, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist 2009; 14:862–70. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009; 20:1842–7. [DOI] [PubMed] [Google Scholar]
- 27.Yang TX, Billah B, Morris DL, Chua TC. Palliative resection of the primary tumour in patients with stage IV colorectal cancer: systematic review and meta-analysis of the early outcome after laparoscopic and open colectomy. Color Dis 2013; 15:e407–19. [DOI] [PubMed] [Google Scholar]
- 28.Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009; 10:44–52. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson KJ, Chua W, Ng W, Roohullah A. Management of asymptomatic primary tumours in stage IV colorectal cancer: Review of outcomes. World J Gastrointest Oncol 2015; 7:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legendre H, Vanhuyse F, Caroli-Bosc FX, Pector JC. Survival and quality of life after palliative surgery for neoplastic gastrointestinal obstruction. Eur J Surg Oncol 2001; 27:364–7. [DOI] [PubMed] [Google Scholar]