Abstract
The burden and prognosis of malignant mesothelioma in the United States have remained largely unchanged for decades, with approximately 3200 new cases and 2400 deaths reported annually. To address care and research gaps contributing to poor outcomes, in March of 2019 the Mesothelioma Applied Research Foundation convened a workshop on the potential usefulness and feasibility of a national mesothelioma registry. The workshop included formal presentations by subject matter experts and a moderated group discussion. Workshop participants identified top priorities for a registry to be (a) connecting patients with high-quality care and clinical trials soon after diagnosis, and (b) making useful data and biospecimens available to researchers in a timely manner. Existing databases that capture mesothelioma cases are limited by factors such as delays in reporting, deidentification, and lack of exposure information critical to understanding as yet unrecognized causes of disease. National disease registries for amyotrophic lateral sclerosis (ALS) in the United States and for mesothelioma in other countries, provide examples of how a registry could be structured to meet the needs of patients and the scientific community. Small-scale pilot initiatives should be undertaken to validate methods for rapid case identification, develop procedures to facilitate patient access to guidelines-based standard care and investigational therapies, and explore approaches to data sharing with researchers. Ultimately, federal coordination and funding will be critical to the success of a National Mesothelioma Registry in improving mesothelioma outcomes and preventing future cases of this devastating disease.
Keywords: asbestos, mesothelioma, registry
1 |. INTRODUCTION
Approximately 3200 new cases of malignant mesothelioma are diagnosed annually in the United States.1 Prognosis remains poor, with about half of the patients dying within a year of diagnosis.2 Many patients do not receive recommended therapy,3 and the number participating in clinical trials is lower than desired.4 Furthermore, our understanding of mesothelioma risk factors beyond occupational asbestos exposure is limited.5–7 To explore whether a national patient registry could address these care and research needs, the Mesothelioma Applied Research Foundation brought together experts in mesothelioma and disease surveillance for a one-day workshop in Bethesda, Maryland on 26 March 2019. The workshop began with formal presentations by subject matter experts, which were followed by a moderated group discussion. Workshop participants reviewed the epidemiology of mesothelioma and clinical and research needs; the strengths and limitations of existing databases, including cancer surveillance programs and the National Mesothelioma Virtual Bank (NMVB); and the lessons learned from a national registry for amyotrophic lateral sclerosis (ALS) and from mesothelioma registries in other countries. They also discussed how a National Mesothelioma Registry could be structured to best meet the needs of patients and the scientific community, taking into account resource constraints. In this document, we provide a summary of the workshop presentations and discussions, supplemented by background information from the scientific literature and national data sources.
2 |. EPIDEMIOLOGY
Despite steep declines in asbestos use in the United States since the 1970s, cases of mesothelioma continue to occur (Figure 1). In 2015, there were 3209 new cases of mesothelioma and 2404 mesothelioma deaths reported in the United States.1 Incidence and mortality rates were higher for men (1.5 cases per 100 000 and 1.2 deaths per 100 000, respectively) than women (0.4 cases per 100 000 and 0.2 deaths per 100 000, respectively). From 2000 to 2015, mesothelioma incidence declined somewhat for men but held steady for women; the overall rate changed slightly from 1.1 to 0.9 cases per 100 000. Data from 1999 to 2017 show mesothelioma mortality rates increased steadily with age: less than 1 death per 100 000 for those under 65 years and greater than 3.5 deaths per 100 000 for those 65 years and older, with the highest mortality (7.6 deaths per 100 000) in the 85 years and older age group.8 Across age groups, the mortality rate of whites (1.4 deaths per 100 000) was similar to that of Hispanics (1.3 deaths per 100 000) and higher than that of blacks or African Americans (0.6 deaths per 100 000).
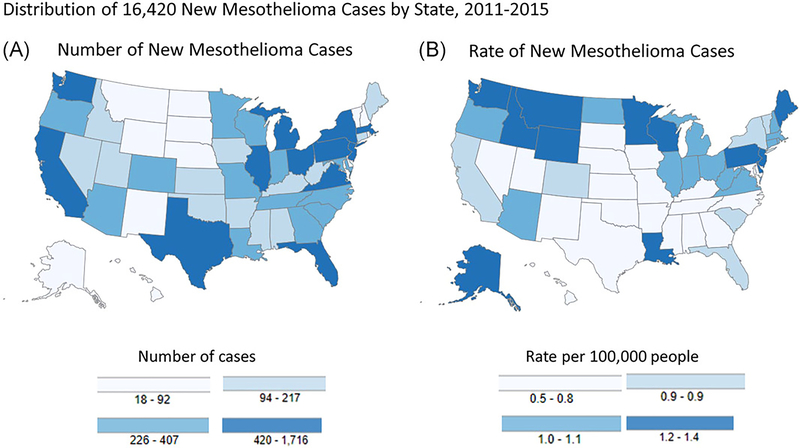
FIGURE 1.
Geographical distribution of new mesothelioma cases (both sexes, all ages, all races, and ethnicities) by state, 2011 to 2015: numbers of cases are depicted in Map A and rates are depicted in Map B. Rates are per 100 000 persons and are age-adjusted to the 2000 US standard population. State rankings based on numbers of cases and incidence rates are different. Source: US Cancer Statistics Working Group1
Regional and occupational variations also are notable. From 2011 to 2015, higher incidence and mortality rates were observed in northern than southern states, with the exception of Louisiana.1 California had the highest number of mesothelioma cases (1283) and deaths (1027) during this period, reflecting the state’s large population. For 26 states, coded occupational information was available for mesothelioma deaths in some years (1999, 2003, 2004, 2007–2013).8 For men, occupational groups with significantly elevated proportionate mortality ratios (PMRs) included insulation workers, hazardous materials removal workers, riggers, marine engineers, ship engineers, plumbers, and pipefitters. For women, significantly elevated PMRs were observed for medical and health services managers, office clerks, and teachers.
3 |. CLINICAL AND RESEARCH NEEDS
The median survival for malignant pleural mesothelioma is less than 1 year, and the prognosis has not improved over the past four decades. A recent analysis of 2004 to 2013 National Cancer Data Base (NCDB) records identified 19 134 cases of malignant pleural mesothelioma.3 Trimodality treatment with chemotherapy, surgical resection, and radiation therapy was associated with the best survival (median, 19.9 months), followed by combination chemotherapy and resection (median, 15.3 months). Less than 3% of patients received trimodal therapy, and 10% received combination chemotherapy and resection. More commonly, patients received no mesothelioma treatment (40%) or chemotherapy alone (31%). The median survival for patients receiving no treatment was 4.8 months and for patients receiving chemotherapy alone was 11.3 months. The population’s demographics, low rate of comorbidities, and early clinical stage of disease suggest that many more of the patients might have been suitable for multimodality treatment.10 The low utilization of multimodality treatment appears to be accompanied by incomplete clinical assessment, as about 30% had “unknown” tumor or node stage and 45% had unknown cell type. Furthermore, associations between survival and higher income and private insurance suggest lack of access to specialized care played a role in the limited use of multimodality therapy. Another study using 2004 to 2014 NCDB data of malignant pleural mesothelioma limited to known histological subtypes also found overall poor compliance with guidelines published by the National Comprehensive Cancer Network and the American Society of Clinical Oncology for multimodality therapy, with high-volume hospitals and academic centers having the highest odds of compliance.11 The authors also observed treatment disparities for women, octogenarians, uninsured, and patients with higher comorbidity scores.
The poor prognosis of mesothelioma with even the best of standard care highlights the need for clinical trials of novel therapeutic approaches. However, a small minority of mesothelioma patients participates in clinical trials. Among all cancer patients, clinical trial participation is estimated to be at most 5%.4,12 Participation among mesothelioma patients is likely lower, as older patients and those with lung cancer are particularly underrepresented in clinical trials.4 It has been suggested that important barriers to participation in cancer clinical trials are mistrust and lack of knowledge of clinical trials.13 One challenge for mesothelioma patients is that available trials tend to be early phase, nonrandomized studies of targeted or cytotoxic therapies, rather than of multimodality approaches.14,15 Furthermore, most mesothelioma patients are treated in the community setting, while most clinical trials are sponsored by academic centers.14 Thus, patients with mesothelioma may not be aware of relevant trials or may approach investigators late in treatment, when they are no longer eligible for trials designed to examine new first-line therapies. Workshop participants noted that recruiting adequate numbers of patients meeting inclusion criteria needed to complete mesothelioma clinical trials was difficult and time consuming. These challenges exist despite excellent available resources such as the NCI’s Clinical Trials Information for Patients and Caregivers website.16
Basic science and epidemiologic studies on mesothelioma provide evidence that can be used for treatment development and disease prevention through improved understanding of modifiable risk factors. Important basic science questions are many and include the optimal method for targeting mesothelin, the role of genetic factors such as germline mutations in disease phenotype, and the genetics of mesothelioma at nonpleural anatomical sites.17–21 Epidemiologically, we still do not fully understand differences in disease incidence and mortality by sex, race/ethnicity, region, and occupation, the potential gene-environment interactions that may influence pathogenesis and treatment outcomes, or the pathogenic role of exposures beyond occupational asbestos, particularly for women.5,7,22–24 Although not well documented in the literature, workshop participants also expressed concern that the complex legal issues surrounding asbestos exposure and mesothelioma in the United States might hinder efforts to address these research issues, as patients might be advised by attorneys that answers provided on research questionnaires about issues such as past exposures or genetic analyses performed on biospecimens might subsequently be used to defend against claims for compensation. Thus, there might be powerful incentives for individuals with mesothelioma to not complete portions of research questionnaires (such as those documenting past exposures) or to not provide biological samples for analysis (such as evaluation for potential genetic risk factors).
A registry would have several options for preventing these unintended outcomes, even if compelled by courts to release information. One approach would be to not collect information with the potential to affect causal attribution in compensation cases. A consequence would be that the registry database would be of limited usefulness in studying risk factors for malignant mesothelioma. Another approach would be to collect information, but anonymize it, so that it could not be linked to individuals. This would potentially have consequences for longitudinal follow-up and usefulness of data for epidemiological purposes. Another suggestion was to hold data with potential impact on compensation cases in a format where it could not be used until after a sufficient period of time had elapsed for cases to be resolved. This might be accomplished by encrypting the data in a format that would not allow decryption until a specific date in the future.
Workshop participants also noted that clinical samples may be sequestered by legal requests, without return to the source, making them unavailable for clinical research use. Although outside the scope of services provided by a registry, responding to legal requests for biopsy samples by providing whole slide scans might provide a way for clinical facilities to retain the physical samples while providing the needed information to requestors.
4 |. EXISTING DATABASES
Table 1 outlines the statutory basis for cancer surveillance in the United States. Cancer is reportable in all states and territories25; data are abstracted from patients’ medical records, entered into the facility’s cancer registry if one is maintained, and then sent to the regional or state central cancer registry.26 Most reports initially come from pathology laboratories, with the remainder from hospitals, physicians’ offices, nursing homes, and other care facilities. After receiving reports, the central cancer registry conducts case consolidation functions that take from 12 to 24 months. Although each state or territory acts independently, standardized methods for coding and data set structure have been established by the North American Association of Central Cancer Registries (NAACCR), permitting data aggregation.27
TABLE 1.
Statutory basis for US cancer surveillance
| Statutory basis for US cancer surveillance |
| Cancer is a reportable disease in all the US states and territories |
| • Healthcare providers and facilities are required to report cancer cases to state cancer registries |
| • Health Insurance Portability and Accountability Act (HIPAA) permits cancer registries to access and maintain identifiable cancer data |
| National Cancer Act of 1971 authorized NCI to conduct population-based cancer surveillance, led to the SEER program in 1973 |
| Cancer Registries Amendment Act of 1992 authorized CDC to provide funds to states and territories to enhance existing cancer registries and establish new ones |
| Caroline Pryce Walker Conquer Childhood Cancer Act of 2008 required CDC to award a grant to enhance and expand tracking of pediatric cancer and include actual occurrences within weeks |
| Survivorship, Treatment, Access, and Research Act of 2018 (STAR) extended and expanded the Caroline Pryce Walker Act |
Note: In the United States, cancer is a reportable illness and cancer surveillance is supported by the NCI and CDC. There is precedent for legislation requiring a specialized program for rapid reporting of a particular type of cancer soon after diagnosis. The Carolyn Pryce Walker Act and subsequent STAR act required rapid reporting of pediatric cancer.
Abbreviations: CDC, Centers for Disease Control and Prevention; National Cancer Institute (NCI); SEER, Surveillance, Epidemiology, and End Results.
Two federal cancer incidence surveillance programs, the National Program of Cancer Registries (NPCR), supported by the Centers for Disease Control and Prevention (CDC), and the Surveillance, Epidemiology, and End Results (SEER) Program, supported by the National Cancer Institute (NCI), receive data annually from central cancer registries.26 Together these two programs cover the entire country. In addition, the National Vital Statistics System (NVSS) maintained by CDC’s National Center for Health Statistics is used for cancer mortality.8 Data on cancer incidence and mortality are disseminated through several online tools, including United States Cancer Statistics (USCS), which makes data publicly available through the Data Visualization tool.1 Figure 2 outlines the flow of information and a timeline for the collection and dissemination of cancer data in the United States.
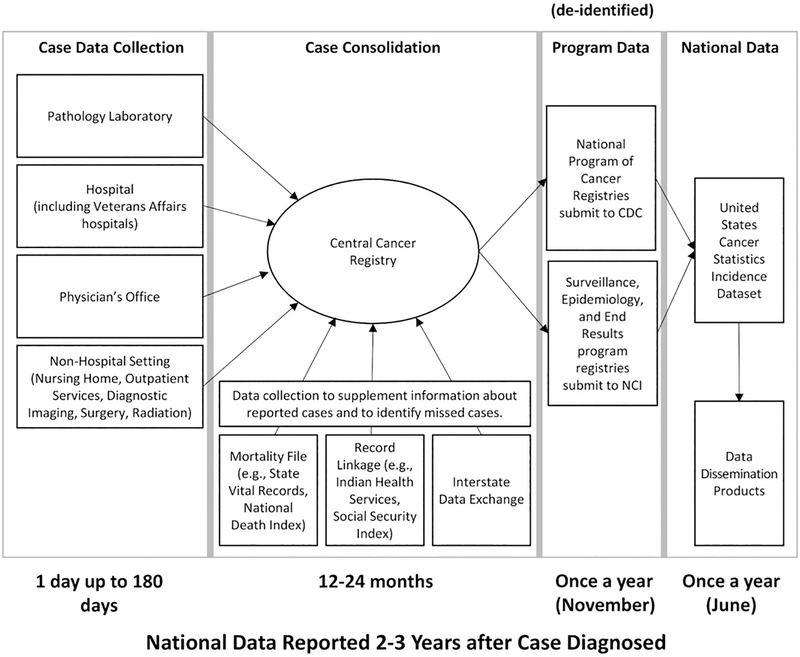
FIGURE 2.
Current system for collecting and reporting cancer data. Various medical facilities report data to central cancer registries in metropolitan areas, regions, states, or territories. Central cancer registries receive funding and technical assistance from the Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries (NPCR), and the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology and End Results (SEER) program. The registries gather additional information, consolidate information about individual patients, and electronically submit deidentified data to the NPCR or SEER programs. The data from NPCR and SEER are combined, which provide 100% national coverage. CDC and NCI assure high data quality, publish official federal government national statistics for cancer and provide tools to view and analyze the data (https://www.cdc.gov/uscs). There is a period of about 2 to 3 years between diagnosis and national reporting of deidentified data. Source: Jane Henley, CDC
Although NPCR, SEER, and NVSS provide useful national data on mesothelioma incidence and mortality, they have several limitations. Due to delays inherent to reporting, consolidation at the central cancer registry, and aggregation at the national level, USCS data reflect cases that were diagnosed 2 to 3 years earlier. This time lag, combined with the fact that the data are deidentified, means these surveillance programs are not positioned to facilitate patient outreach regarding treatment and clinical trials. Furthermore, information on exposure, biomarkers, treatment and outcomes, including complications and quality of life, is limited, reducing the utility for clinical and epidemiological research.
Several other programs demonstrate how such limitations can be addressed. In 2008, the federal Caroline Pryce Walker Conquer Childhood Cancer Act provided funding for more rapid reporting of pediatric cancer cases.28,29 In Louisiana, this funding enabled the state cancer registry to implement electronic pathology reporting for pediatric and young adult cancers using a commercial software package (E-Path Reporter, Artificial Intelligence in Medicine, Toronto, Canada). The software is installed on a pathology laboratory’s computer network, where it scans text, identifies cancer cases, and automatically transmits the information to the central cancer registry. The advantages of this system are that it is efficient, provides data rapidly, and places less of a burden on healthcare facilities. It has allowed the Louisiana Tumor Registry to assist hospitals in NCI’s Community Oncology-Based Research Program (NCORP), identifying patients eligible for clinical trial enrollment within a month of diagnosis. Disadvantages include licensing and maintenance fees for the software and the fact that only pathologically confirmed cases are identified. Free software for secure transmission of public health information is available from CDC in the form of the Public Health Information Network Messaging System,30 but this program relies on International Classification of Diseases (ICD) codes rather than scanning text of pathology reports and automatically reporting cases, so reporting through this system might potentially be less sensitive and specific. Commercial software for electronic radiology reporting can be used to identify cases diagnosed without pathological examination, but most patients with radiographic pleural findings have diagnoses other than mesothelioma, so registry staff must review medical records to confirm the cancer diagnosis. Another potential software resource is the Text Information Extraction System, a natural language processing pipeline and clinical document search engine.31,32
In California, about 300 cases of mesothelioma are identified through routine reporting annually.33 Between 1988 and 2016, just 37% of cases were reported within 6 months of diagnosis.33 The positive experience since 2001 with voluntary E-Path reporting to the Los Angeles County Cancer Surveillance Program provided the stimulus for legislation mandating statewide electronic pathology reporting beginning in 2019.34 As 85% of mesothelioma cases in California are confirmed by pathological examination,33 this system is expected to capture the vast majority of mesothelioma cases within 4 weeks of diagnosis. About a dozen other states now require electronic pathology reporting.
The NMVB was founded in 2006 as a resource for basic and clinical translational researchers.35–40 Funded by the National Institute for Occupational Safety and Health (NIOSH), the NMVB currently contains data and biospecimens (tumor tissue, blood products, and control samples) from over 1600 patients with pleural and peritoneal mesothelioma as well as controls. The five collaborating academic health centers maintain their own specimens but share the deidentified data centrally, an approach that serves to control costs. Like the national cancer surveillance systems currently in place, the NMVB uses NAACCR standardized coding and is not designed to facilitate patient outreach. However, compared with those surveillance systems, the NMVB offers more detailed information on epidemiological factors (such as occupation and past exposures), pathological features, and clinical course, including treatment and follow-up. Furthermore, the availability to researchers of biospecimens is valuable, as they can be used to understand mesothelioma at the genetic and cellular level. To date, 600 patients’ biospecimens have been shared with 40 different universities and institutes. Limitations of the NMVB include that it is not population-based and the small number of participating centers, limiting the ability to study risk factors at the population level. The NMVB has been funded at approximately $1 million per year from NIOSH since its inception.
Additional resources include the NCDB, which is maintained by the American College of Surgeons and the American Cancer Society and captures approximately 70% of incident cancer cases; SEER-Medicare, a linkage between the SEER and Medicare Programs that provides information on primarily elderly patients with cancer41; and other databases that provide information on cancer and noncancer care.42–44 Although they provide important information, these databases’ lag time, deidentification, lack of comprehensive information on exposure, genetic markers, treatment, and/or outcomes, and lack of linked biospecimens limit their utility to the clinical and research communities.
5 |. EXAMPLES OF OTHER REGISTRIES
Multiple countries have established mesothelioma registries.45–49 They vary in their scope (national or regional), outcome of interest (incident cases, occupational cases, or deaths), case-finding methods (passive or active), case definitions (established by ICD codes or expert review panel), extent to which they are linked to other national data sources, and their relationship to a national cancer surveillance system (related to, independent of, or in a country that lacks such a system).50 One of the most comprehensive is Italy’s National Mesothelioma Register (ReNaM), which began in 1993 and was codified into law in 2002.48 ReNaM is a network of regional registries that together cover nearly the entire country. Data collection includes both passive receipt of reports and active queries to healthcare entities. Exposure information is collected through patient or next-of-kin standardized interviews, at times supplemented by consultation with local public health and safety agencies. Linkage to the national Social Security Institute ensures data completeness. Other countries that have incorporated patient interviews to better understand exposures include Australia, France, New Zealand, and South Korea.50
ALS is a progressive neurological disease of unknown etiology. Like mesothelioma, ALS is relatively rare, occurs more frequently in older age groups, and carries a poor prognosis.51 Unlike mesothelioma, ALS is not a reportable condition and is not included in any national disease surveillance system. In 2008, the United States Congress charged CDC and the Agency for Toxic Substances and Disease Registry with establishing a population-based registry to describe the incidence and prevalence of ALS, the demographics of ALS patients, and risk factors for the disease.52 After a pilot period to test case-finding methodologies for this non-notifiable condition, the National ALS Registry was launched in 2010.53 To identify ALS patients, the registry uses national healthcare databases (such as Medicare, Medicaid, and Veterans’ Administration records) and a web-based portal that allows patients to join the registry directly. About 10% to 15% of enrolled patients are identified through the web-based portal. Self-identifying patients must answer validating questions to confirm the diagnosis, after which they are invited to complete surveys on risk factors, clinical course, and health insurance status. In addition, the registry serves to connect patients with investigators recruiting for clinical trials and epidemiologic studies, assisting approximately 50 institutions domestically and abroad, recruiting over 1000 patients to date. The registry also funds etiologic research and, since 2017, has collected and shared biospecimens with the scientific community. The National ALS Registry currently receives $10 million per year in funding from Congress to support its activities.
6 |. RECOMMENDATIONS FOR A NATIONAL MESOTHELIOMA REGISTRY
The primary purpose of a National Mesothelioma Registry would be to improve clinical outcomes, most notably duration of survival with high quality of life. A registry could facilitate early access to guideline-based care and novel therapies by connecting patients with experienced, high-volume hospitals and clinical trial investigators.11,54 Even without the establishment of a registry, an organized public health marketing campaign targeted to patients and caregivers that promotes guideline-based care and clinical trials through the use of the NCI website16 and other available resources might help to address existing barriers. Establishing a registry would come at substantially greater cost, but could potentially add value through more tightly targeted outreach.
To accomplish these aims, the registry will need to receive identifiable patient data in a timely fashion, at the time of diagnosis and before the opportunity to influence treatment decisions has passed. Electronic pathology reporting to the registry is an extremely promising mechanism. However, given that the majority of states are not yet requiring electronic pathology reporting and only a limited number of pathology laboratories are currently using the necessary software, additional data collection approaches will be needed. These might include the use of national healthcare databases, outreach to providers and pathology laboratories, collaboration with state health departments and cancer registries, partnerships with advocacy groups and labor unions for outreach to groups at high risk for mesothelioma, and patient self-referral via a web-based portal. Workshop participants envisioned that the timely submission of patient data to the registry would allow registry staff to contact patients directly to provide information on local and regional resources for care and available clinical trials.
A National Mesothelioma Registry also could support research to advance the detection, treatment, and prevention of mesothelioma. The NMVB and the National ALS Registry provide excellent examples of how the systematic collection of questionnaire data and biospecimens can facilitate basic and epidemiologic research.19,55–62 Again, timely identification of patients will be important, as questionnaire data is best obtained from the patients themselves. Establishing a mechanism for patient follow-up would facilitate research on treatment modalities and outcomes, including survival and quality of life. The registry will need to address thorny issues related to patient consent, protection of confidentiality, compliance with the Health Insurance Portability and Accountability Act Privacy Rule, and avoiding unintended effects on registrants’ cases for compensation. In addition, the registry will need to develop procedures for reviewing investigators’ proposals, criteria for approval, and mechanisms for sharing data and specimens. A centralized or virtual approach to consenting and data collection and storage could be used; if a virtual approach is used, the incorporation of open source tools could facilitate information sharing.
The experience of the National ALS Registry highlights the importance of starting with small-scale pilot initiatives focused on the most pressing needs. For a National Mesothelioma Registry, pilot projects might explore mechanisms for rapid identification of cases at the regional or state level; evaluate the acceptability of different methods of patient outreach; and assess the usefulness of the registry to patients, families, clinicians, and researchers. Lessons learned from the pilot projects can be incorporated into national plans.
Implementation and maintenance of a disease registry is expensive, typically requiring millions of dollars for infrastructure and staffing.63 Although potential sources such as private foundations, pharmaceutical companies, Workers’ Compensation Boards, and asbestos bankruptcy trust funds could be explored, dedicated federal funding undoubtedly will be critical to the long-term stability and success of a National Mesothelioma Registry. The registry could be coordinated by a research center with extramural funding, as modeled by the NMVB, or by a federal institution with intramural funding, as has been established for the National ALS Registry. The level of funding might also influence decisions about whether registry data are held centrally, as with a traditional database, or regionally, as the NMVB has demonstrated to be a viable and cost-saving approach.22,38,40 CDC’s experience with both exposure and health registries, expertise in occupational cancers, and existing partnerships with state and local cancer registries through the NPCR make it a viable candidate to host the registry.64–66 Another potential host is NCI, which leads the National Cancer Program, plays a critical role in the Cancer Moonshot, an initiative to improve cancer detection, treatment, and prevention, and runs the National Clinical Trials Network and SEER.67 Regardless of where the registry is ultimately housed, a collaborative approach that brings together local, state, federal, and private entities will be necessary to maximize the impact of any available funding (Table 2). Members of the public have been provided with the opportunity to share their perspectives on the establishment of a National Mesothelioma Registry by responding to CDC’s Request for Information published in April 2019.68
TABLE 2.
Summary of approach to establishing a National Mesothelioma Registry
| Facilitate rapid collection and consolidation of case data by central cancer registries |
| Provide funding and technical support to cancer registries and state health departments to facilitate rapid case ascertainment, consolidation of data, and rapid reporting to the National Mesothelioma Registry |
| Facilitate automated reporting of cases from pathology laboratories to central cancer registries using software tools such as ePath or the Text Information Extraction System (TIES) |
| Support use of electronic health records (EHRs) to prompt and facilitate e-reporting of cases to cancer registries and to augment available information through structured data capture (clinical, demographic, etc) |
| Promote rapid, direct reporting by patients and healthcare providers to cancer registries as soon as possible after mesothelioma diagnosis. Target populations at high risk for mesothelioma, and providers likely to encounter patients with the disease |
| Rapidly receive data from central cancer registries and use it to improve clinical care and support multidisciplinary research |
| Work with stakeholders and experts to assure that operations and services are accepted and meet users’ need |
| Develop methods for securely receiving and storing identifiable data from cancer registries or receiving deidentified data and working through registries to provide services requiring identified data |
| Develop standardized methods for collecting additional high-quality data about cases needed by researchers (such as detailed information about exposures, clinical presentation, outcomes of treatments) by linking to available data sets or obtaining data directly from patients and families |
| Develop services for registrants such as informational materials and assistance with accessing state-of-the-art care |
| Develop and implement services allowing research teams to contact appropriate patients nationally and consent them for trials and clinical research |
| Aggregate data for use in basic, epidemiological, and clinical and translational research and act as an “honest broker” to provide researchers with access to accurate, deidentified data |
| Collaborate with National Mesothelioma Virtual Bank to facilitate collection and banking of biospecimens needed for research |
Note: Two main areas of activity are shown. One is to work with central cancer registries and states to facilitate automated and electronic reporting and to promote direct reporting by patients and providers. Funding and technical support is needed to facilitate rapid case reporting to registries and to enable the registries to rapidly consolidate data and transmit it to a National Mesothelioma Registry. The second area of activity is for the National Mesothelioma Registry to securely receive and store the data, enhance it by linkage to other data sets and direct data collection, and use it to provide useful services to patients, researchers, and others.
7 |. CONCLUSIONS
The continued burden and poor prognosis of mesothelioma demand novel approaches, whether by enhancing existing resources, such as those offered by the NCI, or developing new ones, such as a National Mesothelioma Registry. By providing a mechanism to connect patients with high-quality care and clinical trials, and by making available a unique database to investigators, a National Mesothelioma Registry would be a useful resource for addressing care and research gaps. Feasibility is suggested by the recent success of another rare disease registry in the United States and national mesothelioma registries in other countries but will depend on rapid case identification and adequate funding.
ACKNOWLEDGMENTS
The authors thank Drs Waqas Amin and Jonathan Silverstein of the University of Pittsburgh School of Medicine for their thoughtful reviews of the manuscript. The workshop was funded by the Mesothelioma Applied Research Foundation and the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC). Drs Becich and Pass receive extramural funding from NIOSH for the Mesothelioma Virtual Bank for Translational Research (CDC NIOSH 1-U19-OH009077).
Funding information: Mesothelioma Applied Research Foundation; National Institute for Occupational Safety and Health (NIOSH), Grant/Award Number: CDC NIOSH 1-U19-OH009077; Centers for Disease Control and Prevention (CDC)
Footnotes
CONFLICT OF INTEREST
Michael J Becich is the founder, and a patent holder and equity owner in SpIntellx, a computational and systems pathology company.
DISCLOSURE BY AJIM EDITOR OF RECORD
John Meyer declares that he has no conflict of interest in the review and publication decision regarding this article.
REFERENCES
- 1.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, June 2018 Atlanta, GA: Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz Accessed 14 May 2019. [Google Scholar]
- 2.Beebe-Dimmer JL, Fryzek JP, Yee CL, et al. Mesothelioma in the United States: a Surveillance, Epidemiology, and End Results (SEER)-Medicare investigation of treatment patterns and overall survival. Clin Epidemiol. 2016;8:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saddoughi SA, Abdelsattar ZM, Blackmon SH. National trends in the epidemiology of malignant pleural mesothelioma: a National Cancer Data Base study. Ann Thorac Surg. 2018;105(2):432–437. [DOI] [PubMed] [Google Scholar]
- 4.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22): 2720–2726. [DOI] [PubMed] [Google Scholar]
- 5.Attanoos RL, Churg A, Galateau-Salle F, Gibbs AR, Roggli VL. Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med. 2018;142(6):753–760. [DOI] [PubMed] [Google Scholar]
- 6.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10): 1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol. 2013;14(7):576–578. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. National Vital Statistics System, 1999–2017. https://wonder.cdc.gov/ Accessed 14 May 2019.
- 9.Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of survival in malignant pleural mesothelioma: a Surveillance, Epidemiology, and End Results (SEER) study of 14,228 patients. PLOS One. 2015;10(12): e0145039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waller DA. The management of malignant pleural mesothelioma in the USA 2004–13-a decade of lost opportunity? J Thorac Dis. 2018; 10(suppl 9):S1044–S1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinoza-Mercado F, Borgella JD, Berz D, et al. Disparities in compliance with national guidelines for the treatment of malignant pleural mesothelioma. Ann Thorac Surg. 2019;108(3):889–896. [DOI] [PubMed] [Google Scholar]
- 12.Unger JM, Hershman DL, Fleury ME, Vaidya R. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol. 2019;108(3):889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne MM, Tannenbaum SL, Gluck S, Hurley J, Antoni M. Participation in cancer clinical trials: why are patients not participating? Med Decis Making. 2014;34(1):116–126. [DOI] [PubMed] [Google Scholar]
- 14.Suveg K, Putora PM, Berghmans T, Glatzer M, Kovac V, Cihoric N. Current efforts in research of pleural mesothelioma-An analysis of the ClinicalTrials.gov registry. Lung Cancer. 2018;124:12–18. [DOI] [PubMed] [Google Scholar]
- 15.Tsao AS, Lindwasser OW, Adjei AA, et al. Current and future management of malignant mesothelioma: a consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol. 2018; 13(11):1655–1667. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Clinical trials information for patients and caregivers. https://www.cancer.gov/about-cancer/treatment/clinicaltrials Accessed 9 October 2019.
- 17.Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol. 2016;34(34):4171–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone A, Pennati M, Parrino B, et al. Novel 1H-pyrrolo[2,3-b] pyridine derivative nortopsentin analogues: synthesis and antitumor activity in peritoneal mesothelioma experimental models. J Med Chem. 2013;56(17):7060–7072. [DOI] [PubMed] [Google Scholar]
- 19.Nasu M, Emi M, Pastorino S, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015; 10(4):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and its hyperacetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin Cancer Res. 2016;22(12):3087–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung YP, Chirieac LR. Novel insights and recent discoveries on the genetics and pathogenesis of malignant mesothelioma. J Thorac Dis. 2018;10(3):1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Gerwen M, Alpert N, Wolf A, et al. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Database. Carcinogenesis. 2019;40(4):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann F, Buck BJ, Metcalf RV, McLaurin BT, Merkler DJ, Carbone M. The presence of asbestos in the natural environment is likely related to mesothelioma in young individuals and women from southern Nevada. J Thorac Oncol. 2015;10(5):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone M, Kanodia S, Chao A, et al. Consensus report of the 2015 Weinman International Conference on Mesothelioma. J Thorac Oncol. 2016;11(8):1246–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coates RJ, Jajosky RA, Stanbury M, Macdonald SC. Summary of notifiable noninfectious conditions and disease outbreaks: introduction to the summary of notifiable noninfectious conditions and disease outbreaks—United States. MMWR Morb Mortal Wkly Rep. 2015;62(54):1–4. [DOI] [PubMed] [Google Scholar]
- 26.Singh SD, Henley SJ, Ryerson AB. Summary of notifiable noninfectious conditions and disease outbreaks: surveillance for cancer incidence and mortality—United States, 2011. MMWR Morb Mortal Wkly Rep. 2015;62(54):11–51. [DOI] [PubMed] [Google Scholar]
- 27.North American Association of Central Cancer Registries (NAACCR). Version 18 Data Standards and Data Dictionary. https://www.naaccr.org/data-standards-data-dictionary/ Accessed 13 June 2019.
- 28.Puckett M, Neri A, Rohan E, et al. Evaluating early case capture of pediatric cancers in seven central cancer registries in the United States, 2013. Public Health Rep. 2016;131(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caroline Pryce Walker Conquer Childhood Cancer Act of 2008. Public Law 110–285. 122 STAT. 2628–2631. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Public Health Information Network Messaging System. https://www.cdc.gov/phin/tools/phinms/index.html Accessed 14 May 2019.
- 31.Linkov F, Silverstein J, Davis M, et al. Integration of cancer registry data into the text information extraction system: leveraging the structured data import tool. J Pathol Inform. 2018;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson RS, Becich MJ, Bollag RJ, et al. A federated network for translational cancer research using clinical data and biospecimens. Cancer Res. 2015;75(24):5194–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.California Cancer Registry. Cancer rates. https://www.cancer-rates.info/ca/ Accessed 22 March 2019.
- 34.California Cancer Registry. Implementing California AB 2325. https://www.ccrcal.org/submitdata/reporting-by-pathologists/implementingab-2325/ Accessed 13 June 2019.
- 35.National Mesothelioma Virtual Bank. https://www.mesotissue.org/ Accessed 14 May 2019.
- 36.Amin W, Parwani AV, Schmandt L, et al. National Mesothelioma Virtual Bank: a standard based biospecimen and clinical data resource to enhance translational research. BMC Cancer. 2008;8(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin W, Singh H, Pople AK, et al. A decade of experience in the development and implementation of tissue banking informatics tools for intra and inter-institutional translational research. J Pathol Inform. 2010;1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin W, Parwani AV, Melamed J, et al. National Mesothelioma Virtual Bank: a platform for collaborative research and mesothelioma biobanking resource to support translational research. Lung Cancer Int. 2013;2013:765748–765749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin W, Linkov F, Landsittel DP, et al. Factors influencing malignant mesothelioma survival: a retrospective review of the National Mesothelioma Virtual Bank cohort. F1000Res. 2018;7:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohanty SK, Mistry AT, Amin W, et al. The development and deployment of Common Data Elements for tissue banks for translational research in cancer–an emerging standard based approach for the Mesothelioma Virtual Tissue Bank. BMC Cancer. 2008;8(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.National Cancer Institute. SEER-Medicare Linked Database. https://healthcaredelivery.cancer.gov/seermedicare/ Accessed 14 May 2019.
- 42.Agency for Healthcare Research and Quality. All-Payer Claims Databases. https://www.ahrq.gov/professionals/quality-patientsafety/quality-resources/apcd/index.html Accessed 13 June 2019.
- 43.New York State Department of Health. Statewide Planning and Research Cooperative System (SPARCS). https://www.health.ny.gov/statistics/sparcs/ Accessed 14 May 2019.
- 44.Society of Thoracic Surgeons. General Thoracic Surgery Database. https://www.sts.org/registriesresearch-center/sts-national-database/sts-general-thoracic-surgery-database Accessed 13 June 2019.
- 45.Galateau-Sallé F, Gilg Soit Ilg A, Le Stang N, et al. The French mesothelioma network from 1998 to 2013. Ann Pathol. 2014;34(1):51–63. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg M, Imbernon E, Rolland P, et al. The French National Mesothelioma Surveillance Program. Occup Environ Med. 2006;63(6): 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann V, Günther S, Müller KM, Fischer M. Malignant mesothelioma–German mesothelioma register 1987–1999. Int Arch Occup Environ Health. 2001;74(6):383–395. [DOI] [PubMed] [Google Scholar]
- 48.Marinaccio A, Binazzi A, Marzio DD, et al. Pleural malignant mesothelioma epidemic: incidence, modalities of asbestos exposure and occupations involved from the Italian National Register. Int J Cancer. 2012;130(9):2146–2154. [DOI] [PubMed] [Google Scholar]
- 49.Mensi C, De Matteis S, Dallari B, Riboldi L, Bertazzi PA, Consonni D. Incidence of mesothelioma in Lombardy, Italy: exposure to asbestos, time patterns and future projections. J Occup Environ Med. 2016; 73(9):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrante P, Binazzi A, Branchi C, Marinaccio A. National epidemiological surveillance systems of mesothelioma cases. Epidemiol Prev. 2016;40(5):336–343. [DOI] [PubMed] [Google Scholar]
- 51.Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis—United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ALS Registry Act of 2008. Public Law 110–373. 122 STAT. 4047–4050. [Google Scholar]
- 53.Agency for Toxic Substances and Disease Registry. National Amyotrophic Lateral Sclerosis (ALS) Registry. https://www.cdc.gov/als/Default.html Accessed 14 May 2019.
- 54.Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(13):1343–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crisanti MC, Wallace AF, Kapoor V, et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8(8):2221–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saloura V, Wang LC, Fridlender ZG, et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-β for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum Gene Ther. 2010;21(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatia K, Modali R, Goedert JJ. Merkel cell polyomavirus is not detected in mesotheliomas. J Clin Virol. 2010;47(2):196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gee GV, Koestler DC, Christensen BC, et al. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127(12):2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dabir S, Kluge A, Kresak A, et al. Low PIAS3 expression in malignant mesothelioma is associated with increased STAT3 activation and poor patient survival. Clin Cancer Res. 2014;20(19):5124–5132. [DOI] [PubMed] [Google Scholar]
- 60.Dabir S, Kresak A, Yang M, Fu P, Wildey G, Dowlati A. CD30 is a potential therapeutic target in malignant mesothelioma. Mol Cancer Ther. 2015;14(3):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Rienzo A, Cook RW, Wilkinson J, et al. Validation of a gene expression test for mesothelioma prognosis in formalin-fixed paraffin-embedded tissues. J Mol Diagn. 2017;19(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention. National Amyotrophic Lateral Sclerosis Registry, Papers. https://www.cdc.gov/als/ALSPapers.html Accessed 28 June 2019.
- 63.Antao VC, Muravov OI, Sapp J, et al. Considerations before establishing an environmental health registry. Am J Public Health. 2015;105(8):1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agency for Toxic Substances and Disease Registry. Exposure and Health Registries. https://www.atsdr.cdc.gov/publications_health_registries.html Accessed 14 May 2019.
- 65.Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR). https://www.cdc.gov/cancer/npcr/index.htm Accessed 14 May 2019.
- 66.National Institute for Occupational Safety and Health. Occupational cancer. https://www.cdc.gov/niosh/topics/cancer/default.html Accessed 14 May 2019.
- 67.National Cancer Institute. https://www.cancer.gov/ Accessed 14 May 2019.
- 68.Centers for Disease Control and Prevention. Mesothelioma Registry Feasibility: Request for Information. https://www.federalregister.gov/documents/2019/04/08/2019-06784/mesothelioma-registryfeasibility-request-for-information Accessed 14 May 2019.