Abstract
Brucella ovis is a non-zoonotic rough Brucella that causes genital lesions, abortions and increased perinatal mortality in sheep and is responsible for important economic losses worldwide. Research on virulence factors of B. ovis is necessary for deciphering the mechanisms that enable this facultative intracellular pathogen to establish persistent infections and for developing a species-specific vaccine, a need in areas where the cross-protecting ovine smooth B. melitensis Rev1 vaccine is banned. Although several B. ovis virulence factors have been identified, there is little information on its metabolic abilities and their role in virulence. Here, we report that deletion of pyruvate phosphate dikinase (PpdK, catalyzing the bidirectional conversion pyruvate ⇌ phosphoenolpyruvate) in B. ovis PA (virulent and CO2-dependent) impaired growth in vitro. In cell infection experiments, although showing an initial survival higher than that of the parental strain, this ppdK mutant was unable to multiply. Moreover, when inoculated at high doses in mice, it displayed an initial spleen colonization higher than that of the parental strain followed by a marked comparative decrease, an unusual pattern of attenuation in mice. A homologous mutant was also obtained in a B. ovis PA CO2-independent construct previously proposed for developing B. ovis vaccines to solve the problem that CO2-dependence represents for large scale production. This CO2-independent ppdK mutant reproduced the growth defect in vitro and the multiplication/clearance pattern in mouse spleens, and is thus an interesting vaccine candidate for the immunoprophylaxis of B. ovis ovine brucellosis.
Keywords: Brucella ovis, metabolism, gluconeogenesis, pyruvate phosphate dikinase, attenuation, laboratory models, vaccine, virulence
Introduction
Brucellosis is an infectious disease caused by several species of the intracellular gram-negative pathogen Brucella spp. This disease affects domestic and wild animals and can be transmitted to humans, producing important economic losses and human suffering in many countries throughout the world [1]. Currently, these bacteria are grouped in a single genus with up to 12 nominal species that often show host preference (https://lpsn.dsmz.de/genus/brucella). The zoonotic brucellae that infect cattle (B. abortus), swine (B. suis biovars 1, 2, and 3) and goats and sheep (B. melitensis) carry a smooth lipopolysaccharide (LPS) and have deserved greater attention because of their great impact on public health and animal production. Sheep can also be infected by B. ovis, a non-zoonotic species that causes ovine epididymitis and is naturally rough (i.e. bears an LPS lacking the O-polysaccharide section) [2, 3]. B. ovis is considered one of the most important causes of ovine infertility and has a significant economic impact on sheep husbandry [4, 5].
Animal vaccination is the most suitable method for controlling brucellosis in areas with moderate to high prevalence of the disease. Since sheep brucellosis can be caused by either B. melitensis or B. ovis and no specific vaccine against B. ovis is available, the attenuated vaccine B. melitensis Rev1 has been used to control infections by both bacteria. However, this vaccine has several drawbacks [6–8], among them its virulence for humans and an induction of persistent antibodies against the LPS O-polysaccharide [9]. Since this is the antigen used in the diagnosis of B. melitensis infections, those antibodies hamper the discrimination of Rev1 vaccinated and B. melitensis infected animals. Owing to this drawback, Rev1 is banned in regions or countries where B. melitensis has been eradicated [10], thus favoring the emergence of B. ovis infections. Therefore, research on vaccines on a B. ovis background is of great interest as such species-specific vaccines would neither interfere in B. melitensis serological tests nor cause human infections [11–13].
Current live attenuated brucellosis vaccines reproduce closely the cell invasion, intracellular trafficking and antigen presentation of virulent brucellae [14] and are thus the best vaccines available against B. abortus and B. melitensis [15]. Indeed, the development of new attenuated vaccines depends on an understanding of the virulence factors involved in infection, a topic that is delayed in B. ovis with respect to its smooth zoonotic counterparts. Several B. ovis attenuated mutants in classic Brucella virulence factors, outer membrane proteins, core LPS glycosyltransferases and an ABC transporter have been described [16–19], some of them providing interesting results as potential vaccines [18, 20–23]. However, recent works emphasize the relevance of bacterial metabolism in the virulence of smooth Brucella species [24, 25], an aspect of the biology of the parasite yet to be explored in B. ovis.
In B. abortus 2308 W, we have shown that disruption of pyruvate phosphate dikinase (PpdK) (catalyzing the bidirectional conversion ATP + pyruvate + Pi ⇌ AMP + phosphoenolpyruvate + PPi) severely affects growth on gluconeogenic substrates and causes attenuation in mice [26], strongly suggesting an important role for the phosphoenolpyruvate (PEP)-Tricarboxylic Acid Cycle (TCA) connections in virulence. Considering the great homogeneity of the Brucella genus, it seemed plausible that this metabolic step is also important in the virulence of other brucellae and, in keeping with this hypothesis, we found that attenuation occurs in a double PckA-PpdK mutant of B. suis 513, a more prototrophic biovar 5 strain that uses both PpdK and phosphoenolpyruvate carboxykinase (PckA; ATP + oxaloacetate → ADP + CO2 + PEP) for PEP synthesis [27]. Accordingly, the aim of this research was to investigate whether genes coding for PpdK and PckA are present in B. ovis PA and to determine their involvement in metabolism and virulence. Additionally, since the CO2 requirement of B. ovis represents a significant obstacle for large-scale production of a live attenuated vaccine, we explored the possibilities of combining deletion of those metabolic genes with genetic modifications of the B. ovis carbonic anhydrases required for CO2-independent growth that we have recently deciphered [28].
Materials and methods
Bacterial strains and culture conditions
B. ovis PA is a virulent strain that, like most B. ovis strains, requires CO2 for growth. It was used as the parental strain to obtain the recombinant B. ovis strains used in this work (Table 1). B. ovis strains were cultivated at 37 °C under a 5% CO2 atmosphere, except for the selection and characterization of CO2-independent strains. Tryptic soy agar (TSA) or tryptic soy broth (TSB) (Pronadisa-Laboratorios Conda), both supplemented with 0.3% yeast extract (YE) (Pronadisa-Laboratorios Conda) and 5% horse serum (HS) (Gibco-Life Technologies), were used as solid (TSA-YE-HS) and liquid (TSB-YE-HS) culture medium, respectively.
Table 1.
Most relevant bacterial strains used in this work.
| Brucella ovis strainsa | Main characteristics | Source |
|---|---|---|
| B. ovis PA | Virulent parental strain; CO2-dependent | BCCN (76-250) |
| Bov::CA | B. ovis PA carrying Tn7Ba2308WCAIBa2308WCAII; CO2-independent | This work |
| BovΔppdK | Derived from B. ovis PA; ppdK deletion; CO2-dependent | This work |
| BovΔppdK pAZI-19 | BovΔppdK complemented in trans with ppdK gene cloned into pRH001; CO2-dependent | This work |
| Bov::CAΔppdK | Derived from Bov::CA; ppdK deletion; CO2-independent | This work |
| Bov::CAΔppdK pAZI-19 | Bov::CAΔppdK complemented in trans with ppdK gene cloned into pRH001; CO2-independent | This work |
aThe B. ovis recombinant strains derive from B. ovis PA, which was obtained from BCCN (Brucella Culture Collection Nouzilly, Institut National de la Recherche Agronomique, Nouzilly, France).
To study the metabolic phenotype of the B. ovis strains, the gluconeogenic minimal medium of Gerhardt and Wilson (referred to here as Gerhardt) was used [29]. Growth was also tested on this medium supplemented with 5% horse serum (Gerhardt-HS), 5% horse serum and methionine 1 mM (Gerhardt-HS-meth) or the latter adding a mixture of vitamins provided by the RPMI supplement R7256 (Sigma Aldrich) (Gerhardt-HS-meth-vit). The composition of the different defined media is detailed in Additional file 1. The pH was adjusted to 7.
When required, media were supplemented with 5% sucrose (Sigma) or with kanamycin (Km; 50 μg/mL) and/or nalidixic acid (Nal; 25 μg/mL) (Sigma). All strains were stored at −80 °C in TSB-YE-HS with 7% DMSO.
DNA manipulations and analysis
Genomic sequences were obtained from the database National Center for Biotechnology Information (NCBI) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Searches for DNA and protein homologies were carried out using NCBI BLAST [30]. Sequence alignments were performed with Clustal Omega [31, 32]. Plasmid DNA was extracted with QIAprep Spin Miniprep (Qiagen). PCR amplification for verification of genetically modified B. ovis strains was performed with Red Taq DNA polymerase master mix (VWR, Leuven, Belgium).
Plasmids and mutagenesis
Construction of the ppdK mutants was done using previously described plasmid [26] and strategy [16, 18]. Briefly, to obtain the in-frame ppdK mutant (BovΔppdK), the suicide plasmid pMZI-2 was introduced into B. ovis PA and both the deletion mutant and the sibling revertant strain generated by allelic exchange were selected by sucrose resistance and Km sensitivity and by PCR using external and internal primers to the deleted fragment. The mutation generated resulted in the loss of the 93% of ppdK (BOV_RS02525 locus of the B. ovis 63/290 genome; accession numbers NC_009505 and NC_009504 for chromosome I and II, respectively).
The CO2-independent strain Bov::CA was obtained using previously described strategy [28]. Briefly, the genes coding for the CAI and CAII carbonic anhydrases of the CO2-independent strain B. abortus 2308 W were cloned into vector pUC18R6KT-miniTn7-KmR [33]. The recombinant plasmid was introduced in B. ovis PA and the desired Bov::CA strain (containing both CA genes inserted in the genome between glmS and recG) was selected according to previously described procedures [28]. Kanamycin resistance cassette was deleted following the protocol set up by Martínez-Gómez et al. [34]. Finally, to obtain the CO2-independent ppdK mutant (Bov::CAΔppdK), the suicide plasmid pMZI-2 was introduced into Bov::CA following the procedure described above.
In trans complementation of both mutants with wild-type ppdK (BovΔppdK pAZI-19 and Bov::CAΔppdK pAZI-19) was performed with plasmid pAZI-19 [26].
Bacterial growth evaluation
Growth curves in TSB-YE-HS were stablished for all strains from initial bacterial suspensions with an optical density (OD600) of 0.05 in 30 mL of culture medium that were incubated under agitation (120 rpm). OD600 values were determined through a 120-h period.
Growth evaluation in minimal media was performed as follows. CO2-independent B. ovis PA was inoculated into 10 mL of TSB-YE-HS in a 50-mL flask and incubated at 37 °C with orbital shaking for 18 h. These exponentially growing bacteria were harvested by centrifugation, resuspended in 5 mL of each test medium at an OD600 of 0.1, and incubated at 37 °C with orbital shaking for 18 h. Then, these preconditioned bacteria were harvested by centrifugation, resuspended at an OD600 of 0.1 in the same test medium in Bioscreen multiwell plates (200 μL/well), and incubated in a Bioscreen C (Lab Systems) apparatus with continuous shaking at 37 °C. Absorbance values at 420–580 nm were automatically recorded at 0.5-h intervals over a 300-h period. All experiments were performed in triplicate. Controls with medium and no bacteria were included in all experiments.
Cell infection assays
Internalization and intracellular survival in J774.A1 murine macrophage-like cell line (DSMZ ACC170) and epithelial HeLa cells (ATCC CCL-2TM) were evaluated according to previously described protocols [35]. Briefly, cell lines were cultured for 24 h in 96-well sterile plates at 37 °C under a 5% CO2 atmosphere (2 × 104 J744.A1 cells/well or 1.5 x 104 HeLa cells/well) and infected with the different B. ovis strains (4 x 106 CFU/well for macrophages or 8 x 106 CFU/well for HeLa cells). Bacteria were discarded after 2-h incubation, and gentamycin was added to kill extracellular bacteria. After 1-h incubation, three wells per B. ovis strain were washed to remove gentamycin, eukaryotic cells were lysed by incubation with H2O, and intracellular bacteria were enumerated by platting serial dilutions of the content of the wells on TSA-YE-HS [t0 post-infection (p.i.)]. The remaining wells were maintained in the presence of gentamycin and three wells per strain were similarly processed to determine intracellular bacteria at t20 and t44 p.i. The results were expressed as mean ± SD of the log CFU/well at each time point and are representative of three independent experiments.
Mouse assays and ethics statement
In vivo virulence experiments were performed with female 6-week-old BALB/c mice (Charles River Laboratories, France), received 1 week before, that were kept with water and food ad libitum at registered facilities of the University of Salamanca (PAE SA-001). All procedures involving animals were designed according to Spanish and European laws regarding the use of animals in research (RD 53/2013 and directive 2010/63/UE). They were approved by the Bioethics Committee of the University of Salamanca and authorized by the competent authority of “Junta de Castilla y León”, Spain. Microbiological protocols were approved by the Biosecurity Committees of the Universities of Salamanca and Navarra.
In the first experiment, mice were inoculated by the intraperitoneal route with 106 or 108 CFU of B. ovis PA or BovΔppdK in 200 µL of phosphate buffered saline (PBS). Bacterial splenic colonization was determined in five mice per group at weeks 3 and 11 p.i. as previously described [20]. These time points were chosen because, in the parental strain week 3 corresponds to the acute phase of infection (highest numbers of splenic bacteria) while week 11 corresponds to a point of the chronic phase when a decrease of CFU in spleen is observed [17, 21].
Subsequently, the level of splenic infection was determined at weeks 1, 3, 5, 7 and 11 in mice inoculated with 108 CFU of the CO2-dependent (B. ovis PA and BovΔppdK) and CO2-independent (Bov::CA and Bov::CAΔppdK) strains. The complemented mutant strains (BovΔppdK pAZI-19 and Bov::CAΔppdK pAZI-19) were also evaluated at weeks 1, 3, 7 and 11.
Statistical analysis
Statistical comparisons between means were performed with one-way ANOVA and the Fisher´s Least Significant Differences test of GraphPad Prism Software (GraphPad Software Inc., San Diego, USA). Statistically significant differences (P < 0.01) were established with a 99% confidence interval.
Results
Comparison of pckA and ppdK orthologues
Concerning pckA, B. ovis 63/290 BOV_RS09880 is annotated as a pseudogene that hypothetically encodes a protein of 458 amino acids, shorter than the 536-amino acid PckA proteins encoded by Agrobacterium tumefaciens C58 (ATU_RS00170) and B. suis 513 (genome without annotation, contig accession number ACBK01000073), both previously shown to be functional [27, 36]. The B. ovis 63/290 putative PckA enzyme keeps the specific domain (IGGTSYAGE-KKS; 190 to 202) required for its activity (Additional file 2). The phosphate-binding (G–G-GKT) and adenine-binding (IIML–D) consensus sites of ATP-dependent proteins (residues 233 to 243 and 345 to 351, respectively) as well as a metal ion binding (G—EG) site (residues 265 to 271) [36, 37] are also conserved (Additional file 2). However, these domains are also present in the protein encoded by the pckA ortholog of B. abortus 2308 W (BAB1_2091), which encodes an inactive protein of 491 amino acids because of a premature stop codon [26] (Additional file 2). Similar to B. abortus, deletion of a cytosine at position 1523 in B. ovis pckA alters the reading frame and thus only the first 418 amino acids are conserved when compared to B. suis 513 (Additional file 2). Accordingly, PckA is not expected to be functional in B. ovis, as previously suggested by Tsolis et al. [38].
A search of the B. ovis 63/290 genome revealed that the locus BOV_RS02525 putatively encodes PpdK. The predicted protein has 887 amino acids, where residues 19 to 376 contain a PEP-binding domain and amino acids 530 to 883 form the TIM-barrel domain characteristic of PEP-utilizing enzymes. When comparing this protein with its homologues in B. abortus 2308 W and B. suis 513, both functional [26, 27], only 3 amino acid changes were found: A177V, V312E, C857Y (Additional file 2). Also, an alanine present at position 605 in B. suis 513 and B. ovis 63/290 is substituted by a glycine in B. abortus 2308 W (Additional file 2). Therefore, PpdK should also be functional in B. ovis and, accordingly, ppdK mutants were constructed in CO2-dependent and CO2-independent virulent B. ovis PA.
Growth characteristics of B. ovis mutants
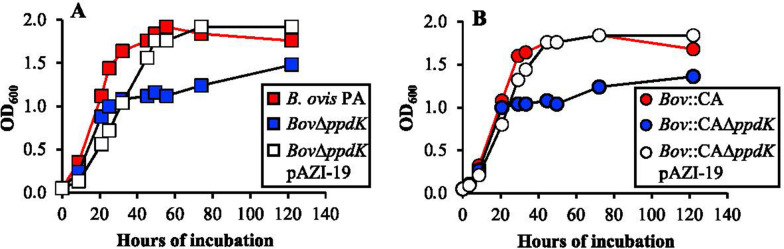
Growth in TSB-YE-HS showed no differences between the CO2-dependent and -independent strains (Figure 1). However, although both ppdK mutants did not show a marked delayed growth during the first 20 h of incubation, their growth drastically slowed down after that moment, with comparatively reduced maximum OD600 scores when compared to their parental strains (Figure 1). Since complementation with plasmid pAZI-19 carrying ppdK restored the wild-type phenotype (Figure 1), these results clearly indicate a role for PpdK in B. ovis PA metabolism under these in vitro conditions.
Figure 1.
Growth of the B. ovis strains in TSB-YE-HS. Growth was evaluated by measuring the evolution over time of the OD600 values in TSB-YE-HS liquid medium of CO2-dependent strains (A) and CO2-independent strains (B). Results are representative of two independent experiments.
As indicated in the Introduction, PpdK catalyzes a bidirectional reaction that can thus be involved in gluconeogenesis when bacteria grow on 3 and 4 C substrates, a function that should be shared by B. ovis PpdK [26, 27]. However, although B. abortus, B. melitensis and B. suis grow on glutamate-lactate-glycerol (Gerhardt’s medium) [26, 27, 39], to investigate the gluconeogenic role of PpdK in B. ovis PA it was first necessary to examine the ability of this strain to grow in Gerhardt’s medium (Additional file 1). Among brucellae B. ovis is notoriously fastidious and requires serum even in rich media. Thus, a first attempt was made by supplementing Gerhardt’s medium with 5% horse serum (Gerhardt-HS, Additional file 1). However, B. ovis PA did not grow in this medium. Some Brucella strains require methionine to grow in minimal media [25], but when this amino acid was also added to Gerhardt-HS (Gerhardt-HS-meth, Additional file 1) the combination did not support growth of B. ovis PA either. Finally, since brucellae show variable auxotrophy for some vitamins, the effect of the vitamin-rich RMPI supplement R7256 was also tested (Gerhardt-HS-meth-vit, Additional file 1). Again, no growth was observed in this medium. These results, that show that B. ovis PA is more auxotrophic than other brucellae and are in agreement with the comparative genome degradation of this species [38], preclude to assess whether the growth defect observed in rich medium corresponds to a shutdown of fueling of C precursors to the TCA (i.e. a catabolic role) or, on the contrary, to a gluconeogenic flux from TCA to the triose-phosphate pathway.
Virulence in cellular models
Since B. ovis is an intracellular pathogen, J774.A1 murine macrophages were used to determine the relevance of PpdK for intracellular survival and multiplication of B. ovis PA, BovΔppdK (CO2-dependent) and Bov::CAΔppdK (CO2-independent) mutants, together with the respective parental strains and the mutant strains complemented with wild-type ppdK, were included in the study. No statistically significant differences of internalization in J744.A1 macrophages were observed among the CO2-dependent (Figure 2A) and -independent strains (Figure 2B). However, while the usual decrease [21, 40] of about 1 log unit in intracellular CFU numbers was observed after 20 h for the parental and complemented strains, both ppdK mutants maintained the intracellular values observed at the beginning of the experiment (Figure 2A, B). As expected, intracellular replication of parental and complemented strains was observed after 20 h (P < 0.0005), with intracellular CFU counts close to those at the beginning. Interestingly, although the ppdK mutants were not killed, their intracellular CFU numbers were similar at 20 and 44 h (Figure 2A, B), showing that they barely multiplied.
Figure 2.
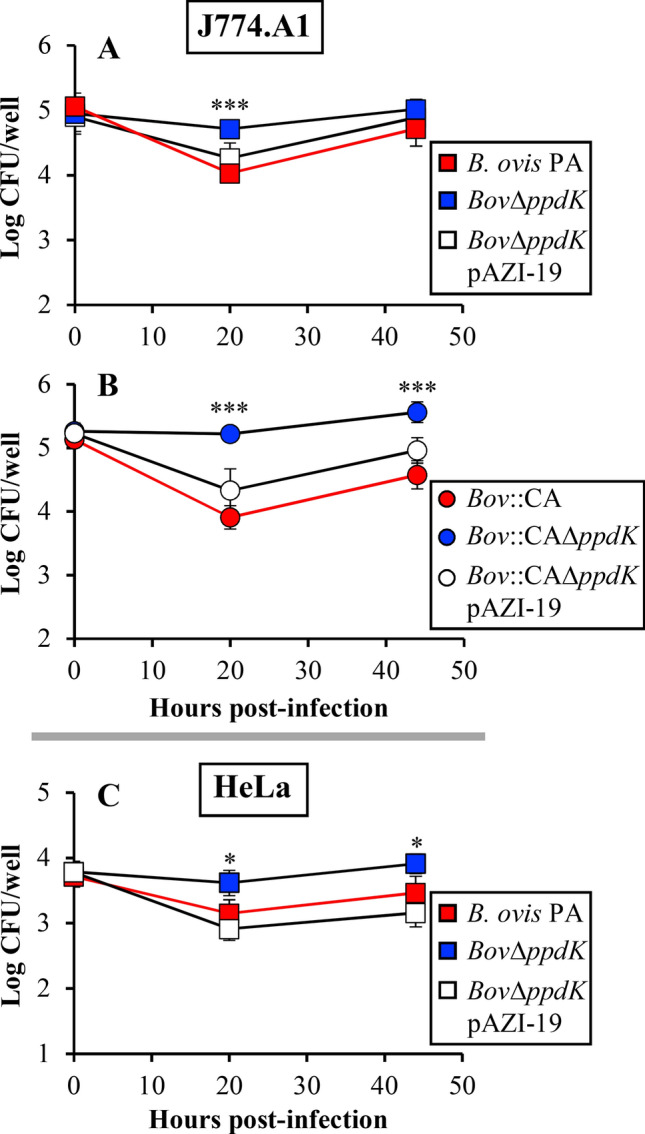
Behaviour of the B. ovis strains in J774.A1 murine macrophages (A, B) and HeLa cells (C). Cells were infected with CO2-dependent strains (A, C) or CO2-independent strains (B) and the intracellular CFU evaluated a t0, t20 and t44 as described in Materials and Methods. The results are expressed as mean ± SD of the log CFU/well and are representative of three independent experiments. Statistically significant differences (P < 0.01) with the corresponding parental strain are marked with asterisks (*P ≤0.01, **P ≤ 0.005; ***P ≤ 0.0005).
Considering the striking phenotype in macrophages, this part of the study was extended to evaluate the behavior of the CO2-dependent strains in a non-professional phagocyte (HeLa epithelial cells). In agreement with previous works with B. ovis PA [21, 40], the parental and complemented strains gave the expected pattern in HeLa cells (Figure 2C). As observed in J774.A1 macrophages, whereas intracellular counts of the ppdK mutant did not decrease at 20 h and were significantly higher (P < 0.01) than those of the parental strain, they did not increase significantly from 20 to 44 h (Figure 2C).
Virulence in mice
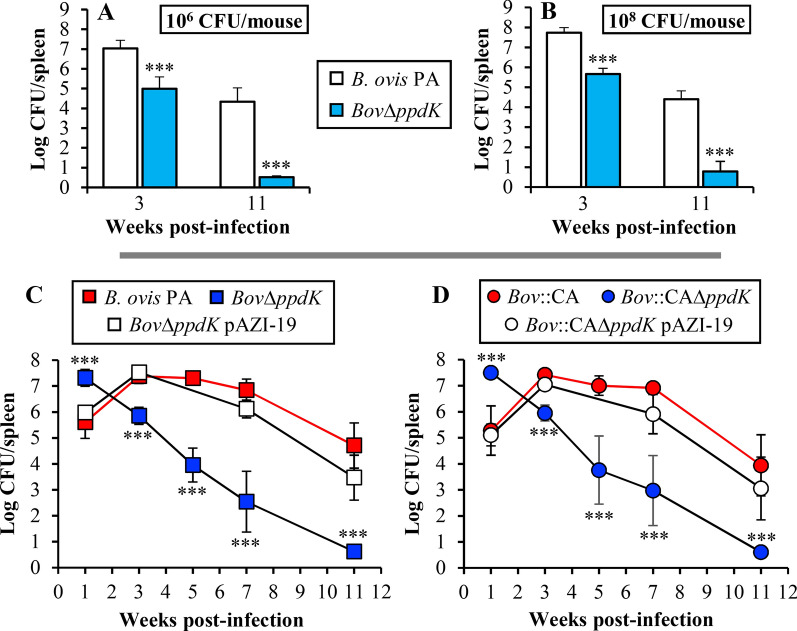
In a first experiment, parental B. ovis PA and its ppdK mutant were inoculated in mice at 106 or 108 CFU/mouse to assess spleen colonization after 3 and 11 weeks. At p.i. week 3, mice inoculated with 106 CFU of the ppdK mutant showed splenic counts c.a. 2 log units lower (P < 0.0005) than those of the parental strain (Figure 3A). At p.i. week 11 (Figure 3A), the attenuation of the ppdK mutant became more evident because, while the parental strain yielded splenic counts in the order of 4.5 log units, bacterial counts were beyond detection in the spleens of mice inoculated with the ppdK mutant (limit of detection of this method = 3.3 CFU/mL of the homogenized spleen [41]). Similar results were observed with the higher dose of inoculation, although in this case a few colonies of the ppdK mutant were detected in one mouse at p.i. week 11 (Figure 3B).
Figure 3.
Spleen colonization kinetics of strains derived from CO2-dependent (A–C) and CO2-independent (D) B. ovis PA. In a first experiment (A, B), animals were inoculated with 106 CFU (A) or 108 CFU (B) of B. ovis PA or the ppdK mutant. The results are expressed as mean ± SD of the log CFU/spleen (n = 5) detected for each strain at weeks 3 and 11 p.i. In a second experiment (C, D), mice were inoculated with 108 CFU of the CO2-dependent and CO2-independent strains and bacterial CFU were determined in spleen at weeks 1, 3, 5, 7 and 11 p.i. The results are expressed as mean ± SD of the log CFU/spleen (n = 5) detected for each strain. For each time point, statistically significant differences (P < 0.01), when compared to mice inoculated with the corresponding parental strain, are marked with asterisks (***P ≤ 0.0005).
Brucella spleen replication profile in mice shows four phases: (i) onset or colonization phase (first 48 h); (ii) acute phase (from day 3 to weeks 2 to 4) where bacteria reach maximal numbers; (iii) chronic steady phase, where the bacterial numbers plateau; and (iv) chronic declining phase, when brucellae are progressively eliminated [41]. When intraperitoneally inoculated, and with small differences depending on the inoculation dose, B. ovis PA reaches the highest bacterial load in spleen after 3 to 4 weeks, the plateau phase continues until week 9 and then bacterial numbers decrease [17, 21, 42]. Using this model, a splenic colonization curve covering weeks 1 to 11 was obtained for all B. ovis strains inoculated at 108 CFU/mouse. As previously reported, CO2-dependent and CO2-independent strains showed similar spleen counts throughout the experiment (Figure 3C, D) [28]. Surprisingly, both ppdK mutants yielded splenic CFU counts about 2 log units higher (P < 0.0005) than those observed for the respective parental strains at p.i. week 1 (Figure 3C, D). However, while the CFU of the parental strains increased at p.i. week 3 and maintained similar levels of infection until week 7, the splenic CFU of the ppdK mutants underwent a progressive decrease after week 1 and were not detected in any mouse at week 11 (Figure 3C, D). Complementation of the ppdK mutants with wild type ppdK restored phenotype of the parental strains (Figure 3C, D). Since these results show that both ppdK mutants failed to reach the chronic steady phase, they strongly suggest that PpdK activity plays a role during infection by wild-type B. ovis PA.
Discussion
Although several works have identified genes encoding virulence factors in B. ovis, there is only indirect information on the metabolic abilities of this species and none on their connection with virulence. Thus, to gain insight into the metabolic properties of B. ovis, the enzymes related to PEP use were studied, a choice made here on the bases of their relevance in B. abortus 2308 W and B. suis 513 (reference strain of biovar 5) [26, 27]. Since no Brucella genome contains a gene encoding Pps (phosphoenolpyruvate synthase), the studied candidates were PckA (phosphoenolpyruvate carboxykinase) and PpdK (pyruvate phosphate dikinase). Previous studies have shown that while B. suis 513 relies on PpdK and PckA for PEP synthesis, B. abortus 2308 W uses only PpdK due to an inactivating frameshift in PckA. These observations have been interpreted to mean that some pathways or reactions remain functional in species or biovars that, like the rodent-associated B. suis 513, are close to the early diverging Brucella clades while becoming inactivated in species adapted to domestic livestock [26, 27]. The results obtained in this work are in line with this interpretation because they show that the picture in B. ovis PA is similar to that of B. abortus with an inactive PckA that is even shorter [26]. Why PpdK is conserved while PckA is not in some Brucella species might be related to the bidirectional nature of the enzymatic reaction catalyzed by PpdK in contrast to the role of PckA, which is involved only in the gluconeogenic direction. However, while both the genomic evidence and our experiments strongly suggest that ppdK encodes a functional enzyme catalyzing the pyruvate ⇌ phosphoenolpyruvate reaction, we have not been able to test this prediction because of the inability of B. ovis to grow on defined media containing only glutamate-lactate-glycerol as C sources, a strategy we have used previously to confirm this activity in B. abortus 2308 W and B. suis 513 [26, 27]. It is noteworthy that deletion of ppdK in these two smooth brucellae does not affect growth in rich medium (TSB) to any extent while the homologous deletion has this effect in B. ovis when growing on TSB-YE-HS. Indeed, it is not possible presently to identify the precise reason(s) for this difference because of the inability of B. ovis to grow on simple chemically defined media. This inability is remarkable because, in fact, Gerhardt’s medium supports growth of B. abortus, B. melitensis and B. suis biovar 1 better than other simple defined media, including those that contain glucose as C source, and this has been related both to the capability of many Brucella strains to carry out gluconeogenesis and to the ability of these bacteria to use glutamate very efficiently through the TCA. Also, the addition of serum and other nutritional complements to Gerhardt’s medium did not allow B. ovis growth, which means that potential nutrients in serum do not compensate for the complex amino acid and other nutrients present in peptones and yeast extract. This observation together with the impact of ppdK deletion in rich medium clearly shows that the metabolism of B. ovis differs from those of at least B. abortus 2308 W and B. suis 513, confirming that the internal diversity of the genus may be reflected in the metabolism [27]. Significantly, B. ovis is defective in the oxidative metabolism of arabinose, galactose, ribose, xylose, glucose, erythritol, fructose and mannose, but all B. ovis strains tested are able to oxidize adonitol, a carbohydrate not metabolized by B. abortus, B. suis and B. melitensis [43]. Tsolis et al. suggested that the metabolic defects of this species could be related to the inactivation by frameshifts, point mutations or gene degradation of several putative sugar transporters predicted to be functional in other Brucella species [38], as it is the case of the glucose/galactose transporter GluP, which is almost 100 bp shorter in B. ovis due to a point mutation [25].
The intracellular multiplication of the ppdK mutant in macrophages also showed differences with the behavior of the B. abortus 2308 W ppdK and B. suis 513 pckA-ppdK mutants. While these two mutants were less fit than the parental strains in the replicative niche and did not multiply to their level at 24 and 48 h post-infection [26 and unpublished results], B. ovis mutants showed intracellular counts at 20 h significantly higher than those of the parental and complemented strains. This behavior parallels the higher numbers of splenic counts detected at post-infection week 1 for both ppdK mutants. Moreover, the level of splenic infection progressively decreased thereafter, which could be related with the lack of significant multiplication of the mutants in phagocytes after 20 h. Resembling the phenotypes of the corresponding mutants in B. abortus 2308 W and B. suis 513, both ppdK mutants failed to reach the chronic steady phase typical of virulent brucellae, yielding significantly lower CFU counts after week 1. These results are compatible with the model proposed before for B. abortus 2308 W and B. suis 513 suggesting that in the replicative niche of these bacteria, glutamate, alanine, and other amino acids are important C sources, while a limited supply of 6 and 5 C would be used for biosynthesis of envelope polymers and for the Pentose Phosphate cycle-dependent biosynthetic reactions [26]. However, considering the above-discussed metabolic differences with the smooth brucellae, it is necessary to study other B. ovis mutants in key enzymes of the glycolytic and gluconeogenic pathways, or the anabolic routes bridging the TCA and the triose-phosphate pathway to support this hypothesis.
It is important to highlight the practical implications of this work. The kinetics of splenic infection observed with the of B. ovis PA ppdK mutants, with the high numbers of bacterial counts observed at week 1 and persistence until at least week 7, suggests that this mutant might be an interesting vaccine candidate against B. ovis infection. A similar profile of splenic infection in mice has been observed for another B. ovis attenuated mutant conferring homologous protection in the murine model, and with B. melitensis Rev1 [20], the classical vaccine used against B. ovis infection in sheep [10]. Moreover, B. abortus 2308 W and B. suis biovar 2 ppdK mutants showed good potential to immunize against virulent B. abortus and B. suis, respectively. In fact, both vaccine candidates reached the level of protection of the reference vaccines in the mouse model while showed reduced residual virulence (Zúñiga-Ripa et al., unpublished results and Aragón-Aranda et al., unpublished results). Therefore, the combination of ppdK deletion with the genetic modifications required for CO2-independent growth results in an interesting tool to study protection against sheep brucellosis caused by B. ovis.
Supplementary information
Additional file 1: Chemically defined media used in this work.
Additional file 2: (A) Amino acid sequence alignment of PckA in A. tumefaciens C58, B. ovis 63/290, B. abortus 2308 and B. suis 513. In bold and underlined, the specific domain (IGGTSYAGE-KKS; 190 to 202) required for the PckA activity, the phosphate-binding (G--G-GKT; 233 to 243) and adenine-binding (IIML—D; 345 to 351) consensus sites of ATP-dependent proteins and the metal ion binding (G----EG; 265 to 271) site. In red, amino acids (419-458) not conserved in B. ovis 63/290 due to the deletion of a cytosine at position 1523 of the pckA gene. B Amino acid sequence alignment of PpdK in B. ovis 63/290, B. abortus 2308 and B. suis 513. In red, the 3 amino acid changes found in B. ovis 63/290 in contrast to B. abortus 2308W and B. suis 513 (A177V, V312E, C857Y).
Acknowledgements
We thank the staff of the animal experimentation facilities of University of Salamanca for their helpful collaboration.
Abbreviations
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- HS
horse serum
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Km
kanamycin
- LPS
lipopolysaccharide
- Nal
nalidixic acid
- NCBI
National Center for Biotechnology Information
- PckA
phosphoenolpyruvate carboxykinase
- PEP
phosphoenolpyruvate
- P.i
post-infection
- PpdK
pyruvate phosphate dikinase
- Pps
phosphoenolpyruvate synthase
- TCA
tricarboxylic Acid Cycle
- TSA
tryptic soy agar
- TSB
tryptic soy broth
- YE
yeast extract
Authors’ contributions
NV, AZ-R and IM conceived and coordinated the study. NV, LP-E and AZ-R, performed experiments. MI and RC-A supervised the genomic studies. NV, AZ-R and IM wrote the manuscript. All authors analyzed the results and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
The work at “Departamento de Microbiología y Genética, Universidad de Salamanca”, was financed by the “Ministerio de Economía y Competitividad” (MINECO) of Spain (Grant AGL2014-58795-C4-4-R, cofinanced with FEDER funds). The work at “Departamento de Microbiología y Parasitología, Instituto de Salud Tropical, Universidad de Navarra”, was supported by MINECO (grant AGL2014-58795-C4-1-R) and “Instituto de Salud Tropical” funders (Obra Social la CAIXA -LCF/PR/PR13/11080005- and Fundación Caja Navarra, Fundación María Francisca de Roviralta, Ubesol and Inversiones Garcilaso de la Vega S.L).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Procedures in mice were in accordance with Spanish and European laws regarding the use of animals in research (RD 53/2013 and directive 2010/63/UE). They were approved by the Bioethics Committee of the University of Salamanca (Permit number CBE 5) and authorized by the competent authority of “Junta de Castilla y León”, Spain.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13567-020-00824-7.
References
- 1.McDermott JJ, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev Sci Tech. 2013;32:249–261. doi: 10.20506/rst.32.1.2197. [DOI] [PubMed] [Google Scholar]
- 2.OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris: France; 2015. Ovine epididimytis (Brucella ovis) pp. 1–14. [Google Scholar]
- 3.Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol. 2014;5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess GW. Ovine contagious epididymitis: a review. Vet Microbiol. 1982;7:551–575. doi: 10.1016/0378-1135(82)90049-9. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter TE, Berry SL, Glenn JS. Economics of Brucella ovis control in sheep: epidemiologic simulation model. J Am Vet Med Assoc. 1987;190:977–982. [PubMed] [Google Scholar]
- 6.Blasco JM, Molina-Flores B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet Clin North Am Food Anim Pract. 2011;27:95–104. doi: 10.1016/j.cvfa.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Blasco JM, Díaz R. Brucella melitensis Rev-1 vaccine as a cause of human brucellosis. Lancet. 1993;342:805. doi: 10.1016/0140-6736(93)91571-3. [DOI] [PubMed] [Google Scholar]
- 8.Ariza J, Bosilkovski M, Cascio A, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:e317. doi: 10.1371/journal.pmed.0040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marín CM, Moreno E, Moriyón I, et al. Performance of competitive and indirect enzyme-linked immunosorbent assays, gel immunoprecipitation with native hapten polysaccharide, and standard serological tests in diagnosis of sheep brucellosis. Clin Diagn Lab Immunol. 1999;6:269–272. doi: 10.1128/cdli.6.2.269-272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasco JM. A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev Vet Med. 1997;31:275–283. doi: 10.1016/S0167-5877(96)01110-5. [DOI] [PubMed] [Google Scholar]
- 11.Da Costa Martins R, Irache JM, Gamazo C. Acellular vaccines for ovine brucellosis: a safer alternative against a worldwide disease. Expert Rev Vaccines. 2012;11:87–95. doi: 10.1586/erv.11.172. [DOI] [PubMed] [Google Scholar]
- 12.Moriyón I, Grilló MJ, Monreal D, et al. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004;35:1–38. doi: 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- 13.Silva APC, Macêdo AA, Costa LF, et al. Brucella ovis lacking a species-specific putative ATP-binding cassette transporter is attenuated but immunogenic in rams. Vet Microbiol. 2013;167:546–553. doi: 10.1016/j.vetmic.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Pandey A, Cabello A, Akoolo L, et al. The case for live attenuated vaccines against the neglected zoonotic diseases Brucellosis and Bovine tuberculosis. PLoS Negl Trop Dis. 2016;10:e0004572. doi: 10.1371/journal.pntd.0004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicoletti P. Brucellosis: past, present and future. Prilozi. 2010;31:21–32. [PubMed] [Google Scholar]
- 16.Martín-Martín AI, Sancho P, de Miguel MJ, et al. Quorum-Sensing and BvrR/BvrS R regulation, the type IV secretion system, cyclic glucans, and BacA in the virulence of Brucella ovis: similarities to and differences from smooth Brucellae. Infect Immun. 2012;80:1783–1793. doi: 10.1128/IAI.06257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caro-Hernández P, Fernández-Lago L, De Miguel MJ, et al. Role of the Omp25/Omp31 family in outer membrane properties and virulence of Brucella ovis. Infect Immun. 2007;75:4050–4061. doi: 10.1128/IAI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soler-Lloréns P, Gil-Ramírez Y, Zabalza-Baranguá A, et al. Mutants in the lipopolysaccharide of Brucella ovis are attenuated and protect against B ovis infection in mice. Vet Res. 2014;45:72. doi: 10.1186/s13567-014-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva TM, Paixao TA, Costa EA, et al. Putative ATP-binding cassette transporter is essential for Brucella ovis pathogenesis in mice. Infect Immun. 2011;79:1706–1717. doi: 10.1128/IAI.01109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancho P, Tejedor C, Sidhu-Muñoz RS, et al. Evaluation in mice of Brucella ovis attenuated mutants for use as live vaccines against B. ovis infection. Vet Res. 2014;45:61. doi: 10.1186/1297-9716-45-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidhu-Muñoz RS, Sancho P, Cloeckaert A, et al. Characterization of cell envelope multiple mutants of Brucella ovis and assessment in mice of their vaccine potential. Front Microbiol. 2018;9:2230. doi: 10.3389/fmicb.2018.02230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva APC, Macêdo AA, Costa LF, et al. Encapsulated Brucella ovis lacking a putative ATP-Binding Cassette transporter (ΔabcBA) protects against wild type Brucella ovis in rams. PLoS One. 2015;10:e0136865. doi: 10.1371/journal.pone.0136865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva APC, Macêdo AA, Silva TMA, et al. Protection provided by an encapsulated live attenuated Δ abcBA strain of Brucella ovis against experimental challenge in a murine model. Clin Vaccine Immunol. 2015;22:789–797. doi: 10.1128/CVI.00191-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbier T, Nicolas C, Letesson JJ. Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Lett. 2011;585:2929–2934. doi: 10.1016/j.febslet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Barbier T, Zúñiga-Ripa A, Moussa S, et al. Brucella central carbon metabolism: an update. Crit Rev Microbiol. 2018;44:182–211. doi: 10.1080/1040841X.2017.1332002. [DOI] [PubMed] [Google Scholar]
- 26.Zúñiga-Ripa A, Barbier T, Conde-Álvarez R, et al. Brucella abortus depends on pyruvate phosphate dikinase and malic enzyme but not on Fbp and GlpX fructose-1,6-bisphosphatases for full virulence in laboratory models. J Bacteriol. 2014;196:3045–3057. doi: 10.1128/JB.01663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zúñiga-Ripa A, Barbier T, Lázaro-Antón L, et al. The fast-growing Brucella suis biovar 5 depends on phosphoenolpyruvate carboxykinase and pyruvate phosphate dikinase but not on Fbp and GlpX fructose-1,6-bisphosphatases or isocitrate lyase for full virulence in laboratory models. Front Microbiol. 2018;9:641. doi: 10.3389/fmicb.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Etayo L, de Miguel MJ, Conde-Álvarez R, et al. The CO2-dependence of Brucella ovis and Brucella abortus biovars is caused by defective carbonic anhydrases. Vet Res. 2018;49:85. doi: 10.1186/s13567-018-0583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhardt P, Wilson JB. The nutrition of Brucellae: growth in simple chemically defined media. J Bacteriol. 1948;56:17–24. doi: 10.1128/JB.56.1.17-24.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 31.Goujon M, McWilliam H, Li W, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llobet E, March C, Giménez P, Bengoechea JA. Klebsiella pneumoniae OmpA confers resistance to antimicrobial peptides. Antimicrob Agents Chemother. 2009;53:298–302. doi: 10.1128/AAC.00657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Gómez E, Ståhle J, Gil-Ramírez Y, et al. Genomic insertion of a heterologous acetyltransferase generates a new lipopolysaccharide antigenic structure in Brucella abortus and Brucella melitensis. Front Microbiol. 2018;9:1092. doi: 10.3389/fmicb.2018.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidhu-Muñoz RS, Sancho P, Vizcaíno N. Brucella ovis PA mutants for outer membrane proteins Omp10, Omp19, SP41, and BepC are not altered in their virulence and outer membrane properties. Vet Microbiol. 2016;186:59–66. doi: 10.1016/j.vetmic.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Liu P, Wood D, Nester EW. Phosphoenolpyruvate carboxykinase is an acid-induced, chromosomally encoded virulence factor in Agrobacterium tumefaciens. J Bacteriol. 2005;187:6039–6045. doi: 10.1128/JB.187.17.6039-6045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osterås M, Driscoll BT, Finan TM. Molecular and expression analysis of the Rhizobium meliloti phosphoenolpyruvate carboxykinase (pckA) gene. J Bacteriol. 1995;177:1452–1460. doi: 10.1128/JB.177.6.1452-1460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsolis RM, Seshadri R, Santos RL, et al. Genome degradation in Brucella ovis corresponds with narrowing of its host range and tissue tropism. PLoS One. 2009;4:e5519. doi: 10.1371/journal.pone.0005519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerhardt P. The nutrition of brucellae. Bacteriol Rev. 1958;22:81–98. doi: 10.1128/MMBR.22.2.81-98.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidhu-Muñoz RS, Sancho P, Vizcaíno N. Evaluation of human trophoblasts and ovine testis cell lines for the study of the intracellular pathogen Brucella ovis. FEMS Microbiol Lett. 2018;365:24. doi: 10.1093/femsle/fny278. [DOI] [PubMed] [Google Scholar]
- 41.Grilló M-JJ, Blasco JM, Gorvel J-PP, et al. What have we learned from brucellosis in the mouse model? Vet Res. 2012;43:1–35. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiménez de Bagüés MP, Marín CM, Barberán M, Blasco JM. Evaluation of vaccines and of antigen therapy in a mouse model for Brucella ovis. Vaccine. 1993;11:61–66. doi: 10.1016/0264-410X(93)90340-4. [DOI] [PubMed] [Google Scholar]
- 43.Meyer ME. Phenotypic comparison of Brucella ovis to the DNA-homologous Brucella species. Am J Vet Res. 1969;30:1757–1764. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Chemically defined media used in this work.
Additional file 2: (A) Amino acid sequence alignment of PckA in A. tumefaciens C58, B. ovis 63/290, B. abortus 2308 and B. suis 513. In bold and underlined, the specific domain (IGGTSYAGE-KKS; 190 to 202) required for the PckA activity, the phosphate-binding (G--G-GKT; 233 to 243) and adenine-binding (IIML—D; 345 to 351) consensus sites of ATP-dependent proteins and the metal ion binding (G----EG; 265 to 271) site. In red, amino acids (419-458) not conserved in B. ovis 63/290 due to the deletion of a cytosine at position 1523 of the pckA gene. B Amino acid sequence alignment of PpdK in B. ovis 63/290, B. abortus 2308 and B. suis 513. In red, the 3 amino acid changes found in B. ovis 63/290 in contrast to B. abortus 2308W and B. suis 513 (A177V, V312E, C857Y).
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.