Abstract
Objective
Many studies on the treatment of tuberculosis (TB) using herbal medicines have been undertaken in recent decades in East Africa. The details, however, are highly fragmented. The purpose of this study was to provide a comprehensive overview of the reported medicinal plants used to manage TB symptoms, and to analyze scientific reports on their effectiveness and safety.
Method
A comprehensive literature search was performed in the major electronic databases regarding medicinal plants used in the management of TB in East Africa. A total of 44 reports were retrieved, and data were collected on various aspects of the medicinal plants such as botanical name, family, local names, part(s) used, method of preparation, efficacy, toxicity, and phytochemistry. The data were summarized into percentages and frequencies which were presented as tables and graphs.
Results
A total of 195 species of plants belonging to 68 families and 144 genera were identified. Most encountered species were from Fabaceae (42.6%), Lamiaceae (19.1%), Asteraceae (16.2%), and Euphorbiaceae (14.7%) families. Only 36 medicinal plants (18.5%) have been screened for antimycobacterial activity. Out of these, 31 (86.1%) were reported to be bioactive with minimum inhibitory concentrations ranging from 47 to 12,500 μg/ml. Most tested plant extracts were found to have acceptable acute toxicity profiles with cytotoxic concentrations on normal mammalian cells greater than 200 μg/ml. The most commonly reported phytochemicals were flavonoids, terpenoids, alkaloids, saponins, cardiac glycosides, and phenols. Only Tetradenia riparia, Warburgia ugandensis, and Zanthoxylum leprieurii have further undergone isolation and characterization of the pure bioactive compounds.
Conclusion
East Africa has a rich diversity of medicinal plants that have been reported to be effective in the management of symptoms of TB. More validation studies are required to promote the discovery of antimycobacterial drugs and to provide evidence for standardization of herbal medicine use.
Keywords: Antimycobacterial, Antitubercular, Medicinal plants, Herbal medicine, Phytochemicals, Mycobacterium tuberculosis
Background
Tuberculosis (TB) is a chronic infectious bacterial disease caused by Mycobacterium tuberculosis (Mtb). It affects mainly the respiratory system but may also affect other organs of the body causing pulmonary and extrapulmonary TB respectively. The World Health Organization (WHO) estimated that a quarter of the world’s population is infected with Mtb and thus at a risk of developing TB [1]. Although TB affects all people, those living with HIV/AIDS are at a higher risk of developing active TB [2]. The burden of TB is still high as it is ranked among the ten diseases of global concern [3]. In 2018, a total of 10 million new cases and 1.49 million deaths due to TB were reported worldwide. In East Africa, 378,000 new cases and 91,000 deaths (24%) occurred. In East Africa, Kenya and Tanzania are still ranked among the 30 countries with a high burden of TB in the world [1].
Treatment of TB remains a challenge due to the emergence of multidrug-resistant Mtb strains and extensively drug-resistant TB cases which poorly respond to the first line antitubercular drugs (rifampicin, isoniazid, pyrazinamide, and ethambutol). These drugs also have side effects and a high potential to interact with antiretroviral drugs resulting in increased toxicity, poor compliance, and treatment failure [4–6]. As a result, many TB patients have resorted to using alternative and complementary medicines with herbal remedies being the most widely used in the management of tuberculosis [7]. Due to limited access to health services and chronic poverty in East Africa, many people not only believe that herbal medicines are efficacious and safe but also affordable, available, and culturally acceptable [8–10]. Thus, there is widespread use of herbal remedies by many people in the East Africa to manage symptoms of TB [7–13]. The WHO also reported that approximately 60% of the world’s population depend on non-conventional therapies for primary health care [14].
The search to discover new effective drugs against Mtb has intensified globally in the last decade as the current therapies become less effective and in an attempt to have a world free of TB by 2035 [1]. With natural products being the leading sources of novel drugs, ethnobotanical surveys and scientific validation studies have been conducted on East African flora in the past decades [7–10]. Several plant species have been documented and some of their extracts, fractions, and isolated pure compounds have been tested for efficacy and safety [15–18]. However, this information is highly fragmented.
Comprehensive data on medicinal plants used in the management of TB is important for the conservation of these species as some of them are either rare or endangered. It also provides more evidence that increases the confidence in the utilization of these herbal remedies for primary health care as well as their regulation by relevant authorities in case of ineffectiveness and toxicity [19, 20]. The analysis and synthesis of the results may also help in identifying existing gaps and challenges in the current research and stimulates future research opportunities. This can lead to identification of novel molecules that can be developed into new antitubercular drugs with better efficacy and safety profiles [21]. This review was therefore undertaken to compile a comprehensive report on the ethnobotany, ethnopharmacology, and phytochemistry of medicinal plants used in management of symptoms of TB in the East African region so as to generate knowledge on the current status and future opportunities for drug discovery against TB.
Methods
Reporting and protocol registration
This systematic review was reported according to the Preferred Reporting Items for the Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The protocol used in this study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) and can be accessed at their website (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=187098) with the registration number CRD42020187098.
Literature search strategy
Relevant literature pertaining the ethnobotany, phytochemistry, efficacy and safety of medicinal plants utilized in management of symptoms of TB in Uganda, Kenya, Tanzania, Rwanda, Burundi and South Sudan were retrieved from Scopus, Web of Science Core Collection, PubMed, Science Direct and Google Scholar [23–25]. Key search words such as tuberculosis, mycobacteria, tuberculosis symptoms, tuberculosis treatment, vegetal, antituberculosis, antitubercular, antimycobacterial, cough, traditional medicine, ethnobotany, alternative medicine, and ethnopharmacology combined with either Uganda, Kenya, Tanzania, Rwanda, Burundi, or South Sudan were used. All publishing years were considered, and reports in the returned results were carefully scrutinized. More searches were carried out at the Google search engine using more general search terms, such as mycobacteria, tuberculosis, antituberculosis, antimycobacterial, cough, vegetal species, vegetal extract, traditional medicine, alternative medicine, plants, plant extract, vegetal, herbal, complementary therapy, natural medicine, ethnopharmacology, ethnobotany, herbal medicine, herb, herbs, decoction, infusion, macerate, and concoction combined with either Uganda, Kenya, Tanzania, Rwanda, Burundi, or South Sudan. The searches were done independently by the authors for each country and the outputs were saved where possible on databases and the authors received notifications of any new searches meeting the search criteria from Science Direct, Scopus, and Google scholar.
Inclusion and exclusion criteria
Only full-text original research articles published in peer-reviewed journals, books, theses, dissertations, patents, and conference papers on plants used in the management of symptoms of TB in Uganda, Kenya, Tanzania, Rwanda, Burundi, and South Sudan written in English and dated until April 2020 were considered.
Study selection
At first, literature screening of the extracted articles involved examining the titles and abstracts for relevant articles for inclusion. This was conducted independently by 6 authors. Then, the full-text articles were evaluated against the inclusion/exclusion criteria. The article selection process resulted in 44 studies included in this systematic review (Figure S1).
Data collection
A data collection tool was designed in Microsoft Excel (Microsoft Corporation, USA) to capture data on different aspects of medicinal plant species used in TB management. These included botanical name, plant family, local name(s), part(s) used, growth habit, mode of preparation and administration, method of extraction, efficacy, toxicity and phytochemical screening of crude extracts, isolated pure compounds, and efficacy and toxicity. Careful review of the articles was done, and data were captured using the tool. The collected data were checked for completeness, processed independently for each country by the authors and later analyzed.
Data analysis
Missing information in some studies (local names and growth habit of the plants), and misspelled botanical names were retrieved from the Google search engine and botanical databases (The Plant List, International Plant Names Index, NCBI taxonomy browser, and Tropicos) respectively.
Descriptive statistical methods were used to analyze the collected data. Results were expressed as ranges, percentages, and frequencies and subsequently presented as tables and charts. The analyses were performed using SPSS statistical software (Version 20, IBM Inc.)
Results and discussion
Ethnobotanical studies
With the current antitubercular drugs becoming less effective in the management of multidrug-resistant Mtb strains, medicinal plants can provide the novel molecules for development of new efficacious and safe drugs [26, 27]. From the electronic survey in multidisciplinary databases, 44 reports on medicinal plants used for management of symptoms of TB in East Africa were retrieved. A total of 195 species of plants belonging to 68 families and 144 genera were identified (Table 1). Some of these documented plant species have also been reported in other regions across the world for management of TB. For example, Psidium guajava, Catha edulis, Carica papaya, Citrus limon, Lantana camara, Aloe vera, Biden pilosa, Piliostigma thonningii, Tamarindus indica, Ficus platyphyla, and Vernonia cinereal in Nigeria, South Africa, Ethiopia, India, and Mexico [60–64]. This implies that plants continue to occupy a critical niche in the environment due to their rich possession of secondary metabolites (phytochemicals) that have potential to be used as medicines for several ailments that affect man. Therefore, the use of herbal medicines in the provision of primary health care remains an integral component of all health systems globally [14].
Table 1.
Medicinal plants used in treatment of symptoms of TB in East Africa
| Botanical name | Family | Local Names | Habit | Part used | Country | Author (s) |
|---|---|---|---|---|---|---|
| Acacia ataxacantha DC | Fabaceae | Not reported | Tree | Roots | Kenya | [28] |
| Acacia hockii De Wild. | Fabaceae | Kasana (Luganda), Kashiono | Tree | Leaves, Stem bark | Uganda | [7, 10] |
| Acacia horrida (L.) | Fabaceae | Lerai (Samburu) | Tree | Stem bark | Kenya | [29] |
| Acacia mearnsii De Wild. | Fabaceae | Burikoti | Tree | Stem bark | Uganda | [10] |
| Acacia nilotica (L.) Willd. Ex Delile | Fabaceae | Sunut | Tree | Fruit | South Sudan | [30] |
| Acacia polyacantha Willd. | Fabaceae | Egirigirioi | Tree | Stem bark | Uganda | [10] |
| Acacia senegal | Fabaceae | Lderekesi (Samburu) | Tree | Stem bark | Kenya | [29] |
| Acacia spectabilis A. Cunn. Ex Benth. | Fabaceae | Gasiya (Luganda) | Tree | Leaves | Uganda | [7] |
| Acanthus pubescens (Thomson ex Oliv.) Engl. | Acanthaceae | Matovu, Itojo | Herb | Roots | Uganda, Kenya | [10, 12] |
| Achyranthes aspera L. | Amaranthaceae | Muhurura | Herb | Flower | Uganda | [10] |
| Achyrospermum carvalhoi Gürke | Lamiaceae | Kanyamafundo | Shrub | Leaves | Uganda | [10] |
| Acokanthera friesiorum | Apocynaceae | Chipilikwa (Samburu) | Tree | Leaves | Kenya | [29] |
| Adenia gummifera | Passifloraceae | Chepnyalildet (Nandi) | Climber | Roots | Kenya | [31] |
| Adhatoda engleriana Lindau C.B. Clarke | Acanthaceae | Iringoringo (Chagga) | Herb | Roots | Tanzania | [32] |
| Ageratum conyzoides L. | Asteraceae | Namirembe (Luganda) | Herb | Whole plant | Uganda | [7] |
| Alangium chinense (Lour.) Harms | Cornaceae | Omusiisa (Luganda) | Herb | Stem bark | Uganda | [7] |
| Albizia anthelmitica | Fabaceaa | Lamurtana (Samburu) | Tree | Stem bark | Kenya | [29] |
| Albizia coriaria Welw. Ex Oliv | Fabaceae | Mugavu (Luganda), Etek (Lango), Musita (Lusoga), Omusesa (Runyangkore), Omubele (Wanga) | Tree | Stem bark | Uganda, Kenya | [7–10, 12, 33] |
| Albizia species | Fabaceae | Ennongo (Luganda) | Tree | Stem bark | Uganda | [7] |
| Albizia versicola | Fabaceae | Not reported | Tree | Leaves | Tanzania, Kenya | [12] |
| Albizia zygia (DC.) Macbr. | Fabaceae | Ekegonchori (Kuria) | Tree | Roots | Kenya | [12] |
| Allium sativum L. | Alliaceae | Kitungu saumu (Luo), Garlic (Luganda) | Herb | Leaves | Uganda, Kenya | [10, 12] |
| Aloe vera (L.) Burm. f. | Asphodelaceae | Kigaji (Luganda) | Herb | Leaves | Uganda | [7] |
| Aloe secundiflora Engl. | Aloaceae | Sukuroi (Samburu), Osukuroi (Masai), Kiluma (Kamba) | Herb | Leaves | Kenya | [12, 34] |
| Amaranthus spinosus | Amaranthaceae | Kidodo (Luganda) | Herb | Leaves | Uganda | [10] |
| Anogeissus leiocarpus (DC.) Guill. & Perr. | Combretaceae | Sahab | Tree | Stem bark | South Sudan | [30, 35] |
| Antiaris toxicaria Lesch. | Moraceae | Kirundu (Luganda) | Tree | Stem bark | Uganda | [7] |
| Asparagus africanus Lam. | Asparagaceae | Mukira gwango (Luganda) | Climber | Stem bark | Uganda | [10] |
| Aspilia africana (Pers.) C.D. Adams | Asteraceae | Makaayi (Luganda) Emaruoit | Herb | Root bark, Leaves | Uganda | [7, 10] |
| Aspilia pluriseta Schweinf. | Asteraceae | Rirangera | Herb | Roots | Kenya | [28] |
| Azadirachta indica L. | Meliaceae | Muarubaini (Kamba) | Tree | Seeds | Kenya | [12] |
| Azadirachta indica A. Juss. | Meliaceae | Neem tree (Luganda) | Tree | Leaves, stem bark | Uganda | [7, 10] |
| Balanites aegyptiaca (L.) Delile | Zygophyllaceae | Olngosua (Maasai), Ekorete | Shrub | Stem bark | Tanzania, Kenya; Uganda | [10, 12] |
| Bersama abyssinica Fres. | Melianthaceae | Kipsigriet (Sabaot), Kibuimetiet (Nandi) | Tree | Leaves | Kenya | [36] |
| Bidens pilosa L. | Asteraceae | Sere, Labika (Luganda), Kalala (Lusoga), ononot (Lango) | Herb | Flowers, Leaves | Uganda, Rwanda, Burundi | [7, 10, 37, 38] |
| Blighia unijugata Baker | Sapindaceae | Enkuza nyana (Luganda) | Tree | Stem bark | Uganda | [7] |
| Boscia senegalensis (Pers.) Lam. | Capparaceae | Kursan; Mukheit | Shrub | Not reported | South Sudan | [35] |
| Bridelia micrantha (Hochst.) Baill. | Euphorbiaceae | Katazamitti (Luganda), Umugimbu, | Tree | Stem bark, Root | Uganda, Burundi | [7, 38] |
| Brillantaisia owariensis P. Beauv. | Acanthaceae | Icuga | Herb | Leaves | Uganda | [10] |
| Cadaba farinosa Forssk | Capparaceae | Lumuriai (Samburu), Akado marateng (Luo) | Shrub | Not reported | Kenya | [39] |
| Callistemon citrinus (Curtis) Skeels | Myrtaceae | Mwabalabutonya (Luganda) | Shrub | Leaves, Stem bark | Uganda | [7, 9, 10] |
| Canarium schweinfurthii Engl. | Burseraceae | Muwafu (Luganda), Mubafu (Lusoga, Rutoro) | Tree | Stem bark, stem, roots | Uganda, Kenya | [7, 9, 12] |
| Canephora pierre ex A. Froehner | Rubiaceae | Emwanyi (Luganda) | Shrub | Stem bark | Uganda | [7] |
| Capparis erythrocarpos Isert | Capparaceae | Muzingani omwelu, Kitunku ekitono | Shrub | Roots | Uganda | [10] |
| Capparis tomentosa Lam. | Capparaceae | Muzingani omwelu, Kitunku ekitono | Shrub | Roots | Uganda | [10] |
| Carica papaya L. | Caricaceae | Amapapali, Paapali essajja (Luganda), Mupapali omusaiza (Lusoga), Apapalu (Lango) | Shrub | Leaves, Stem | Uganda | [7, 9, 10] |
| Carissa edulis (Forsk.) Vahl | Apocynaceae | Muyonza, Ekamuriei (Ateso) | Shrub | Roots | Uganda | [10] |
| Cassine buchananii Loes. | Celastraceae | Mbaluka (Luganda) | Tree | Stem bark, Leaves | Uganda | [8] |
| Catha edulis Forsk. | Celastraceae | Chemgangoi (Sabaot) | Shrub | Stem bark | Kenya | [36] |
| Celosia trigyna L. | Amaranthaceae | Kakubaggiri (Luganda) | Herb | Leaves | Uganda | [7] |
| Chaetacme aristata Planch. | Ulmaceae | Embutami (Luganda) | Tree | Leaves | Uganda | [7] |
| Cinnamomum zeylanicum Blume | Lauraceae | Mudalasini (Luganda) | Tree | Stem bark | Uganda | [7] |
| Cissampelos pereira L. | Menispermaceae | Karigi munana | Liana | Roots | Kenya | [28] |
| Cissus quinquangularis L. | Vitaceae | Sukurtuti | Herb | Roots | Kenya | [12, 34] |
| Citrus limon (L.) Osbeck | Rutaceae | Nimawa | Tree | Fruit | Uganda | [9] |
| Combretum molle R.Br. ex. G. Don. | Combretaceae | Ndagi, Loro (Lango) | Tree | Stem bark | Uganda | [7, 8, 10] |
| Commiphora species | Burseraceae | Oltemuai (Sabaot) | Shrub | Not reported | Kenya | [40] |
| Commiphora edulis (Klotzsch) | Burseraceae | Not reported | Shrub | Stem bark, Leaves | Kenya | [12, 26] |
| Commiphora ellenbeckii Engl. | Burseraceae | Not reported | Shrub | Stem bark, Leaves | Kenya | [26] |
| Commiphora mildbraedii Engl. | Burseraceae | Not reported | Shrub | Stem bark, Root bark | Kenya | [26] |
| Cordia africana Lam | Boraginaceae | Not reported | Tree | Roots | Tanzania, Kenya | [12] |
| Crassocephalum vitellinum | Apiaceae | Akayungubira | Herb | Leaves | Burundi | [38] |
| Crossopteryx febrifuga (Afzel. ex G.Don) Benth. | Rubiaceae | Not reported | Tree | Roots | Tanzania, Kenya | [12] |
| Croton dichogamus Pax. | Euphorbiaceae | Oloiborrbenek (Massai) | Shrub | Roots | Tanzania, Kenya | [12] |
| Croton macrostachyus Hochst. ex Del | Euphorbiaceae | Omutswitswi (Wanga), Mukinduri (Kikuyu) | Tree | Leaves, Roots | Kenya | [33] |
| Croton sylvaticus | Euphorbiaceae | Not reported | Tree | Roots | Tanzania | [41] |
| Croton zambesicus | Euphorbiaceae | Um-Gilagla | Tree | Fruit | South Sudan | [42, 43] |
| Cryptolepis sanguinolenta | Apocynaceae | Kafulu (Luganda) | Shrub | Roots | Kenya, Uganda | [12, 44] |
| Cymbopogon citratus D.C. ex Stapf | Poaceae | Kisubi (Luganda), Akisube (Ateso), Lum cai (Lango) | Herb | Leaves | Uganda | [7] |
| Cyperus latifolius Poir. | Cyperasaceae | Ekekeriaut | Herb | Roots | Uganda | [10] |
| Cyperus rotundus L. Subsp. rotundus | Cyperasaceae | Ekekeriaut | Herb | Roots | Uganda | [10] |
| Cyphostemma adenocaule | Vitaceae | Lordo (Samburu) | Herb | Not reported | Kenya | [34] |
| Dalbergia melanoxylon Guill. & Perr. | Fabaceae | Not reported | Tree | Stem bark | Kenya | [28] |
| Datura stramonium | Solanaceae | Not reported | Herb | Leaves | Rwanda | [45] |
| Desmodium salicifolium (Poir.) D.C. | Fabaceae | Enkolimbo (Luganda) | Herb | Leaves | Uganda | [7] |
| Desmodium repandum (Vahl) DC. | Papilionaceae | Ituza | Herb | Leaves | Uganda | [10] |
| Dichrostachys cinerea (L.) Wight and Arn | Fabaceae | Chinjiri (Digo) | Tree | Roots | Kenya | [28] |
| Dodonaea angustifolia L. f. | Sapindaceae | Musambya (Luganda) | Shrub | Leaves | Uganda | [10] |
| Dracaena steudneri Engl. | Asparagaceae | Kajjolyenjovu (Luganda) | Tree | Stem bark | Uganda, Kenya | [7, 9, 10, 12] |
| Dychrostachys glomerata (DG) (Forssk.) | Fabaceae | Not reported | Tree | Leaves, Roots | Uganda, Kenya, Tanzania | [10, 12, 29] |
| Embelia schimperi Vatke | Myrsinaceae | Sachuonet (Ogiek) | Tree | Stem bark | Kenya | [46] |
| Entada abbysinica A. Rich. | Fabaceae | Laginaria (Luo) Mwolola (Luganda) | Shrub | Roots, Stem bark, Leaves | Uganda, Kenya, Tanzania | [7, 10, 12, 29] |
| Erythrina abyssinica Lam. ex DC. | Fabaceae | Ejjirikiti (Luganda), Kiko Omoko (Rutoro), Oluo (Lugbara), Owila kot (Lango), Muyirikiti (Lusoga), Omotembe (Kisii)Muhuti (Kikuyu), Umurinzi | Tree | Stem bark, leaves | Uganda, Kenya, Tanzania, Rwanda, Burundi | [7–10, 12, 38, 45, 47] |
| Eucalyptus species | Myrtaceae | Kalintusi (Luganda) | Tree | Leaves, Stem bark | Uganda, Kenya, Tanzania, Rwanda | [7–10, 12, 47, 48] |
| Euclea divinorum Hiern | Ebenaceae | Emus, Kasalagala/Muda (Lusoga) | Shrub | Roots | Uganda | [10] |
| Euphorbia ingens E.Mey. ex Boiss. | Euphorbiaceae | Not reported | Tree | Roots | Kenya | [28] |
| Euphorbia schimperiana Scheele | Euphorbiaceae | Kazagamira (Luganda) | Tree | Leaves | Uganda | [7] |
| Faidherbia albida (Del.) Chevi. | Fabaceae | Haraz | Tree | Leaves | South Sudan | [42] |
| Ficus glumosa Delile | Moraceae | Muwo (Luganda) | Shrub | Stem bark | Uganda | [7] |
| Ficus natalensis Hochst. | Moraceae | Omutuba (Luganda), Mugaire (Lusoga) | Tree | Stem bark | Uganda | [7] |
| Ficus platyphylla Delile | Moraceae | Mudodwe | Shrub | Stem bark | Uganda | [10] |
| Ficus saussureana | Moraceae | Omuwo (Luganda) | Shrub | Stem bark | Uganda | [8] |
| Fleurya aestuans (L.) Gaudich. ex Miq. | Urticaceae | Munyango (Luganda) | Herb | Leaves | Uganda | [7] |
| Garcinia buchananii Baker | Clusiaceae | Musaali (Luganda) | Tree | Stem bark, Root bark | Uganda, Kenya, Tanzania | [7, 10, 12] |
| Gnaphalium purpureum L. | Asteraceae | Omuya (Luganda) | Herb | Leaves | Uganda | [7] |
| Gnidia buchananii Gilg | Thymelaeaceae | Not reported | Herb | Roots | Kenya | [49] |
| Gomphocarpus physocarpus E. Mey. | Apocynaceae | Gashaho | Herb | Leaves | Uganda | [10] |
| Gutenbergia cordifolia Benth. ex Oliv. | Asteraceae | Ekoutapem | Herb | Roots, Leaves | Uganda | [10] |
| Harrisonia abyssinica Oliv. | Simaroubaceae | Mutagataga (Meru), Osiro (Luo), Orongoriwe (Kuria), Lushaike | Shrub | Stem bark | Uganda, Kenya | [10, 50, 51] |
| Harungana madagascariensis Lam.ex Pior | Hypericaceae | Mukabiiransiko (Luganda) | Tree | Stem bark, Leaves | Uganda | [8] |
| Helichrysum odoratissimum (L.) | Asteraceae | Lweza (luganda) | Herb | Leaves | Uganda | [10] |
| Heterotis canescens | Melastomataceae | Umusomaw’a-bungere, | Herb | Leaves | Burundi | [38] |
| Hibiscus fuscus Garcke | Malvaceae | Lusaala (Luganda) | Herb | Leaves | Uganda | [7] |
| Hoslundia opposita Vahl | Lamiaceae | Cheroronit, Cherungut (Nandi), Nfodo (Lusoga) | Shrub | Leaves | Uganda, Kenya | [10, 31] |
| Hypericum revolutum Vahl | Clusiaceae | Mushungwa | Tree | Leaves | Uganda | [10] |
| Hypoestes verticillaris (L.f.) Sol. | Acanthaceae | Narubat (Ogiek) | Herb | Roots | Kenya | [46] |
| Iboza multiflora (Benth.) E. A. Bruce | Lamiaceae | Iseja | Shrub | Leaves | Uganda | [10] |
| Iboza riparia (Hochst.) N. E. Br. | Lamiaceae | Muravumba | Shrub | Leaves | Uganda | [10] |
| Indigofera emarginella Steud. ex A. Rich. | Fabaceae | Olutunga nsonzi (Luganda) | Shrub | Leaves, Stem bark | Uganda | [7] |
| Indigofera lupatana Baker F | Fabaceae | Not reported | Shrub | Roots | Kenya | [28] |
| Kalanchoe glaucescens Planch. ex Benth | Crassulaceae | Ekiyondo ekyeru (Luganda) | Herb | Leaves | Uganda | [7, 9] |
| Kalanchoe integra | Crassulaceae | Not reported | Shrub | Leaves | Rwanda | [48] |
| Khaya senegalensis | Meliaceae | Not reported | Tree | Leaves, Stem bark | South Sudan | [52] |
| Lagenaria sphaerica (Sond.) Naudin | Cucurbitaceae | Mutanga | Herb | Leaves | Uganda | [10] |
| Lantana camara L. | Verbenaceae | Kayukiyuki (Luganda), Owinybilo (Lango), Kanpanga (Ateso) | Shrub | Leaves | Uganda | [7, 10, 53] |
| Lantana trifolia | Verbenaceae | Not reported | Shrub | Leaves | Rwanda | [48] |
| Leonotis nepetifolia (L.) R. Br. | Lamiaceae | Susuni | Shrub | Leaves | Uganda | [10] |
| Leucas calostachys Oliv. | Lamiaceae | Kakuba musulo (Luganda) | Shrub | Leaves, Whole plant | Uganda | [8] |
| Lippia grandifolia Hochst. ex A. Rich | Verbenaceae | Olugumaguma (Luganda) | Herb | Leaves | Uganda | [7] |
| Lonchocarpus eriocalyx Harms | Fabaceae | Not reported | Tree | Stem bark | Kenya | [11, 28] |
| Maesa lanceolata Forssk. | Myrsinaceae | Muhanga | Tree | Roots | Uganda | [10] |
| Mangifera indica L. | Anacardiaceae | Muyembe (Luganda), Aeme (Lango) | Tree | Stem bark | Uganda, Kenya | [7, 9, 10, 12, 47] |
| Maytenus senegalensis (Lam.) | Celastraceae | Naligwalimu (Luganda), Muwaiswa, Eterka, Itereka (Lango) | Shrub | Root bark, Leaves | Uganda | [7, 10] |
| Microglossa pyrifolia (Lam.) | Asteraceae | Kabilili akatono (Luganda) | Shrub | Roots | Uganda | [10] |
| Microgramma lycopodiodes (L.) Copel | Polypodiaceae | Kukumba (Luganda) | Herb | Roots, Leaves | Uganda | [8] |
| Milicia excelsa (Welw.) C.C. Berg | Moraceae | Muvule (Luganda) | Tree | Leaves | Uganda | [7] |
| Momordica foetida Schumach. | Cucurbitaceae | Bombo (Luganda), Luiwula/Mwishwa | Herb | Leaves | Uganda, Rwanda | [7, 10, 45] |
| Momordica rostrata A. Zimm. | Cucurbitaceae | Chepkologolio (Ogiek) | Herb | Roots | Kenya | [46] |
| Morella kandtiana (Engl.) Verdc. & Polhill | Myricaceae | Mukikimbo (Luganda) | Herb | Roots, Leaves, Whole plant | Uganda | [8] |
| Morinda lucida Benth. | Rubiaceae | Kabaja nsayi (Luganda) | Tree | Stem bark | Uganda | [7] |
| Moringa oleifera Lam. | Moringaceae | Moringa (Luganda) | Tree | Fruit, Stem | Uganda | [7, 10] |
| Mucuna pruriens (L.) DC. | Papilionaceae | Lugenyu (Luganda) | Vine | Leaves | Uganda | [10] |
| Myrica kandtiana Engl. | Myricaceae | Enkikimbo(Luganda) | Tree | Fruit, Leaves, Stem bark, Root bark | Uganda | [7] |
| Myrsine africana L. | Myrsinaceae | Seketeti (Samburu) | Shrub | Not reported | Kenya | [34] |
| Nauclea latifolia Sm | Rubiaceae | Karmadoda | Tree | Fruit | South Sudan | [54] |
| Ocimum basilicum | Lamiaceae | Umusurasura | Herb | Leaves | Burundi | [38] |
| Ocimum suave Willd. | Lamiaceae | Muhumuzanganda (Luganda) | Herb | Leaves | Uganda | [10] |
| Olea capensis L. | Oleaceae | Pekeriondet (Sabaot) | Tree | Stem bark | Kenya | [36] |
| Olinia rochetiana | Penaeaceae | Kaptolongit (Sabaot) | Tree | Roots | Kenya | [36] |
| Ormocarpum trichocarpum (Taub.) Harms | Papilionaceae | Eseperuae | Tree | Roots | Uganda | [10] |
| Pappea capensis (Spreng) Eckl. & Zeyh. | Sapindaceae | Muba (Kikuyu), Enkorrirri, Oltimigomi (Maasai) | Shrub | Stem bark, Root bark | Kenya | [55, 56] |
| Parinari curatellifolia Planch. ex Benth. | Chrysobalanaceae | Umunazi | Tree | Stem bark, roots | Burundi | [38] |
| Pavetta crassipes K. Schum. | Rubiaceae | Not reported | Shrub | Roots | Tanzania, Kenya | [12] |
| Pentas longiflora Oliv. | Rubiaceae | lsagara | Herb | Roots | Rwanda | [37] |
| Persea americana Mill. | Lauraceae | Ovacado (Luganda) | Tree | Stem bark | Uganda | [7, 9] |
| Phaseolus lunatus L. | Fabaceae | Kayindiyindi (Luganda) | Herb | Leaves | Uganda | [7] |
| Phaseolus vulgaris L. | Fabaceae | Bijanjaro (Luganda) | Herb | Husks | Uganda | [7] |
| Phyllanthus reticulatus Poir. | Phyllanthaceae | Mutulika (Luganda) | Shrub | Leaves | Uganda | [7] |
| Piliostigma thonningii | Fabaceae | Chebutiandet (Sabaot) | Tree | Leaves | Kenya | [36] |
| Piptadenistrum africana | Fabaceae | Mpewere (Luganda) | Tree | Stem bark | Uganda | [7, 9, 10] |
| Plectranthus barbatus Andrews | Lamiaceae | Ekibankulata (Luganda), Ebiriri omutano (Ateso) | Shrub | Leaves | Uganda | [7, 10] |
| Plectranthus hadiensis | Lamiaceae | Kibwankulanta (Luganda) | Shrub | Whole plant, Leaves | Uganda | [8] |
| Plumbago dawei | Plumbaginaceae | Lkiarianthus (Samburu) | Herb | Stem bark | Kenya | [29] |
| Plumbago zeylanica L. | Plumbaginaceae | Musajjabanda (Luganda), Mukya (Kamba) | Herb | Leaves | Uganda, Kenya | [7, 34, 57] |
| Podocarpus usambarensis Pilg. | Podocarpaceae | Kamusenene (Luganda) | Tree | Leaves | Uganda | [7] |
| Prunus africana (Hook.f.) Kalkman | Rosaceae | Ntaseesa, Ngwabuzito (Luganda, Rutoro),Sirumandu (Lugisu) | Tree | Stem bark | Uganda | [7] |
| Pseudospondia microcarpa (A. Rich.) Engl. | Anacardiaceae | Muziru (Luganda) | Tree | Stem bark | Uganda | [7] |
| Psidium guajava L. | Myrtaceae | Mpera (Chagga) | Tree | Fruit, Leaves, Stem bark, Root bark | Uganda, Kenya, Tanzania | [7, 12] |
| Pycnostachys ericirosenii R.E.Fr. | Lamiaceae | Musindikwa (Luganda) | Shrub | Leaves | Uganda | [10] |
| Rhamnus prinoides L’Herit. | Rhamnaceae | Munanira (Luganda) | Shrub | Leaves | Uganda | [10] |
| Rhoicissus tridentata (L.f.) Wild. & R.B.D. Drumm. | Vitaceae | Mumara (Luganda) | Shrub | Leaves | Uganda | [10] |
| Rhus natalensis Bernh. ex Krauss | Anacardiaceae | Lmisigiyoi, Muthigiu (Kikuyu) | Tree | Roots, Leaves | Kenya | [51] |
| Rhus vulgaris Meikle | Anacardiaceae | Kakwansokwanso (Luganda) | Herb | Stem bark, Leaves | Uganda | [7] |
| Ribes uva-crispa L. | Grossulariaceae | Entuntunu (Luganda) | Shrub | Leaves | Uganda | [7] |
| Rosmarinus officinalis L. | Lamiaceae | Not reported | Herb | Leaves | South Sudan | [52] |
| Rubia cordifolia L. | Rubiaceae | Kasalabakesi (Luganda) Urumurwa (Kuria) | Herb | Leaves, Whole plant | Uganda, Kenya, Tanzania | [7, 9, 10, 12, 16] |
| Rumex abyssinicus Jacq. | Polygonaceae | Not reported | Herb | Leaves | Rwanda | [48] |
| Sapium ellipticum (Hochst.) Pax | Euphorbiaceae | Omusasa (Luganda) | Shrub | Stem bark | Uganda | [7] |
| Securidaca longipedunculata Fresen. | Polygalaceae | Mukondwa, Awee ilila (Lango), Mukondwa (Lusoga), Eliloi (Ateso) | Tree | Roots | Uganda | [8, 10] |
| Senna siamea (Lam.) Irwin & Barneby | Fabaceae | Gasiya seed | Tree | Stem bark | Uganda | [10] |
| Sesamum calycinum | Pedaliaceae | Lutungotungo (Luganda) | Herb | Leaves, Whole plant | Uganda | [8] |
| Solanum aculeastrum Dunal | Solanaceae | Mutura (Kikuyu), Ekitengo (Luganda) | Shrub | Fruit, Roots, Leaves | Uganda, Kenya | [7, 8, 12] |
| Solanum incanum L. | Solanaceae | Entengotengo Ennene (Luganda), Ocokocok (Lango), Ntonka (Lusoga), Mutongu (Kamba),Entulelei (Maasai) | Shrub | Fruit | Uganda, Kenya | [7, 12] |
| Solanum mauense Bitter | Solanaceae | Ng’onyoyiek (Ogiek) | Shrub | Seeds | Kenya | [46] |
| Spathodea campanulata P. Beauv. | Bignoniaceae | Kifabakazi (Luganda) | Tree | Stem bark | Uganda | [7] |
| Syzygium cumini (L.) Skeels | Myrtaceae | Jambula (Luganda) | Tree | Stem bark | Uganda | [7, 9] |
| Tamarindus indica L. | Fabaceae | Mukoge (Luganda), Cwao (Lango) | Tree | Leaves | Uganda | [10] |
| Teclea nobilis Del. | Rutaceae | Luzo | Shrub | Leaves | Uganda | [10] |
| Tetradenia riparia (Hochst.) Codd | Lamiaceae | Ekyewamala (Luganda) | Herb | Leaves | Uganda, Rwanda | [7, 37] |
| Terminalia laxiflora Engl. & Diels | Combretaceae | Darout | Tree | Stem bark | South Sudan | [30] |
| Tithonia diversifolia (Hemsl.) A. Gray | Asteraceae | Ekimyula, Okelokelo (Lango) | Shrub | Stem bark | Uganda | [7] |
| Toddalia asiatica (L.) Lam | Rutaceae | Simborichet (Sabaot), Mururue (Kikuyu), Oleparmunyo (Maasai), Kawule (Luganda) | Shrub | Roots, Leaves | Uganda, Kenya | [7, 8, 10, 36] |
| Tragia brevipes Pax | Euphorbiaceae | Nakepian | Climber | Roots | Uganda | [10] |
| Tragia subsessilis Pax | Euphorbiaceae | Totoananyia | Herb | Roots | Uganda | [10] |
| Trichilia dregeana Sond. | Meliaceae | Sekoba (Luganda) | Tree | Stem bark, | Uganda | [7] |
| Triumfetta flavescens Hochst. ex A. Rich. | Malvaceae | Luwugula (Luganda) | Shrub | Stem | Uganda | [7] |
| Vachellia drepanolobium (Harms ex Sjostedt) P.J.H. Huter | Fabaceae | Oluai (Maasai) | Tree | Stem bark, Root bark | Kenya | [55] |
| Vernonia cinerea (L.) Less. | Asteraceae | Kayayana, Lukohe (Luganda), Yat Kwong (Lango) | Herb | Leaves | Uganda | [7] |
| Vernonia amygdalina Del. | Asteraceae | Mululuza (Luganda) Lubilili | Shrub | Leaves | Uganda | [7, 10] |
| Warburgia ugandensis Sprague | Canellaceae | Abaki, Sokoni (Samburu), Muthiga (Kikuyu) | Tree | Stem bark | Uganda, Kenya, Tanzania | [7–10, 12, 16, 57–59] |
| Zanthoxylum chalybeum Engl. | Rutaceae | Ntale ya ddungu (Luganda), Eusuk (Ateso), Agodaman (Lango), Oloisuki (Maasai), Rukuts (Karimojong), Outiku (Lugbara) | Tree | Stem bark | Uganda, Kenya, Tanzania | [5, 8–10, 12] |
| Zanthoxylum gillettii (De Wild.) P.G. Waterman | Rutaceae | Sagawatiet, Shihumba/Shikuma | Tree | Stem bark | Kenya | [31] |
| Zanthoxylum leprieurii | Rutaceae | Not reported | Tree | Stem bark | Uganda | [5] |
| Zehneria scabra | Cucurbitaceae | Umushishiro, | Herb | Leaves | Burundi | [38] |
| Zingiber officinale | Zingiberaceae | Tangawizi (Luo), Ntangawuzi (Luganda) | Herb | Stem | Uganda, Kenya | [7, 9, 10, 12] |
Languages: Ateso, Lango, Luganda, Lugbara, Lugisu, Lusoga, Karimojong, and Rutoro (Uganda); Digo, Kamba, Kikuyu, Kisii, Kuria, Luo, Maasai, Meru, Nandi, Ogiek ,Sabaot, Samburu, and Wanga (Kenya); and Chagga (Tanzania). Local names with language(s) not indicated were not specified by the authors
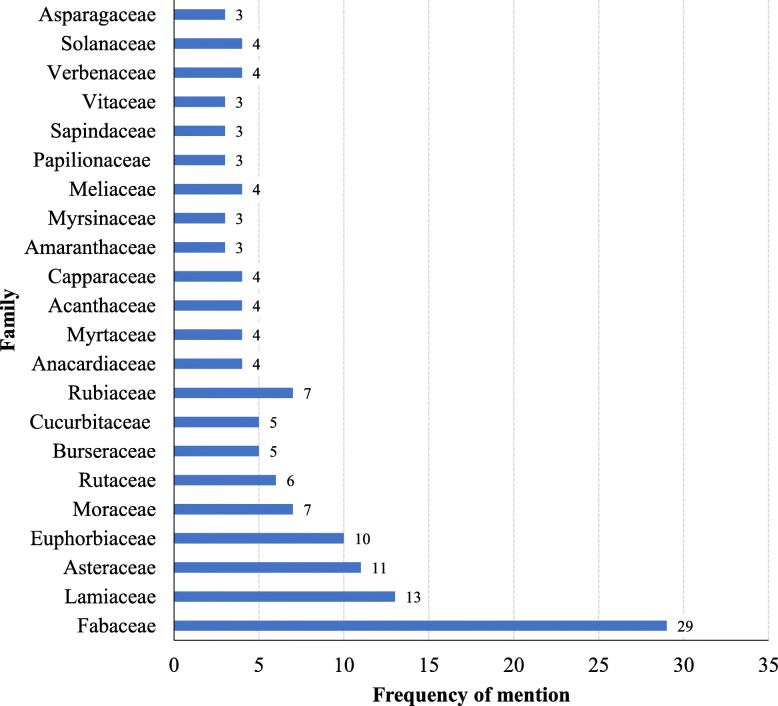
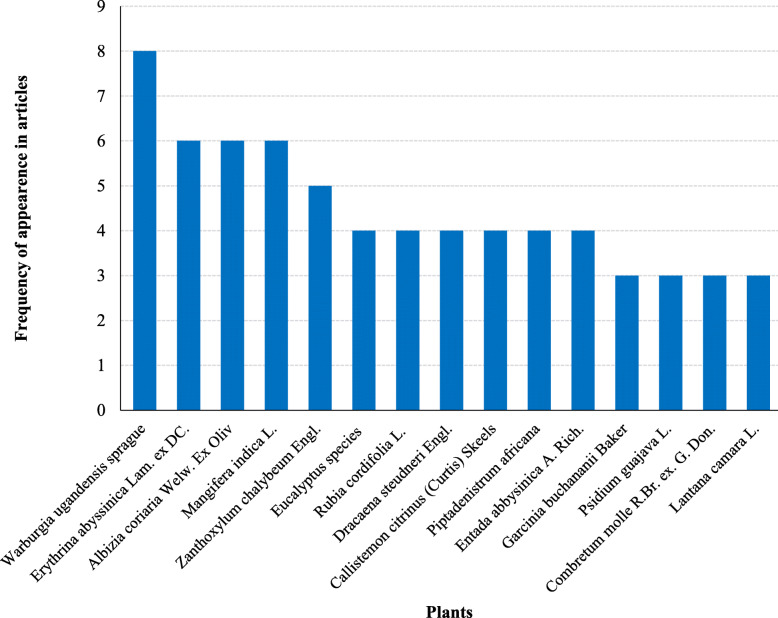
Most encountered species were from the family Fabaceae (42.6%), Lamiaceae (19.1%), Asteraceae (16.2%), Euphorbiaceae (14.7%), Moraceae (10.3%), Rubiaceae (10.3%), Rutaceae (8.8%), Burseraceae (7.4%), and Cucurbitaceae (7.4%) (Fig. 1). Fabaceae, Asteraceae, and Lamiaceae were also reported to provide the largest number of plants species used for TB management in South Africa, Ghana, Nigeria, Ethiopia, and India [64–72]. From these families, 15 species were the most cited in East Africa (Fig. 2). These families were reported from at least four countries of East Africa. This could probably be attributed to the abundant distribution of the analogue active substances among species from these families [23, 24]. The family Fabaceae has biosynthetic pathways that produce majorly flavonoids, terpenoids, and alkaloids as secondary metabolites [73–75]. It is these phytochemicals that are responsible for the antimycobacterial activity against different mycobacterial strains [67, 70, 76, 77]. Other families reported in East Africa to house medicinal plants for management of TB and have also been reported in other countries include Acanthaceae, Apocynaceae, Cariaceae, Combretaceae, Malvaceae, Moraceae, Myrtaceae, Rhamnaceae, Rubiaceae, Solanaceae, and Zingiberaceae [64, 72, 78–81].
Fig. 1.
Major botanical families from which TB remedies are obtained in East Africa
Fig. 2.
The most cited plant species used for treatment of TB and its symptoms in East Africa
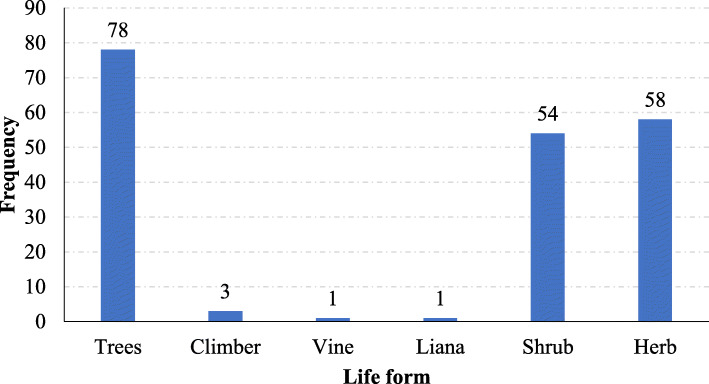
Geographically, none of the documented plant species was reported to be used in the management of TB across all the East African countries. However, two plant species (Erythrina abyssinica and Eucalyptus species) are used by at least 4 countries. A total of 30 plant species were reported to be used by at least two countries. Uganda had the highest number of species mentioned followed by Kenya and then Tanzania (Table 1). The differences in species utilization could be attributed to the differences in soil chemistry, rainfall, topography, and climate that results into differences in phytochemical composition of the same species growing in different geographical areas [82]. Additionally, it could also be due to differences in knowledge and experiences as result of different social and cultural backgrounds that exists across the countries. Uganda had many ethnobotanical surveys conducted to document medicinal plants used in the management of tuberculosis as compared to other countries. Most of these medicinal plants were growing as trees (40.0%), herbs (29.7%), shrubs (27.7%), and rarely as climbers, vines, or lianas (Fig. 3).
Fig. 3.
Growth habit of the plants used for preparation of antitubercular remedies in East Africa
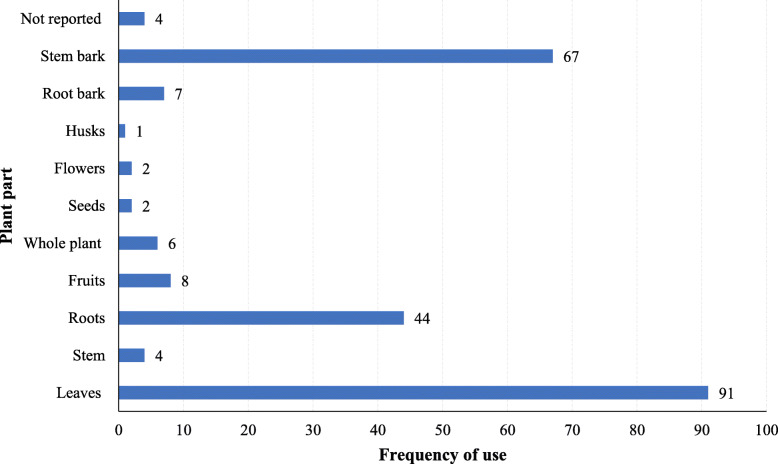
Analysis of ethnomedicinal recipes revealed that mainly leaves (38.6%), stem bark (28.4%), and roots (18.6%) were used for preparing herbal remedies. Root bark, whole plants, fruits, flowers, seeds, and husks were rarely used (Fig. 4). Harvesting of leaves and stem bark allows sustainable utilization of the plants hence promoting their conservation as opposed to use of roots and whole plants. Additionally, leaves are the primary sites for secondary metabolic pathways in plants while stem barks act as major concentration areas (deposition sites) for the synthesized metabolites [9, 57].
Fig. 4.
Frequency of plant parts used for preparation of antitubercular remedies in East Africa
Most articles reviewed reported that traditional herbal medicine practitioners usually combined different plant species while preparing herbal medicines. However, they did not report how the herbal medicine from individual plant species can be prepared. Decoction was by far the commonest method of herbal medicine preparation cited. Others included cold infusions, drying and pounding into a powder, burning into ash, chewing, and steaming. Use of more than one plant in combination is more effective than single plant perhaps due to the synergistic interactions that occur among the different phytochemicals that result into increased bioactivity (efficacy). But also, the benefit of phytochemicals from one species counteracting the toxicity of another species could be another explanation.
The major route of administration was oral (via the mouth) although sometimes inhalation and topical application were also reported depending on the preparation method used and the toxicity of the plant(s). Cups, bottles, and tablespoons were the most commonly used for determining the posology of herbal remedies [7, 10, 12].
Efficacy and safety studies
Some ethnobotanical studies reported that herbal medicine preparations were effective in the treatment of TB, while some were used in the management of multidrug-resistant tuberculosis [7, 12, 47]. This could be due to the synergistic interaction between the various phytochemicals present in the herbal preparations [27, 83]. However, as much as these herbal medicines might have genuine bioactivity, sometimes they are used concurrently with conventional therapies as supplements and at times adulterated. Therefore, it is important to scientifically validate the claimed efficacy and safety of both the herbal preparations and the individual medicinal plants. Out of the 195 species documented, only 36 plant species (18.5%) have been studied for their antimycobacterial activity. A WHO report [14] indicated that only approximately 10% of the world’s flora have been studied as regards their medicinal potential. This has greatly hindered the discovery of potential lead compounds that could be developed into new antitubercular drugs.
Out of the 36 screened medicinal plants, 31 species (86.1%) were reported to be bioactive with some species exhibiting quite considerable antimycobacterial activity although the current standard drugs had superior bioactivity (Table 2). This is comparable to India where 70% of 365 plants which were studied showed antimycobacterial activity [87]. Among the promising plant species (with minimum inhibitory concentration less than 0.5 mg/ml) were Erythrina abyssinica, Entada abyssinica, Bidens pilosa, Callistemon citrinus, Khaya senegalensis, Lantana camara, Piptadenistrum africana, Rosmarinus officinalis, Tetradenia riparia, and Zanthoxylum leprieurii. Isolated pure compounds from three of the promising plant species had much higher activity against Mtb than the crude extracts and fractions. Indeed, some of the compounds from Zanthoxylum leprieurii had minimum inhibitory concentrations lower than those of standard antitubercular drugs (Table 3). Crude extracts and fractions usually have less pharmacological activity than standard drugs because of the interference from other inactive substances in the matrix that reduce the overall concentration of the active molecules in the tested dose. This explains why isolation of pure compounds is a critical step in natural product drug discovery process. The five documented medicinal plants that were found to be inactive are Acacia ataxacantha, Dalbergia melanoxylon, Indigofera lupatana, Lonchocarpus eriocalyx, and Solanum incanum. This could probably be attributed to the absence of inherent bioactive phytochemicals against Mtb in the plant species. This could be brought about by absence or impaired biosynthetic metabolic pathways due to unfavorable growth conditions in the habitat from where the plants grow. This implies that herbal remedies for TB containing each of these plants singly may not be effective. Therefore, other benefits provided by these species in the concoctions of TB such as detoxification of other toxic phytochemicals, preservation of the herbal medicine, or potentiation of the pharmacological activity of other phytochemicals could be investigated.
Table 2.
Efficacy, toxicity, and phytochemical studies on medicinal plants used for treatment of TB in East Africa
| Plant | Extraction method (solvent) | MIC (μg/ml) on H37Rv strain | MIC (μg/ml) on TMC-331 strain | Toxicity of crude extracts (μg/ml) | Class of compounds | Author(s) |
|---|---|---|---|---|---|---|
| Acacia ataxacantha | Maceration (methanol) | Not active | Not tested | IC50 = 90.39 | Phenols, terpenoids | [28] |
| Acacia horrida | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Alkaloids, cardiac glycosides, tannins, saponins, terpenoids | [29] |
| Acacia senegal | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Cardiac glycosides, tannins, saponins, terpenoids, flavonoids | [29] |
| Acokanthera friesiorum | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Cardiac glycosides, Tannins, flavonoids | [29] |
| Albizia anthelmitica | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Alkaloids, saponins, tannins, flavonoids | [29] |
| Aspilia pluriseta | Maceration (methanol) | Active at 1 g/ml (MIC not determined) | Not tested | IC50 = 24.51 | Phenol, terpenoids, flavonoids | [28] |
| Bidens pilosa | Maceration (ethanol) | 100 | Not tested | Not tested | Not tested | [37] |
| Callistemon citrinus | Maceration (methanol, chloroform) | 325 (methanol), 48 (chloroform) (Iso = 4.0; R = 2.0) | 78 (methanol), 158 (chloroform), Iso = 4.0 | Not tested | Flavonoids, alkaloids, triterpenoids, saponins | [15] |
| Cissampelos pareira | Maceration (methanol) | Active at 1 g/ml (MIC not determined) | Not tested | IC50 = 179 | Anthraquinones, phenols, terpenoids, flavonoids | [28] |
| Commiphora edulis | Maceration (ethyl acetate, DCM, water) | 6250 (Ethyl acetate), 780 (methanol), Not active (water) | Not tested | IC50 = 393 (DCM), 1734 (ethyl acetate) | Flavonoids, terpenoids | [26] |
| Commiphora ellenbeckii | Maceration (ethyl acetate, methanol, water) | 12500 (Ethyl acetate), 3125 (methanol), 780 (water), 15 (rif) | Not tested | IC50 = 608 (methanol), 1509 (water) | Alkaloids, saponins, tannins, phenols, flavonoids, terpenoids | [26] |
| Commiphora mildbraedii | Maceration (ethyl acetate, methanol, water) | 6250–9250 (Ethyl acetate), 390–780 (methanol), not active (water), 15 (Rif) | Not tested | IC50 = 339 (ethyl acetate), 452 (methanol) | Alkaloids, saponins, tannins, phenols, flavonoids, terpenoids | [26] |
| Cordia sinensis | Soxhlet (methanol) | < 500 (Iso < 500) | Not tested | Not tested | Saponins, terpenoids, flavonoids, tannins | [29] |
| Cryptolepsis sanguinolenta | Methanol chloroform | 1170 (methanol) (Iso = 0.25; R = 0.25) | 1580 (methanol) (Iso = 0.25) | LD50 = 758 mg/kg | Alkaloids, tannins, flavonoids | [84] |
| Dalbergia melanoxylon | Maceration (methanol) | Not active | Not tested | IC50 = 120.04 | Phenols, terpenoids | [28] |
| Dichrostachys cinerea | Maceration (methanol) | Active at 1 g/ml, (MIC not determined) | Not tested | IC50 = 201.22 | Phenols, terpenoids | [28] |
| Entadda abyssinica | Maceration (methanol) | 500 (Iso = 0.25) | Not tested | Not tested | Flavonoid, alkaloids, saponins, tannins | [12, 29] |
| Erythrina abyssinica | Maceration (methanol) | 390 (Rif = 0.25; Iso = 0.25) | 2350 (Iso = 9.38) | LD50 = 776.2 mg/kg | Flavonoids, alkaloids, tannins | [44] |
| Euphorbia ingens | Maceration (methanol) | Active at 1 g/ml (MIC not determined) | Not tested | IC50 = 105.55 | Phenols, terpenoids | [28] |
| Euphorbia scarlatica | Soxhlet (methanol) | < 500 (Iso < 500) | Not tested | Not tested | Alkaloids, cardiac glycosides, terpenoids, flavonoids | [29] |
| Gnidia buchananii | Maceration (methanol) | Active at 1 g/ml (MIC not determined) | Not tested | IC50 = 76.24 | Phenols, terpenoids, | [28] |
| Indigofera lupatana | Maceration (methanol) | Not active | Not tested | IC50 = 60.37 | Phenols, terpenoids | [28] |
| Khaya senegalensis | Maceration (ethyl acetate, chloroform) | 6.25 | Not tested | IC50 = 1000 | Not tested | [52] |
| Lantana camara | Maceration (methanol, chloroform) | 20 (Rif = 1) | 15 (Iso = 0.25) | LD50 > 500 mg/kg | Not reported | [53] |
| Lonchocarpus eriocalyx | Maceration (methanol) | Not active | Not tested | IC50 = 201.87 | Terpenoids, phenols, flavonoids | [28] |
| Loranthus acaciae | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Alkaloids, cardiac glycosides, saponins, flavonoids | [29] |
| Mangifera indica | Methanol | 3130 (methanol) (Iso = 0.25; R = 0.25) | 590 (methanol) (Iso = 0.25) | Not tested | Phenols, terpenoids | [16] |
| Pentos longiflora | Maceration (ethanol) | 1000 | Not tested | Not tested | Not tested | [37] |
| Piptadenistrum africana | Maceration (chloroform) | 395 (chloroform) | 395 (chloroform) | Not tested | Flavonoids, tannins | [15] |
| Plumbago dawei | Soxhlet (methanol) | < 1000 (Iso < 500) | Not tested | Not tested | Cardiac glycosides, tannins, terpenoids, flavonoids | [29] |
| Rosmarinus officinalis L. | Maceration (chloroform) | 6.25 | Not tested | IC50 = 100 | Not tested | [52] |
| Salvadora persica | Soxhlet (methanol) | < 500 (Iso < 500) | Not tested | Not tested | Alkaloids, cardiac glycosides, terpenoids, flavonoids | [29] |
| Solanum incanum | Methanol chloroform | Not active | Not active | Not tested | Not reported | [16] |
| Tetradenia riparia | Maceration (ethanol) | 500 | Not tested | Not tested | Not tested | [37] |
| Warburgia ugandensis | Methanol chloroform | 4690 (methanol), 2350 (chloroform) (Iso = 0.25; R = 0.25) | 2350 (methanol), 590 (chloroform) (Iso = 0.25) | Not tested | Flavonoids, tannins, terpenoids | [85, 86] |
| Zanthoxylum leprieurii | Methanol | 47.5 (Iso = 4.0; R = 2.0) | 75.3 (Iso = 4.0) | Not tested | Alkaloids | [5] |
IC50 median cytotoxic concentration, LD50 median lethal dose, Iso isoniazid, Rif rifampicin, H37Rv pan sensitive Mtb strain, TMC331 rifampicin-resistant Mtb strain, MIC minimum inhibitory concentration. Extracts in [26] were tested against Mycobacteria smegmatis
Table 3.
Isolation and characterization studies on medicinal plants used for management of TB in East Africa
| Plant | Pure compounds with antitubercular activity | Chemical class | MIC of pure compounds (μg/ml) | Author(s) |
|---|---|---|---|---|
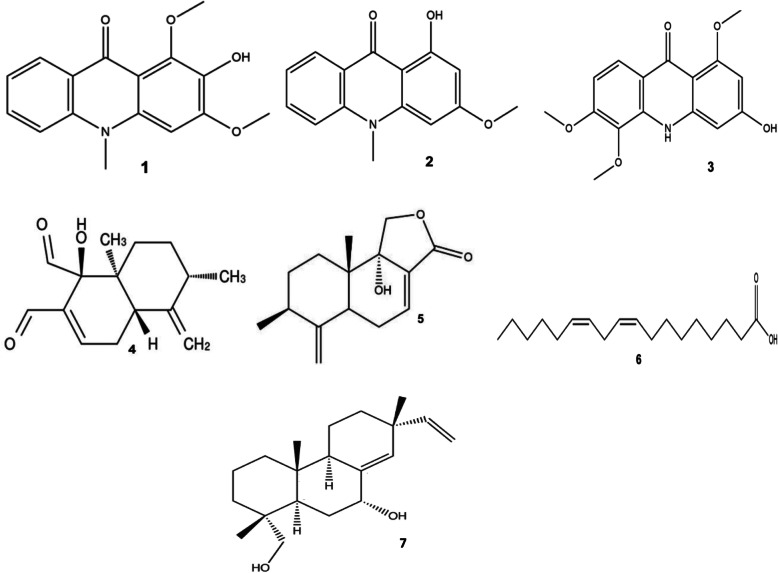
| Zanthoxylum leprieurii | 2-hydroxy-1, 3-dimethoxy-10-methyl-9-acridone (1), 1-hydroxy-3-methoxy-10-methyl-9-acridone (2), 3-hydroxy-1, 5, 6-trimethoxy-9-acridone (3) | Acridone alkaloids | 1.5 (1), 0.2 (2), 0.4 (3); tested against H37Rv | [5] |
| Warburgia ugandensis Sprague | Muzigadial (4), muzigadiolide (5), linoleic acid (6) | Sesquiterpenes | 64 (4), 128 (5), 16 (6); tested against M. smegmatis | [58, 85] |
| Tetradenia riparia | 15- sandaracopimaradiene-7α, 18-dio1 (7) | Diterpenediol | 25–100 | [37] |
MIC minimum inhibitory concentration. No toxicity studies of the pure compounds were conducted.
All toxicity studies reviewed evaluated only the acute toxicity profiles of the medicinal plants either in vitro or in vivo but not both. Of the bioactive extracts screened, less than half of them were tested for their acute toxicity. Selectivity index (SI) is used as the best estimate of the relative toxicity of a compound to normal mammalian cells as compared to the pathogen and hence its suitability for being a drug candidate. According to the SI criterion, compounds with higher SI are regarded to have better toxicity profiles than those with lower SI [88]. From the retrieved data, only two plant species (Khaya senegalensis and Rosmarinus officinalis) had acceptable selectivity indices to warrant drug discovery from them. In this study, the SI of only five plant species could be calculated (Table 4) because they were the only plant species with both the inhibitory concentration on Mtb and cytotoxic concentration on normal mammalian cell lines (IC50) reported. Hence, there is need to emphasize dual testing of both toxicity and efficacy of natural products for drug development purposes.
Table 4.
Selectivity indices of some antitubercular plant species reported in East Africa
| Plant | Solvent | MIC on Mtb strain (μg/ml) | IC50 (μg/ml) | SI | Comment |
|---|---|---|---|---|---|
| Commiphora edulis | Dichloromethane | 1560 | 393 | 0.25 | More toxic to human cells than the Mtb; not useful |
| Ethyl acetate | 3125 | 1734 | 0.55 | More toxic to human cells than the Mtb; not useful | |
| Commiphora ellenbeckii | Water | 780 | 1509 | 1.93 | More toxic to Mtb than human cells but the SI is low. May be optimized for lead candidate identification |
| Methanol | 3125 | 608 | 0.19 | More toxic to human cells than the Mtb; not useful | |
| Commiphora mildbraedii | Methanol | 390 | 452 | 1.16 | More toxic to Mtb than human cells but the SI is close to 1. No practical application |
| Ethyl acetate | 6250 | 339 | 0.054 | More toxic to human cells than the Mtb; not very useful | |
| Khaya senegalensis | Chloroform | 6.25 | 1000 | 160 | More toxic to Mtb than human cells with high SI. Promising for development of lead candidate |
| Rosmarinus officinalis L. | Chloroform | 6.25 | 100 | 16 | More toxic to Mtb than human cells with high SI. Promising for development of lead candidate |
IC50 cytotoxic concentration normal cells, SI selectivity index
Two other systems of acute toxicity classification: The National Cancer Institute (NCI) and Organization for Economic cooperation and development (OECD) guidelines 423 were used to assess the toxicity profiles of the different extracts [89, 90]. There was no single plant species among those tested for acute toxicity that was reported to be highly toxic (with IC50 less than 20 μg/ml). All the plant species with promising bioactivity that were tested for toxicity had acceptable acute toxicity profiles. These included Rosmarinus officinalis, Lantana camara, Khaya senegalensis, and Erythrina abyssinica (Table 2). Aspilia pluriseta, Cissampelos pareira, Euphorbia ingens, and Gnidia buchananii had moderate toxicity with IC50 between 20 and 200 μg/ml. According to OECD 2001 guidelines, Lantana camara, Erythrina abyssinica, and Cryptolepis sanguinolenta had slight toxicity as their median lethal doses (LD50) were above 500 mg/kg. These results justify the general public belief that traditional medicines are relatively safer as compared to the current conventional therapies. However, toxicity testing should be done on all potential medicinal plants and their phytochemicals before concluding that they are safe for human treatment [91–94]. This is because toxicity of herbal medicines may be due to presence of inherent poisonous chemicals in the plant species, misidentification of the plant species, adulteration or contamination during harvesting, preparation, and storage [95, 96]. Acute toxicity tests determine a single high dose that kills 50% of the cells or animals in a population. They may not be evident enough to depict the real toxicity situation for herbal remedies taken for a longer time in chronic conditions like TB [18, 97]. Therefore, this may necessitate sub-chronic and chronic toxicity tests to be carried out on a medicinal plant species with a potential lead compound [95].
Phytochemistry of the reported plants
Phytochemical investigation reveals the chemical nature of the pure compounds that are responsible for the pharmacological activity as well as the toxicity of medicinal plants [19, 64, 98–101]. Chromatographic and spectroscopic techniques are used to identify and elucidate the chemical structures of compounds [102–107]. In this study, maceration was the commonly used method of extraction as compared to Soxhlet. Majority of the hexane extracts were reported to be inactive against mycobacterial strains while almost all methanolic extracts were active. Methanol being a polar solvent extracts polar phytochemical while hexane (a non-polar solvent) extracts non-polar compounds. It is reasonable to assert that the antimycobacterial activity of the extracts is largely due to polar phytochemicals. There were variations in bioactivity of different parts of the same plant with no specific patterns. This could be due to differences in their rate of accumulating the bioactive substances.
The phytochemicals that were frequently screened for have been alkaloids, saponins, cardiac glycosides, flavonoids, terpenoids, and phenols. All these secondary metabolites were reported to be present in different bioactive extracts. The most commonly reported phytochemicals were flavonoids, terpenoids, and alkaloids [15, 17, 26, 29, 70, 106, 108]. Flavonoids and alkaloids were reported to be absent in three out of the five inactive plants (Table 2). Out of the 31 bioactive plant species, only three (Tetradenia riparia, Warburgia ugandensis, and Zanthoxylum leprieurii) have been further characterized to identify the pure compounds responsible for their antimycobacterial activity [5, 37, 58, 85] (Table 3). This is attributed to the complexity and the rigorous nature of the process that require extraction, screening, isolation, and characterization [100, 109, 110]. Low extraction yield, compound instability, high costs, low technology especially in developing countries, limited access to advanced chromatographic, and spectroscopic equipment and inadequate funding have made it difficult to undertake herbal medicine research [21, 111, 112]. This is further complicated by the microbiological nature of the Mtb that require bioassays to be conducted in biosafety level 3 laboratories that are not readily available in East Africa [60, 113]. More robust and effective techniques are required to fasten the drug discovery process against TB [3, 77, 92, 114].
A total of seven pure compounds have been isolated and characterized with bioactivity against Mtb (Fig. 5). These are 2-hydroxy-1,3-dimethoxy-10-methyl-9-acridone (1), 1-hydroxy-3- methoxy-10-methyl-9-acridone (2), 3-hydroxy-1, 5, 6-trimethoxy-9-acridone (3), muzigadial (4), muzigadiolide (5), linoleic acid (6), and 15-sandaracopimaradiene-7α, 18-dio1 (7). Compounds 1, 2, and 3 are acridone alkaloids; 4, 5, and 6 are sesquiterpenes, while 7 is a diterpenediol [5, 37, 85]. In Asia and America, several studies have reported pure compounds isolated from medicinal plants to have promising antimycobacterial activity [78, 115–117]. For example, Bisbenzylisoquinoline alkaloids from Tiliacora triandra (tiliacorinine, tiliacorine and 2′-nortiliacorinine) were found to have comparable antimycobacterial activity (MIC = 0.7–6.2 μg/ml) to the standard first line drugs against sensitive and resistant Mtb strains [108]. Rukachaisirikul et al. [118] reported that 5- hydroxysophoranone (an isoflavone from Erythrina stricta) had promising antimycobacterial activity (MIC = 12.5 μg/ml) against Mtb H37Ra. Vasicine acetate and 2-acetyl benzylamine isolated from hexane extract of Adhatoda vasica Ness. (Acanthaceae) inhibited one sensitive and multidrug-resistant strain at 50 and 200 μg/ml respectively [119]. Since flavonoids and alkaloids were reported to be absent in three out of the five inactive plants [28] and majority of the isolated bioactive pure compounds belong to the class of alkaloids, terpenoids, and flavonoids [5, 85, 118], it implies that these classes of phytochemicals are the ones most likely to be responsible for the observed antimycobacterial activity.
Fig. 5.
Structure of antitubercular molecules isolated in claimed medicinal plants in East Africa. The numbers 1–7 correspond to the molecules mentioned in Table 3
Conclusion
East Africa has a rich diversity of medicinal plants that have been reported to be effective in the management of symptoms of TB. Most of the plants are from the family Fabaceae, Lamiaceae, and Asteraceae. A large proportion of the documented plants have not been scientifically validated for their efficacy and safety. Although the standard drugs had superior activity, majority of the validated plants were found to possess acceptable acute toxicity profile on animal cells and considerable bioactivity with isolated pure compounds showing promising efficacy against Mtb. We recommend more scientific validation studies to be conducted on the remaining plants in order to standardize herbal medicine use and also promote drug discovery and development against TB. More isolation and characterization studies will enrich the chemical diversity of both the natural product and synthetic chemical libraries from which possible lead candidates could be developed. Currently, we are working on isolation and characterization of bioactive compounds from selected medicinal plants from family Fabaceae identified from this study. These include Erythrina abyssinica, Albizia coriaria, and Entada abyssinica.
Supplementary information
Additional file 1: Figure S1. PRISMA flow diagram used for the review.
Acknowledgements
The authors are grateful to the World Bank and the Inter-University Council of East Africa (IUCEA) for the scholarship awarded to SBO, MPO, and TO through the Africa Centre of Excellence II in Phytochemicals, Textiles and Renewable Energy (ACE II PTRE) at Moi University, Kenya, which made this communication possible. The authors commend preceding authors for their fruitful quest for knowledge on medicinal plants utilized by rural communities of East Africa, the reports of which the current study was based.
Abbreviations
- IC50
Median cytotoxic concentration
- LD50
Median lethal dose
- Iso
Isoniazid
- MIC
Minimum inhibitory concentration
- Rif
Rifampicin
- H37Rv
Pan sensitive Mtb strain
- TMC331
Rifampicin-resistant Mtb strain
- SI
Selectivity Index
- TB
Tuberculosis
- WHO
World Health Organization
Authors’ contributions
SBO, AK, IK, EK, MPO, TO, and LB designed the study. SBO, IK, EK, MPO, TO, and LB performed literature search for medicinal plants in Uganda, Burundi, Rwanda, Kenya, Tanzania, and South Sudan, respectively. SBO and TO analyzed the collected data. TO, MPO, and LB verified the plant names in botanical databases and local languages. SBO, MPO, TO, and LB wrote the first draft of the manuscript. AK, IK, and EK reviewed the draft manuscript. All authors revised and approved the final manuscript.
Funding
This research received no external funding.
Availability of data and materials
This is a review article and no raw experimental data were collected. All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s41182-020-00256-1.
References
- 1.WHO. Global Tuberculosis Report 2019. World Health Organization, Geneva, Switzerland. 2019. 297p. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1. Acessed 04 March 2020.
- 2.Hiraiwa M, Kim J, Lee H, Inoue S, Becker AL, Weigel KM, et al. Amperometric immunosensor for rapid detection of Mycobacterium tuberculosis. J Micromech Microeng. 2015;25:055013. doi: 10.1088/0960-1317/25/5/055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan T, Sampson NS. Hit generation in TB drug discovery: from genome to granuloma. Chem Rev. 2018;118:1887–1916. doi: 10.1021/acs.chemrev.7b00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosio LD, Centis R, Sotgiu G, Pontali E, Spanevello A, Migliori GB. New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Res. 2015;1:00010–02015. doi: 10.1183/23120541.00010-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunalema L, Fotso GW, Waako P, Tabuti J, Yeboah SO. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement Altern Med. 2017;17:89. doi: 10.1186/s12906-017-1602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godebo A, Abiy H, Toma A. Recent advances in the development of anti-tuberculosis drugs acting on multidrug-resistant strains: a review. Int J Res Pharm Biosci. 2015;2:1–18. [Google Scholar]
- 7.Bunalema L, Obakiro S, Tabuti JRS, Waako P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J Ethnopharmacol. 2014;151:999–1004. doi: 10.1016/j.jep.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Schultz F, Anywar G, Wack B, Quave CL, Garbe L. Ethnobotanical study of selected medicinal plants traditionally used in the rural greater Mpigi region of Uganda. J Ethnopharmacol. 2020;256:112742. doi: 10.1016/j.jep.2020.112742. [DOI] [PubMed] [Google Scholar]
- 9.Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira central Forest reserve, Uganda. J Ethnobiol Ethnomed. 2016;12:5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabuti JRS, Kukunda CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharmacol. 2010;127:130–136. doi: 10.1016/j.jep.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Jeruto P, Lukhoba C, Ouma G, Otieno D, Mutai C. An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J Ethnopharmacol. 2008;116:370–376. doi: 10.1016/j.jep.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Orodho JA, Kirimuhuzya C, Otieno JN, Magadula JJ, Okemo P. Local management of tuberculosis by traditional medicine practitioners in Lake Victoria region. Open Complement Med J. 2011;3:1–9. [Google Scholar]
- 13.Anywar G, Kaduidi E, Byamukama R, Mukonzo J, Schubert A, Oryem-Origa H. Indigenous traditional knowledge of medicinal plants used by herbalists in treating opportunistic infections among people living with HIV/AIDS in Uganda. J Ethnopharmacol. 2020;246:112205. doi: 10.1016/j.jep.2019.112205. [DOI] [PubMed] [Google Scholar]
- 14.WHO Global Report on Traditional and Complementary Medicine. 2019. https://www.who.int/traditional-complementary-integrative-medicine/WhoGlobalReportOnTraditionalAndComplementaryMedicine2019.pdf?ua=1. Accessed 04 March 2020.
- 15.Bunalema L, Tabuti J, Sekagya Y, Ogwang S, Waako P. Anti-tubercular activity of Callistemon citrinus and Piptadenistrum africanum on resistant strains of Mycobacterium tuberculosis using microplate alamar blue assay. Spat DD. 2015;5:235–240. [Google Scholar]
- 16.Magadula JJ, Otieno JN, Nondo RS, Kirimuhuzya C, Kadukuli E, Orodho JA, et al. Eur J Med Plants. 2012;2:125–131. [Google Scholar]
- 17.Mariita M. Efficacy of medicinal plants used by communities around Lake Victoria region and the Samburu against mycobacteria, selected bacteria and Candida albicans. Nairobi: Kenyatta University; 2011. [Google Scholar]
- 18.Obakiro SB, Bunalema L, Nyatia E, Waako JP. Ulcerogenic potential of Eucalyptus globulus L. leaf extract in Wistar albino rats. J Pharmacol Toxicol. 2018;4:46–51. [Google Scholar]
- 19.Omara T. Plants ised in antivenom therapy in rural Kenya: ethnobotany and future perspectives. J Toxicol. 2020;2020:1–9. doi: 10.1155/2020/1828521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alamgeer. Younis W, Asif H, Sharif A, Riaz H, Bukhari IA, et al. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chin Med. 2018;13:48. doi: 10.1186/s13020-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuniga ES, Early J, Parish T. The future for early-stage tuberculosis drug discovery. Future Microbiol. 2015;10:217–229. doi: 10.2217/fmb.14.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omara T, Kagoya S, Openy A, Omute T, Ssebulime S, Kiplagat KM, et al. Antivenin plants used for treatment of snakebites in Uganda: ethnobotanical reports and pharmacological evidences. Trop Med Health. 2020;48:6. doi: 10.1186/s41182-019-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omara T, Kiprop AK, Ramkat RC, Cherutoi J, Kagoya S, Nyangena DM, et al. Medicinal plants used in traditional management of cancer in Uganda: a review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evidence-Based Complement Alternat Med. 2020;2020:1–26. doi: 10.1155/2020/3529081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omara T. Antimalarial plants used across Kenyan communities. Evidence-Based Complement Alternat Med. 2020;2020:1–31. doi: 10.1155/2020/4538602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimbeshaho F, Mwangi CN, Orina F, Chacha M, Moody JO, Kigondu EM. Antimycobacterial activities, cytotoxicity and phytochemical screening of extracts for three medicinal plants growing in Kenya. J Med Plants Res. 2020; (in press).
- 27.Ayaz M, Ullah F, Sadiq A, Ullah F, Ovais M, Ahmed J, et al. Interactions synergistic interactions of phytochemicals with antimicrobial agents : potential strategy to counteract drug resistance. Chem Biol Interact. 2019;308:294–303. doi: 10.1016/j.cbi.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 28.Njeru SN, Obonyo MA. Potency of extracts of selected plant species from Mbeere, Embu County-Kenya against Mycobacterium tuberculosis. J Med Plant Res. 2016;10:149–157. [Google Scholar]
- 29.Mariita RM, Ogol CKPO, Oguge NO, Okemo PO. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop J Pharm Res. 2010;9:379–385. [Google Scholar]
- 30.Musa MS, Abdelrasool FE, Elsheikh EA, Ahmed LAMN, Mahmoud ALE, Yagi SM. Ethnobotanical study of medicinal plants in the Blue Nile state, South-Eastern Sudan. J Med Plant Res. 2011;5:287–4297. [Google Scholar]
- 31.Kimathi KN, Ogutu PA, Mutai C, Jeruto P. Ethnobotanical study of selected medicinal plants used against bacterial infections in Nandi county. Kenya. 2019;7:103–108. [Google Scholar]
- 32.Watt JM, Breyer-Brandwijk G. Medicinal and poisonous plants of southern and eastern Africa. 2. Edinburgh & London: E. & S. Livingstone Ltd; 1962. [Google Scholar]
- 33.Shiracko N, Owuor BO, Gakuubi MM, Wanzala W. A survey of ethnobotany of the AbaWanga people in Kakamega county, Western province of Kenya. Indian J Tradit Knowle. 2016;15:93–102. [Google Scholar]
- 34.Fratkin E. Traditional medicine and concepts of healing among samburu pastoralists of Kenya. J Ethnobiol. 1996;16:63–97. [Google Scholar]
- 35.Ghazali GE, Abdalla WE, El H, Khalid S, Khalafalla M. Medicinal plants of Sudan, part V: medicinal plants of Ingassana area. Khartoum, Sudan: National Center for Research, Ministry of Science and Technology; 2003. pp. 1–19. [Google Scholar]
- 36.Okello SV, Nyunja RO, Netondo GW, Onyango JC. Ethnobotanical study of medicinal plants used by sabaots of Mt. Elgon Kenya. Afr J Tradit Complement Altern Med. 2010;7:1–10. doi: 10.4314/ajtcam.v7i1.57223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Puyvelde L, Ntawukiliyayo JD, Portaels F, Hakizamungu E. In vitro inhibition of mycobacteria by Rwandese medicinal plants. Phytother Res. 1994;8:65–69. [Google Scholar]
- 38.Ngezahayo J, Havyarimana F, Hari L, Stévigny C, Duez P. Medicinal plants used by Burundian traditional healers for the treatment of microbial diseases. J Ethnopharmacol. 2015;173:338–351. doi: 10.1016/j.jep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Gafna DJ, Dolos K, Mahiri IO, Mahiri JG, Obando JA. Diversity of medicinal plants and anthropogenic threats in the Samburu central sub-county of Kenya. Afr J Tradit Complement Altern Med. 2017;14:72–79. [Google Scholar]
- 40.Kiringe JW. A survey of traditional health remedies used by the Maasai of southern Kaijiado district, Kenya. Ethnobot Res Appl. 2006;4:61–73. [Google Scholar]
- 41.Kokwaro JO. Medicinal plants of East Africa. 3. Nairobi: East Africa Literature Bureau; 1976. [Google Scholar]
- 42.El-Kamalia HH, El-Khalifa KF. Folk medicinal plants of riverside forests of the southern Blue Nile district, Sudan. Fitoterapia. 1999;70:493–497. [Google Scholar]
- 43.Burham BO. Chemical constituents of selected Sudanese medicinal and aromatic plants. 2007. [Google Scholar]
- 44.Bunalema L, Kirimuhuzya C, Tabuti JRS, Waako P, Magadula JJ, Otieno N, et al. The efficacy of the crude root bark extracts of Erythrina abyssinica on rifampicin resistant mycobacterium tuberculosis. Afr Health Sci. 2011;11:587–593. [PMC free article] [PubMed] [Google Scholar]
- 45.Desouter S. Human and veterinary pharmacopoeia, vol. 22. Tervuren; 1991. p. 252.
- 46.Amuka O, Okemo PO, Alex K, Mbugua PK. Ethnobotanical survey of selected medicinal plants used by Ogiek communities in Kenya against microbial infections. Ethnobot Res Appl. 2014;12:627–641. [Google Scholar]
- 47.Orodho JA, Okemo P, Tabuti JB, Otieno N, Magadula JJ, Kirimuhuzya C. Indigenous knowledge of communities around Lake Victoria Basin regarding treatment and management of tuberculosis using medicinal plants. Int J Med Sci. 2014;6:16–23. [Google Scholar]
- 48.Kayonga A, Habiyaremye FX. Traditional medicine and Rwandan medicinal plants. Contribution to ethnobotanic study of Rwandan Flora. Gisenyi prefecture. Curfametra: Univ. Nat. University Research Center on pharmacopoeia and traditional medicine; 1987. p. 121. [Google Scholar]
- 49.Sospeter NN, Meshack AO. Potency of extracts of selected plant species from Mbeere, Embu County-Kenya against Mycobacterium tuberculosis. J Med Plants Res. 2016;10:149–157. [Google Scholar]
- 50.Cyrus WG, Daniel GW, Nanyingi MO, Njonge FK, Mbaria JM. Antibacterial and cytotoxic activity of Kenyan medicinal plants. Mem Inst Oswaldo Cruz. 2008;103:650–652. doi: 10.1590/s0074-02762008000700004. [DOI] [PubMed] [Google Scholar]
- 51.Nanyingi MO, Mbaria JM, Lanyasunya AL, Wagate CG, Koros KB, Kaburia HF, et al. Ethnopharmacological survey of Samburu district, Kenya. J Ethnobiol Ethnomed. 2008;12:1–12. doi: 10.1186/1746-4269-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abuzeid N, Kalsum S, Larsson M, Glader M, Andersson H, Raffetseder J, et al. Antimycobacterial activity of selected medicinal plants traditionally used in Sudan to treat infectious diseases. J Ethnopharmacol. 2014;157:134–139. doi: 10.1016/j.jep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Kirimuhuzya C, Waako P, Joloba M, Odyek O. The anti-mycobacterial activity of Lantana camara a plant traditionally used to treat symptoms of tuberculosis in South-Western Uganda. Afr Health Sci. 2009;9:40–45. [PMC free article] [PubMed] [Google Scholar]
- 54.EL-Kamali HH. Ethnopharmacology of medicinal plants used in North Kordofan (Western Sudan) Ethnobot Leaf. 2009;13:203–210. [Google Scholar]
- 55.Nankaya J, Nampushi J, Petenya S, Balslev H. Ethnomedicinal plants of the Loita Maasai of Kenya. Environ Dev Sustain. 2019. 10.1007/s10668-019-00311-w.
- 56.Nankaya J, Gichuki N, Lukhoba C, Balslev H. Medicinal plants of the Maasai of Kenya: a review. Plants. 2020;9:1–17. doi: 10.3390/plants9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asiimwe S, Kamatenesi-Mugisha M, Namutebi A, Borg-Karlsson AK, Musiimenta P. Ethnobotanical study of nutri-medicinal plants used for the management of HIV/AIDS opportunistic ailments among the local communities of western Uganda. J Ethnopharmacol. 2013;150:639–648. doi: 10.1016/j.jep.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Mbwambo Z, Erasto P, Innocent E, Masimba P. Antimicrobial and cytotoxic activities of fresh leaf extracts of Warburgia ugandensis. Tanzan J Health Res. 2009;11:75–78. [Google Scholar]
- 59.Okello D, Kang Y. Ethnopharmacological potentials of Warburgia ugandensis on antimicrobial activities. Chin J Integr Med. 2019. 10.1007/s11655-019-3042-6. [DOI] [PubMed]
- 60.Buwa LV, Afolayan AJ. Antimicrobial activity of some medicinal plants used for the treatment of tuberculosis in the eastern Cape Province, South Africa. Afr J Biotechnol. 2009;8:6683–6687. [Google Scholar]
- 61.Babalola IT, Adelakun EA. Compendium of medicinal plants for the ethno-therapeutic management of tuberculosis and other respiratory diseases. J Pharmacog Phytochem. 2018;7:1983–1994. [Google Scholar]
- 62.Semenya SS, Maroyi A. Medicinal plants used for the treatment of tuberculosis by Bapedi traditional healers in three districts of the Limpopo province, South Africa. Afr J Tradit Complement Altern Med. 2012;10:316–323. doi: 10.4314/ajtcam.v10i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvin A, Miller KI, Neilan BA. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res. 2014;169:483–495. doi: 10.1016/j.micres.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semenya SS, Maroyi A. Ethnobotanical survey of plants used by Bapedi traditional healers to treat tuberculosis and its opportunistic infections in the Limpopo Province, South Africa. South Afr J Bot. 2019;122:401–421. [Google Scholar]
- 65.Green E, Samie A, Obi CL, Bessong PO, Ndip RN. Inhibitory properties of selected south African medicinal plants against Mycobacterium tuberculosis. J Ethnopharmacol. 2010;130:151–157. doi: 10.1016/j.jep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 66.Famewo EB, Clarke AM, Wiid I, Ngwane A, Van Helden P, Afolayan AJ. Anti-mycobacterium tuberculosis activity of polyherbal medicines used for the treatment of tuberculosis in eastern cape, South Africa. Afr Health Sci. 2017;17:780–789. doi: 10.4314/ahs.v17i3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibekwe NN, Ameh SJ. Plant natural products research in tuberculosis drug discovery and development: a situation report with focus on Nigerian biodiversity. Afr J Biotechnol. 2014;13:2307–2320. [Google Scholar]
- 68.Mann A, Amupitan JO, Oyewale AO, Okogun JI, Ibrahim K, Oladosu P, et al. Evaluation of in vitro antimycobacterial activity of Nigerian plants used for treatment of respiratory diseases. Afr J Biotechnol. 2008;7:1630–1636. [Google Scholar]
- 69.Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-manu D, Addo PGA, Kissi-Twum A. Antimycobacterial and cytotoxic activity of selected medicinal plant extracts. J Ethnopharmacol. 2016;182:10–15. doi: 10.1016/j.jep.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gemechu A, Giday M, Worku A, Ameni G. In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med. 2013;13:291. doi: 10.1186/1472-6882-13-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandit R, Singh PK, Kumar V. Natural remedies against multi-drug resistant Mycobacterium tuberculosis. J Tuberculosis Res. 2015;3:171–183. [Google Scholar]
- 72.Rai R. Herbal remedies in cure of tuberculosis prevalent among ethnic communities in Central India. Trop Plant Res. 2016;3:344–353. [Google Scholar]
- 73.Mongalo NI, McGaw LJ, Segapelo TV, Finnie JF, Van Staden J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia sericea Burch. Ex DC. (Combretaceae) – a review. J Ethnopharmacol. 2016;94:789–802. doi: 10.1016/j.jep.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 74.Saleh-e-In MM, Van Staden J. Ethnobotany, phytochemistry and pharmacology of Arctotis arctotoides (L.f.) O. Hoffm.: a review. J Ethnopharmacol. 2018;220:294–320. doi: 10.1016/j.jep.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Sharma A, Flores-Vallejo RC, Cardoso-Taketa A, Villarreal ML. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J Ethnopharmacol. 2017;208:264–329. doi: 10.1016/j.jep.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 76.Ngadino S, Koerniasari E, Sudjarwo SA. Evaluation of antimycobacterial activity of Curcuma xanthorrhiza ethanolic extract against Mycobacterium tuberculosis H37Rv in vitro. Vet World. 2018;11:368–372. doi: 10.14202/vetworld.2018.368-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuyiringire N, Tusubira D, Munyampundu JP, Tolo CU, Muvunyi CM, Ogwang PE. Application of metabolomics to drug discovery and understanding the mechanisms of action of medicinal plants with anti-tuberculosis activity. Clin Transl Med. 2018;7:29. doi: 10.1186/s40169-018-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-baadani WA, Satyanarayan ND. Anti-tubercular evaluation of Rivea hypocrateriformis (Der.) choisy against Mycobacterium tuberculosis H37Rv strain. J Pharmacognosy Phytochem. 2018;7:2679–2682. [Google Scholar]
- 79.Lawal IO, Grierson DS, Afolayan AJ. Phytotherapeutic information on plants used for the treatment of tuberculosis in eastern Cape Province. South Africa Evidence-based Complement Altern Med. 2014:1–11. 10.1155/2014/735423. [DOI] [PMC free article] [PubMed]
- 80.Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-Manu D, Addo PGA. Medicinal plants used to treat TB in Ghana. Int J Mycobacteriol. 2015;4:116–123. doi: 10.1016/j.ijmyco.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Ogbole OO, Ajaiyeoba EO. Traditional management of tuberculosis in Ogun state of Nigeria: the practice and ethnobotanical survey. Afr J Tradit Complement Altern Med. 2010;7:79–84. doi: 10.4314/ajtcam.v7i1.57270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stewart ZP, Pierzynski GM, Middendorf BJ, Prasad PVV. Approaches to improve soil fertility in sub-Saharan Africa. J Exp Bot. 2020;71:632–641. doi: 10.1093/jxb/erz446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ge F, Zheng F, Liu S, Guo N, Ye H, Song Y, et al. In vitro synergistic interactions of oleanolic acid in combination with isoniazid, rifampicin or ethambutol against Mycobacterium tuberculosis. J Med Microbiol. 2010;59:567–572. doi: 10.1099/jmm.0.014837-0. [DOI] [PubMed] [Google Scholar]
- 84.Kirimuhuzya C. Efficacy of Cryptolepis sanguinolenta root extract on slow-growing rifampicin resistant Mycobacterium tuberculosis. J Med Plants Res. 2012;6:1140–1146. [Google Scholar]
- 85.Wube AA, Bucar F, Gibbons S, Asres K. Sesquiterpenes from Warburgia ugandensis and their antimycobacterial activity. Phytochem. 2005;66:2309–2315. doi: 10.1016/j.phytochem.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 86.Kirimuhuzya C, Bunalema L, Tabuti JRS, Kakudidi EK, Orodho J, Magadula J, et al. A presentation at the 14th NAPRECA symposium held at ICIPE, Kasarani, Nairobi, Kenya. 2011. The in vitro antimycobacterial activity of medicinal plants used by traditional medicine practitioners (TMPs) to treat tuberculosis in the Lake Victoria basin in Uganda. [Google Scholar]
- 87.Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007;110:200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 88.Kaminsky R, Caecilia S, Reto B. An “in vitro selectivity index” for evaluation of cytotoxicity of antitrypanosomal compounds. In vitro Toxicol. 1996;9:315–24.
- 89.OECD. OECD guideline for testing of chemicals: acute oral toxicity – acute toxic class method. OECD Guideline for Testing of Chemicals, no. December: 1–14. 2001. doi: 10.1787/9789264070943-en.
- 90.Geran RI, Greenberg HM, McDonald M, Abbott BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemoth Rep. 1972;33:1–17. [Google Scholar]
- 91.Keter L, Too R, Mwikwabe N, Mutai C, Orwa J, Mwamburi L, et al. Risk of fungi associated with aflatoxin and fumonisin in medicinal herbal products in the Kenyan market. Sci World J. 2017;1892972. [DOI] [PMC free article] [PubMed]
- 92.Pan S, Zhou S, Gao S, Yu Z, Zhang S, Tang M, et al. “New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-based complement. Altern Med. 2013. 10.1155/2013/627375. [DOI] [PMC free article] [PubMed]
- 93.Ko RJ. A U.S. perspective on the adverse reactions from traditional Chinese medicines. J Chin Med Assoc. 2004;67:109–116. [PubMed] [Google Scholar]
- 94.Chuluun B, Iamchaturapatr J, Rhee J. Phytoremediation of organophosphorus and organochlorine pesticides by Acorus gramineus. Environ Eng Res. 2009;14:226–236. [Google Scholar]
- 95.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomlinson B, Chan TY, Chan JC, Critchley JA, But PP. Toxicity of complementary therapies: an eastern perspective. J Clin Pharmacol. 2000;40:451–456. doi: 10.1177/00912700022009206. [DOI] [PubMed] [Google Scholar]
- 97.Aniagu S, Nwinyi F, Akumka DD, Ajoku GD, Dzarma S, Izebe KS. Toxicity studies in rats fed nature cure bitters. Afri J Biotechnol. 2005;4:72–78. [Google Scholar]
- 98.Agyare C, Boakye YD, Bekoe EO, Hensel A, Dapaah SO, Appiah T. Review: African medicinal plants with wound healing properties. J Ethnopharmacol. 2016;177:85–100. doi: 10.1016/j.jep.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Owor RO, Bedane KG, Zühlke S, Derese S, Ong'amo GO, Ndakala A, et al. Anti-inflammatory flavanones and flavones from Tephrosia linearis. J Nat Prod. 2020. 10.1021/acs.jnatprod.9b0092. [DOI] [PubMed]
- 100.Gavamukulya Y, Maina EN, Meroka A, Madivoli ES, El-Shemy HA, Magom G, et al. Liquid chromatography single quadrupole mass spectrometry (LC/SQ MS) analysis reveals presence of novel antineoplastic metabolites in ethanolic extracts of fruits and leaves of Annona muricata. Pharmacognosy J. 2019;11:660–668. [Google Scholar]
- 101.Andima M, Coghi P, Yang LJ, Wong VKW, Ngule CM, Heydenreich M, et al. Antiproliferative activity of secondary metabolites from Zanthoxylum zanthoxyloides Lam : in vitro and in silico studies. Pharmacognosy Comm. 2020;10:44–51. [Google Scholar]
- 102.Bauer A, Brönstrup M. Industrial natural product chemistry for drug discovery and development. Nat Prod Rep. 2014;31:35–60. doi: 10.1039/c3np70058e. [DOI] [PubMed] [Google Scholar]
- 103.Saraswathi VS, Saravanan D, Santhakumar K. Isolation of quercetin from the methanolic extract of Lagerstroemia speciosa by HPLC technique, its cytotoxicity against MCF-7 cells and photocatalytic activity. J Photochem Photobiol B. 2017;171:20–26. doi: 10.1016/j.jphotobiol.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 104.Chraibi MM, Farah A, Lebrazi S, El Amine O, Iraqui Houssaini M, Fikri-Benbrahim K. Antimycobacterial natural products from Moroccan medicinal plants: chemical composition, bacteriostatic and bactericidal profile of Thymus satureioides and Mentha pulegium essential oils. Asian Pac J Trop Biomed. 2016;6:836–840. [Google Scholar]
- 105.Hoerr V, Duggan GE, Zbytnuik L, Poon KKH, Große C, Neugebauer U, et al. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 2016;16:82. doi: 10.1186/s12866-016-0696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Esquivel-ferriño PC, Favela-hernández JMJ, Garza-gonzález E, Waksman N, Ríos MY, Camacho-corona MR. Antimycobacterial activity of constituents from Foeniculum vulgare var. Dulce grown in Mexico. Molecules. 2012;17:8471–8482. doi: 10.3390/molecules17078471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao J, Evangelopoulos D, Bhakta S, Gray AI, Seidel V. Antitubercular activity of Arctium lappa and Tussilago farfara extracts and constituents. J Ethnopharmacol. 2014;155:796–800. doi: 10.1016/j.jep.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 108.Sureram S, Senadeera SPD, Hongmanee P, Mahidol C, Ruchirawat S, Kittakoop P. Antimycobacterial activity of bisbenzylisoquinoline alkaloids from Tiliacora triandra against multidrug-resistant isolates of Mycobacterium tuberculosis. Bioorg Med Chem Lett. 2012;22:2902–2905. doi: 10.1016/j.bmcl.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 109.Gao F, Ye L, Wang Y, Kong F, Zhao S, Xiao J. Benzofuran-isatin hybrids and their in vitro anti-mycobacterial activities against multi-drug resistant Mycobacterium tuberculosis. Eur J Med Chem. 2019;183:111678. doi: 10.1016/j.ejmech.2019.111678. [DOI] [PubMed] [Google Scholar]
- 110.Machelart A, Song O, Hoffmann E, Brodin P. Host-directed therapies offer novel opportunities for the fight against tuberculosis. Drug Discov Today. 2017;22:1250–1257. doi: 10.1016/j.drudis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 111.WHO. Global Tuberculosis Report 2017. WHO, Geneva, Switzerland. 2017. 262p. https://reliefweb.int/sites/reliefweb.int/files/resources/9789241565516-eng.pdf. Accessed 4 Mar 2020.
- 112.Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-manu D, Addo PGA. Current perspectives in drug discovery against tuberculosis from natural products. Int J Mycobacteriol. 2017;4:165–183. doi: 10.1016/j.ijmyco.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manjunatha UH, Smith PW. Perspective: challenges and opportunities in TB drug discovery from phenotypic screening. Bioorganic Med Chem. 2015;23:5087–5097. doi: 10.1016/j.bmc.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 115.León-Díaz R, Meckes M, Said-Fernández S, Molina-Salinas GM, Vargas-Villarreal J, Torres J, et al. Antimycobacterial neolignans isolated from Aristolochia taliscana. Mem Inst Oswaldo Cruz. 2010;105:45–51. doi: 10.1590/s0074-02762010000100006. [DOI] [PubMed] [Google Scholar]
- 116.Bocanegra-Garcia V, Garcia A, Palma-Nicolás JP, Palos I, Rivera G. A case study based insight into modern strategies. Intech open. 2011. Antitubercular drugs development: recent advances in selected therapeutic targets and rational drug design; pp. 207–242. [Google Scholar]
- 117.Vyas DH, Tala SD, Dhaduk MF, Akbari JD, Joshi HS. Synthesis, antitubercular and antimicrobial activities of some new pyrazoline and isoxazole derivatives. J Indian Chem Soc. 2007;84:1140–1144. [Google Scholar]
- 118.Rukachaisirikul T, Saekee A, Tharibun C, Watkuolham S. Biological activities of the chemical constituents of Erythrina stricta and Erythrina subumbrans. Arch Pharm Res. 2007;30:1398. doi: 10.1007/BF02977363. [DOI] [PubMed] [Google Scholar]
- 119.Ignacimuthu S, Shanmugam N. Antimycobacterial activity of two natural alkaloids, vasicine acetate and 2-acetyl benzylamine, isolated from Indian shrub Adhatoda vasica ness . Leaves. J Biosci. 2010;35:565–570. doi: 10.1007/s12038-010-0065-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. PRISMA flow diagram used for the review.
Data Availability Statement
This is a review article and no raw experimental data were collected. All data generated or analyzed during this study are included in this published article.