Abstract
Background
The pivotal trials of adjuvant high-dose interferon alpha (HDI) in E1684, E1690, E1694 and E2696 enrolled almost 2000 patients, and established HDI as the standard of care for adjuvant therapy of resected high-risk melanoma. Here, we present an updated analysis of these four trials.
Methods
Survival and disease status were updated in September 2016. These data represent a median follow-up of 17.9 years for E1684, 12.2 years for E1690, 16.0 years for E1694, and 16.5 years for E2696.
Results
Our analysis confirms the RFS benefit of HDI in E1684 at a median follow-up of 17.9 years. The RFS benefit of E1694 remains evident at a median follow-up of 16 years. We furthermore confirm the RFS benefit of adjuvant HDI vs. observation in a pooled analysis of E1684 and E1690. No OS benefit is apparent in this pooled analysis. Updated results for E1690 and E2696 do not differ from those previously reported. In addition, we report, for the first time, a significant difference in melanoma specific survival (MSS) between patients treated with HDI vs. GMK vaccine in E1694.
Conclusions
In patients with resected high-risk melanoma, adjuvant HDI demonstrates improved RFS in E1684 and E1694, and MSS in a pooled analysis of HDI in E1694. These findings represent the most mature level of evidence for the benefit of HDI with respect to RFS and MSS. HDI is the only approved adjuvant treatment with data available in patients with resected stage IIB/IIC melanoma, and remains a reasonable treatment option in this population.
Keywords: Melanoma, High Dose Interferon, Adjuvant Therapy, Melanoma Specific Survival, Recurrence Free Survival, Overall Survival
Precis
At 16 years follow-up, E1694, the pivotal trial of high-dose interferon in resected high-risk melanoma is associated with melanoma specific survival. E1684 and E1694 are associated with a recurrence free survival benefit at median follow-up of 17.9 and 16 years, respectively.
INTRODUCTION
The incidence of melanoma continues to increase, with an estimated 91,270 cases to be diagnosed in 2018 and 9,320 deaths attributable to melanoma in the United States1. Patients with stage III melanoma have 5-year survival rates of 93, 83, 69 and 32% for stage IIIA, IIIB, IIIC and IIID, respectively, though the IIID subgroup is a new category in the American Joint Committee on Cancer (AJCC) 8th edition2. For the past 20 years, the standard of care for patients with advanced locoregional disease has been surgery followed by adjuvant therapy. High-dose interferon (HDI) α−2b was approved in 1995 for adjuvant therapy in patents with AJCC stage IIB, IIC and stage III melanoma. This was based on the pivotal trial E1684 3, which showed increased recurrence free survival (RFS) and overall survival (OS) in patients with resected melanoma who received HDI vs. observation. E1690 4, E1694 5 and E2696 6 subsequently confirmed the RFS benefit, and E1694 showed an OS benefit in comparison to the GMK vaccine that has since been shown to be inactive5. S0008 was a SWOG-led phase III trial that randomized 432 patients with high-risk resected melanoma (stage IIIA- IIIC) to treatment with standard HDI or biochemotherapy (BCT). At median follow-up of 7.2 years, BCT improved RFS (HR=0.75; 95% CI, 0.58–0.97; p = .015), with a median RFS of 4.0 years versus 1.9 years for HDI and a 5-year RFS of 48% versus 39%. Median OS was not significantly different between the two treatment arms.
More recent studies have evaluated the efficacy and led to the approval of several newer immunotherapeutic and targeted agents in the adjuvant setting. Adjuvant high-dose ipilimumab demonstrated RFS and OS benefit compared to placebo in patients with resected high-risk stage III melanoma7. More recently, adjuvant nivolumab demonstrated RFS benefit compared to ipilimumab in patients with resected stage IIIB-IV melanoma8, as did pembrolizumab versus placebo in patients with resected stage IIIA-IIIC melanoma9. Furthermore, adjuvant dabrafenib plus trametinib was shown to have RFS benefit compared to placebo in patients with resected stage III-IV BRAF V600E/K mutation positive melanoma10.
Here, we present updated results from four Eastern Cooperative Oncology Group (ECOG) and Intergroup trials to evaluate the mature effects of treatment on RFS and OS in patients with AJCC stage IIB, IIC or III melanoma. Patients on these trials were randomized to treatment with HDI, low-dose IFN-α 2b (LDI), observation, or the ganglioside GM2/keyhole limpet hemocyanin vaccine (GMK). E1684 randomized 287 patients to receive 52 weeks of HDI versus observation. At the time of the initial report in 1996, the median follow-up was 6.9 years, and patients in the HDI arm had significantly improved OS and RFS compared to observation. Median OS was 3.82 years in the HDI group versus 2.78 years in the observation group (p=0.024), and median RFS was 1.72 years versus 0.98 years (p=0.002), respectively 3. Updated results were published at 12.6 years, and the RFS benefit of HDI vs. observation was maintained (HR=1.38 for observation vs. HDI, p=0.020). Over time, the OS benefit was somewhat diminished (HR=1.22 for observation vs. HDI, p=0.180), although the role of competing causes of death in an aging population of median age over 60 years has not been dissected.
E1690 enrolled 642 patients, and was first reported in 2000 with a median follow-up of 4.3 years 4. This trial evaluated the impact of treatment with HDI and LDI vs. observation, and HDI was associated with a significant RFS benefit compared to observation (HR=1.28, p=0.025), whereas LDI was not. Neither regimen impacted OS, though this may have been due to high crossover of patients from observation to HDI (off protocol) at the time of regional recurrence, as stage IIB patients were not required to undergo lymphadenectomy 4. Updated median follow-up at 6.6 years revealed that the RFS benefit trended towards but did not reach statistical significance (HR=1.24, p=0.090), with no OS benefit for HDI vs. observation.
E1694 randomized 880 patients to treatment with GMK or HDI. The protocol was closed early based on interim analyses that showed the inferiority of GMK and futility for the hypothesized improvement in outcomes by GMK. At a median follow-up of 16 months, HDI was superior to GMK in terms of RFS (HR=1.47 for GMK vs. HDI, p=0.0002) and OS (HR=1.52 for GMK vs. HDI, p=0.001)5. At 2.1 years, HDI remained superior to the GMK vaccine in terms of both RFS (HR=1.33, p=0.006) and OS (HR=1.32, p=0.040)11.
E2696 randomized 107 patients with resectable stage IIB-III or IV melanoma to treatment with GMK plus concurrent HDI, GMK plus sequential HDI, or GMK alone. At 24 months, the RFS in the arms containing HDI was higher than for patients who received GMK alone (HR=1.75 for GMK vs. concurrent HDI; HR =1.96 for GMK vs. sequential HDI). The treatment benefit was statistically significant after adjusting for several prognostic factors, 6 although this effect diminished at later follow-up 11. At a median follow-up of 2.8 years, GMK with concurrent HDI vs. GMK plus sequential HDI did not reveal an RFS or OS benefit.
It is important to note that though several newer immunotherapeutic and targeted agents have become the standard of care for patients with resected stage III-IV melanoma, the data from the completed HDI trials remain, to date, the only data available for patients with resected stage IIB/IIC melanoma. Here, we present the relapse and survival status of patients enrolled in these four adjuvant studies, with available data as of September 2016.
PATIENTS AND METHODS
The survival and disease status of patients enrolled in the four randomized trials of adjuvant treatment of melanoma between 1985 and 2000 continue to be actively followed. E1684 and E2696 were conducted by ECOG, and E1690 and E1694 were ECOG-led Intergroup trials. A pooled updated analysis was published in 2004, with a data cut off of April 2001 11. The most current outcomes data including relapse and survival are as of September 2016, and were extracted from the ECOG database.
Patients
Eligibility criteria for the four studies included in this analysis were relatively consistent, and have been previously reported3–6,11. Briefly, patients were required to be at least 18 years of age and to have AJCC 6th edition stage IIB (deep primary tumor in the absence of regional lymph node involvement) or stage III melanoma (regional lymph node involvement either at presentation or recurrence. Patients enrolled on E2696 could have had in-transit or subcutaneous metastases, or extracapsular extension (AJCC stage IIIC or IV). Patients were required to have adequate hematological and end organ function, may not have had prior chemotherapy, radiation or immunotherapy, and were required to have ECOG performance status ≤1. Patients were required to undergo complete wide excision with adequate margins, and complete regional lymphadenectomy was required for patients enrolled on all studies but E1690. All studies had complete pathology review in the ECOG Melanoma Committee Pathology Office at the University of Pittsburgh.
Statistical Methods
Primary outcomes of RFS, OS and MSS were evaluated. RFS was defined as time from randomization to disease recurrence or to death in the absence of recurrence. The time last assessed was used for censored cases. MSS was defined as time from randomization to melanoma-specific death. The event time for the cases who died of other causes was censored at the time of death. Two sensitivity analyses were performed. The first utilized a complete case approach, using only patients with a non-missing cause of death. For the second approach those with unknown cause of death who died within 1 year of progression were considered MSS events, otherwise their follow-up times were censored. Follow-up time was computed from time from randomization to last known alive date for the patients who are alive at the time of data extraction.
The Kaplan-Meier method12 was used to compute the product-limit estimates of RFS, OS and MSS. The log-rank test was used to compare RFS, OS, MSS between treatment groups. P-values comparing RFS, OS, MSS are based on the log-rank test, unless otherwise specified. The Cox proportional hazards model was used to estimate HRs and 95% confidence intervals (shown in square brackets in the text) to evaluate associations between prognostic factors, treatment and outcomes 13. The proportional hazards assumption was assessed graphically using log-log plots and plots of scaled Schoenfeld residuals against time. While there was slight non-proportionality near the end of the follow-up period, the assumption was overall reasonable. Continuous variables were dichotomized using a median value. A pooled RFS and OS analysis of E1684 and E1690 was stratified by study.
In developing multivariate Cox models variables with p-values less than 0.2 in univariate models were considered for inclusion. Variables with more than 30% missing data were excluded. Patients with non-missing values for all candidate variables were included in the model-selection process. The final models were then re-fit using patients with complete data for the selected covariates. Forward and backward selection, penalized Cox regression and the Akaike Information Criterion14 were used in model selection and to assess model fit. To assess the effect of missing data, models with a factor level indicating missingness were fit and compared these models to those that excluded patients with missing values. In all cases the estimates were similar and there did not appear to be an effect associated with missingness. All reported p-values are based on two-sided tests. As the updated analysis presented here was considered a secondary analysis, the multiplicity from multiple testing was not corrected. In general, we considered p-values ≤ 0.05 as significant and between ≥ 0.05 and < 0.1 as marginally significant. However, p-values need to be interpreted with caution, especially given different sample sizes. Analyses were conducted using R software version 3.4.3.
RESULTS
A total of 1916 patients were randomized in the four ECOG trials. Excluding 4 patients (3 duplicate registrations in E1694 and 1 with no follow-up data), 1912 patients were included in this analysis. Median follow-up times were 17.9 years for E1684, 12.2 years for E1690, 16.0 years for E1694, and 16.5 years for E2696.
Patient Characteristics
Patient baseline demographics and disease characteristics are summarized by protocol (Table 1). Briefly, the median age overall was 49 years (range, 17–85). Most patients were male (63%) and white (98%), with an ECOG performance status of 0 (83%). The most common site of primary disease was the trunk (43%), and 45% of melanomas had thickness >3 mm. The majority of tumors were T4 (37%), and almost a third (31%) were ulcerated. The studies span a several years, and thus in some cases, patient characteristics collected at baseline differ. This discrepancy is the source of most of the missing data.
TABLE 1.
Baseline Patient Characteristics by Trial
| Characteristic | E1684 N = 286 |
E1690 N = 642 |
E1694 N = 877 |
E2696 N = 107 |
Total N = 1912 |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 114 (40) | 227 (35) | 314 (36) | 45 (42) | 700 (37) |
| Male | 171 (60) | 415 (65) | 563 (64) | 62 (58) | 1211 (63) |
| Missing data | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Race | |||||
| White | 279 (98) | 636 (99) | 852 (97) | 104 (97) | 1871 (98) |
| Black | 2 (1) | 3 (0) | 9 (1) | 0 (0) | 14 (1) |
| Other/unknown | 1 (0) | 3 (0) | 10 (1) | 1 (1) | 15 (1) |
| Missing data | 4 (1) | 0 (0) | 6 (1) | 2 (2) | 12 (1) |
| ECOG PS | |||||
| 0 | 254 (89) | 560 (87) | 686 (78) | 80 (75) | 1580 (83) |
| 1 | 31 (11) | 81 (13) | 190 (22) | 27 (25) | 329 (17) |
| Missing data | 1 (0) | 1 (0) | 1 (0) | 0 (0) | 3 (0) |
| Age, y | |||||
| Median (range) | 48 (17–79) | 47 (17–78) | 50 (19–85) | 48 (20–74) | 49 (17–85) |
| Missing data | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 2 (0) |
| Age, y | |||||
| <49 | 153 (53) | 357 (56) | 392 (45) | 60 (56) | 962 (50) |
| ≥49 | 132 (46) | 285 (44) | 484 (55) | 47 (44) | 948 (50) |
| Missing data | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 2 (0) |
| Site of primary tumor | |||||
| Head/neck | 29 (10) | 77 (12) | 113 (13) | 13 (12) | 232 (12) |
| Upper limb | 41 (14) | 97 (15) | 113 (13) | 14 (13) | 265 (14) |
| Lower limb | 56 (20) | 149 (23) | 175 (20) | 16 (15) | 396 (21) |
| Other | 26 (9) | 18 (3) | 29 (3) | 7 (7) | 80 (4) |
| Trunk | 129 (45) | 298 (46) | 365 (42) | 36 (34) | 828 (43) |
| Missing data | 5 (2) | 3 (0) | 82 (9) | 21 (20) | 111 (6) |
| Pigmentation | |||||
| Melanotic | 242 (85) | 500 (78) | 628 (72) | 47 (44) | 1,417 (74) |
| Amelanotic | 15 (5) | 52 (8) | 55 (6) | 10 (9) | 132 (7) |
| Missing data | 29 (10) | 90 (14) | 194 (22) | 50 (47) | 363 (19) |
| Ulceration | |||||
| Yes | 47 (16) | 231 (36) | 296 (34) | 27 (25) | 601 (31) |
| No | 221 (77) | 376 (59) | 416 (47) | 33 (31) | 1,046 (55) |
| Missing data | 18 (6) | 35 (5) | 165 (19) | 47 (44) | 265 (14) |
| Disease at study entry | |||||
| Primary | 96 (34) | 290 (45) | 561 (64) | 47 (44) | 994 (52) |
| Recurrent | 190 (66) | 350 (55) | 313 (36) | 60 (56) | 913 (48) |
| Missing data | 0 (0) | 2 (0) | 3 (0) | 0 (0) | 5 (0) |
| Micrometastases | |||||
| Yes | 6 (2) | 18 (3) | 0 (0) | 0 (0) | 24 (1) |
| No | 23 (8) | 289 (45) | 0 (0) | 0 (0) | 312 (16) |
| Missing data | 257 (90) | 335 (52) | 877 (100) | 107 (100) | 1576 (82) |
| Satellite metastases | |||||
| Yes | 0 (0) | 0 (0) | 29 (3) | 0 (0) | 29 (2) |
| No | 0 (0) | 0 (0) | 396 (45) | 0 (0) | 396 (21) |
| Missing data | 286 (100) | 642 (100) | 452 (52) | 107 (100) | 1487 (78) |
| Extranodal extension | |||||
| None/microscopic | 15 (5) | 215 (33) | 282 (32) | 0 (0) | 512 (27) |
| Yes/extensive | 13 (5) | 75 (12) | 44 (5) | 0 (0) | 132 (7) |
| Missing data | 258 (90) | 352 (55) | 551 (63) | 107 (100) | 1268 (66) |
| Tumor thickness, mm | |||||
| <3 | 133 (47) | 301 (47) | 376 (43) | 49 (46) | 859 (45) |
| ≥3 | 121 (42) | 320 (50) | 394 (45) | 29 (27) | 864 (45) |
| Missing data | 32 (11) | 21 (3) | 107 (12) | 29 (27) | 189 (10) |
| Primary tumor stage | |||||
| T1 | 43 (15) | 93 (14) | 107 (12) | 11 (10) | 254 (13) |
| T2 | 59 (21) | 142 (22) | 182 (21) | 22 (21) | 405 (21) |
| T3 | 64 (22) | 113 (18) | 156 (18) | 25 (23) | 358 (19) |
| T4 | 88 (31) | 273 (43) | 325 (37) | 20 (19) | 706 (37) |
| Missing data | 32 (11) | 21 (3) | 107 (12) | 29 (27) | 189 (10) |
| Lymph node stage | |||||
| N0 | 31 (11) | 33 (5) | 20 (2) | 33 (31) | 117 (6) |
| N1 | 106 (37) | 123 (19) | 245 (28) | 73 (68) | 547 (29) |
| N2 | 70 (24) | 69 (11) | 90 (10) | 0 (0) | 229 (12) |
| N3 | 57 (20) | 75 (12) | 50 (6) | 0 (0) | 182 (10) |
| Missing data | 22 (8) | 342 (53) | 472 (54) | 1 (1) | 837 (44) |
| Size of primary tumor, mm | |||||
| <1.4 | 97 (34) | 267 (42) | 323 (37) | 34 (32) | 721 (38) |
| ≥1.44 | 151 (53) | 280 (44) | 272 (31) | 23 (21) | 726 (38) |
| Missing data | 38 (13) | 95 (15) | 282 (32) | 50 (47) | 465 (24) |
| Size of largest lymph node, mm | |||||
| <0.2 | 19 (7) | 53 (8) | 130 (15) | 0 (0) | 202 (11) |
| ≥0.2 | 18 (6) | 120 (19) | 230 (26) | 0 (0) | 368 (19) |
| Missing data | 249 (87) | 469 (73) | 517 (59) | 107 (100) | 1342 (70) |
| WBC count, ×109/L | |||||
| <6.9 | 121 (42) | 282 (44) | 419 (48) | 63 (59) | 885 (46) |
| ≥6.9 | 135 (47) | 353 (55) | 404 (46) | 44 (41) | 936 (49) |
| Missing data | 30 (10) | 7 (1) | 54 (6) | 0 (0) | 91 (5) |
| Lactate dehydrogenase | |||||
| Abnormal | 40 (14) | 46 (7) | 187 (21) | 5 (5) | 278 (15) |
| Normal | 193 (67) | 562 (88) | 597 (68) | 101 (94) | 1453 (76) |
| Missing data | 53 (19) | 34 (5) | 93 (11) | 1 (1) | 181 (9) |
Abbreviations: ECOG PS Eastern Cooperative Oncology Group performance status; WBC, white blood cell.
All data are shown as the number (%) unless otherwise specified.
New events since the 2004 update
A pooled analysis update was published in 2004 11, at median follow-up of 12.6 years for E1684, 6.6 years for E1690, 2.1 years for E1694 and 2.8 years for E2696. Since this update in 2004, there have been 137 new relapses and 356 new deaths across all four studies. There have been 2 relapses and 11 deaths on E1684, 12 relapses and 45 deaths on E1690, 112 relapses and 262 deaths on E1694, and 11 relapses and 38 deaths on E2696 (Table 2).
TABLE 2.
Outcomes by Trial
| Trial | E1684 | E1690 | E1694 | E2696 | All Trials |
|---|---|---|---|---|---|
| Median follow-up, y | 18.2 | 12.3 | 16.3 | 16.5 | 14.8 |
| Observation, no. | 140 | 212 | 352 | ||
| Recurrent disease/dieda | 106 | 133 | 239 | ||
| Died | 100 | 123 | 223 | ||
| High-dose IFN, no. | 146 | 215 | 438 | 799 | |
| Recurrent disease/dieda | 97 | 125 | 222 | 444 | |
| Died | 99 | 121 | 234 | 454 | |
| GMK, no. | 439 | 35 | 474 | ||
| Recurrent disease/dieda | 249 | 23 | 272 | ||
| Died | 257 | 21 | 278 | ||
| Other, no. | 215 | 72 | 287 | ||
| Recurrent disease/dieda | 131 | 41 | 172 | ||
| Died | 116 | 42 | 158 | ||
| Total | 286 | 642 | 877 | 107 | 1912 |
Abbreviations: GMK, ganglioside GM2/keyhole limpet hemocyanin vaccine; IFN, interferon.
Recurrent disease/died indicates the number of patients who developed disease recurrence or died in the absence of disease recurrence.
RFS Analysis
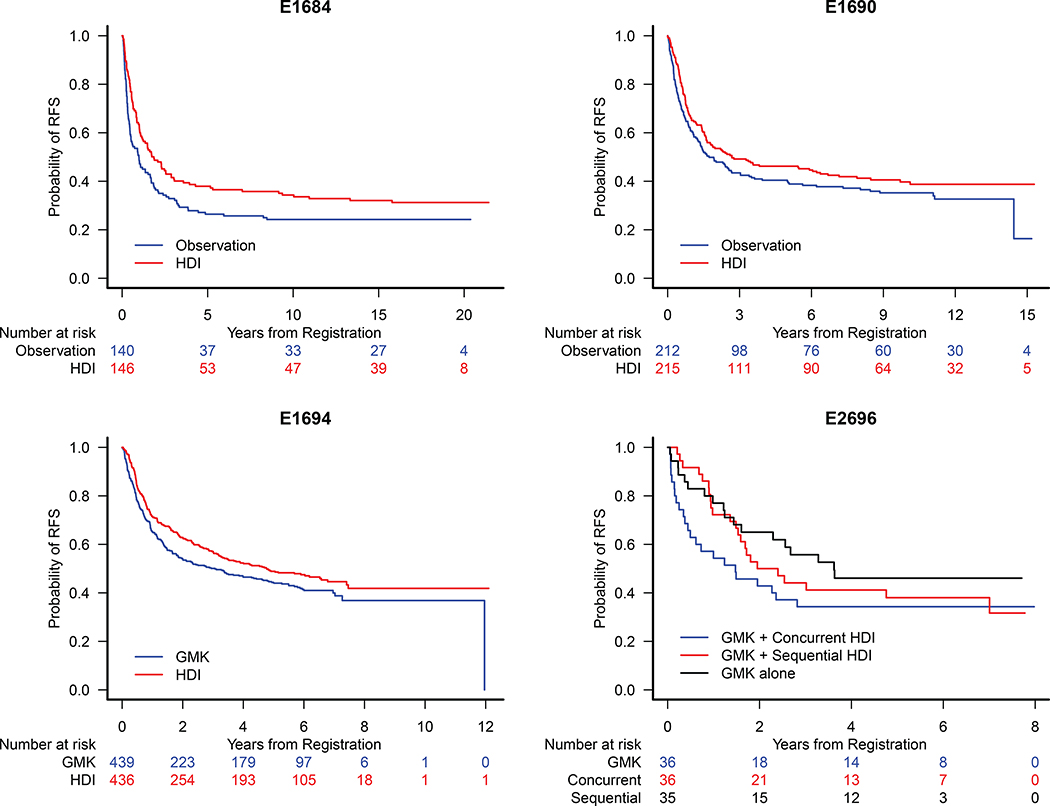
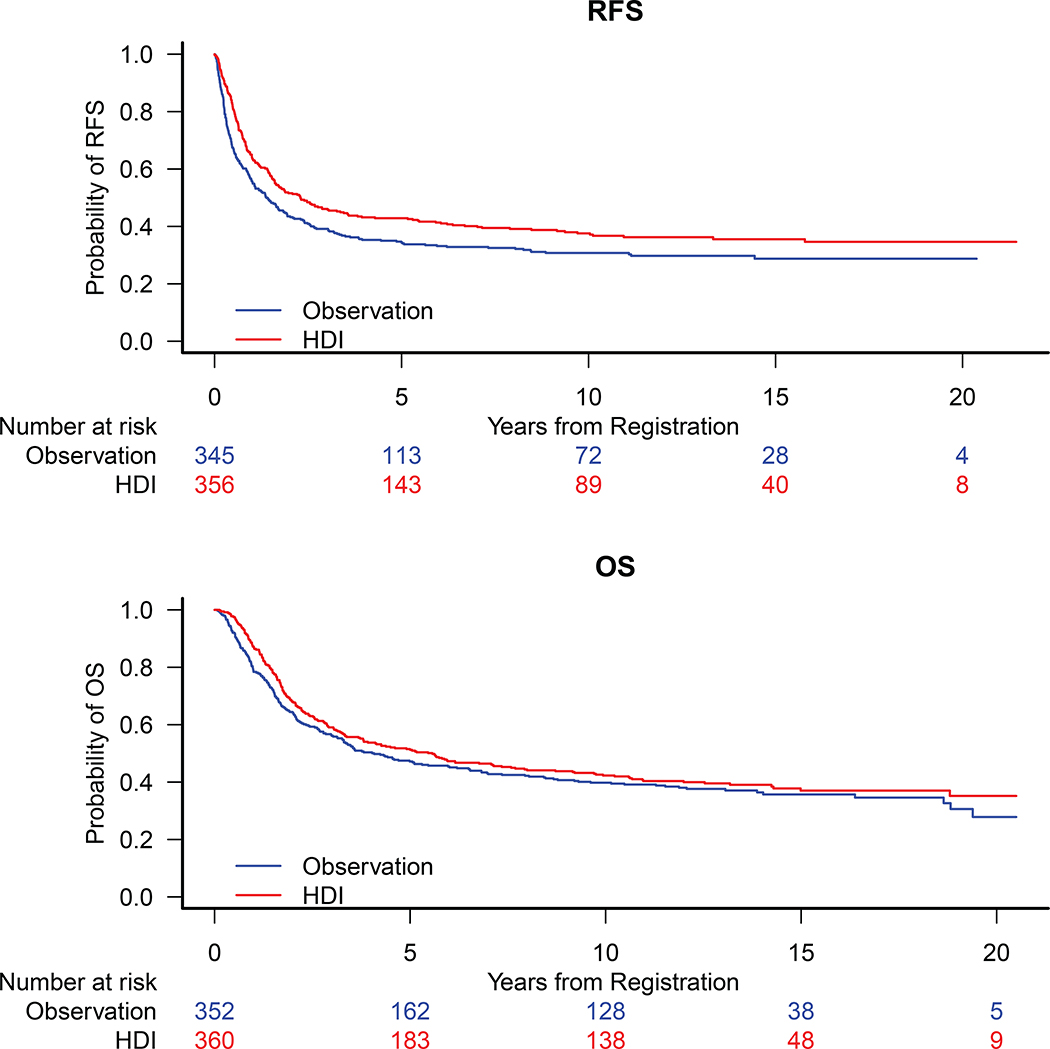
The RFS benefit of HDI vs. observation remained significant on E1684, HR= 0.73 [0.56, 0.97], p=0.028 (Fig. 1A), and was no longer significant on E1690, HR=0.82 [0.64, 1.05], p=0.12 (Fig. 1B). The RFS benefit of HDI vs. GMK was significant, HR=0.82 [0.68, 0.98], p=0.034 (Fig. 1C) on E1694. No significant benefit of HDI was observed on E2696 (Fig. 1D). In a pooled analysis of E1684 and E1690, 361 patients (HDI) vs. 352 patients (observation) with median follow-up of 13.3 years, there remains a significant benefit of HDI, HR= 0.78 [0.64, 0.94], p=0.008 (Fig. 2A).
Figure 1.
Kaplan Meier Estimates of Recurrence Free Survival (RFS) for Patients Treated on A) E1684: HR = 0.73 [95% CI: 0.56, 0.97], B) E1690: HR=0.82 [95% CI: 0.64, 1.05], C) E1694: HR=0.82 [95% CI: 0.69, 0.99] and D) E2696: Concurrent vs. GMK alone HR = 0.75 [95% CI: 0.42, 1.34], sequential vs. GMK alone HR = 0.61 [95% CI:0.33, 1.12].
Figure 2.
Kaplan Meier Estimates of A) Recurrence Free Survival (RFS) HR = 0.78 [95% CI: 0.64, 0.94] and B) Overall Survival (OS) HR = 0.88 [95% CI: 0.73, 1.07] for Patients Treated on E1684 and E1690 Based on the Pooled Analysis.
OS Analysis
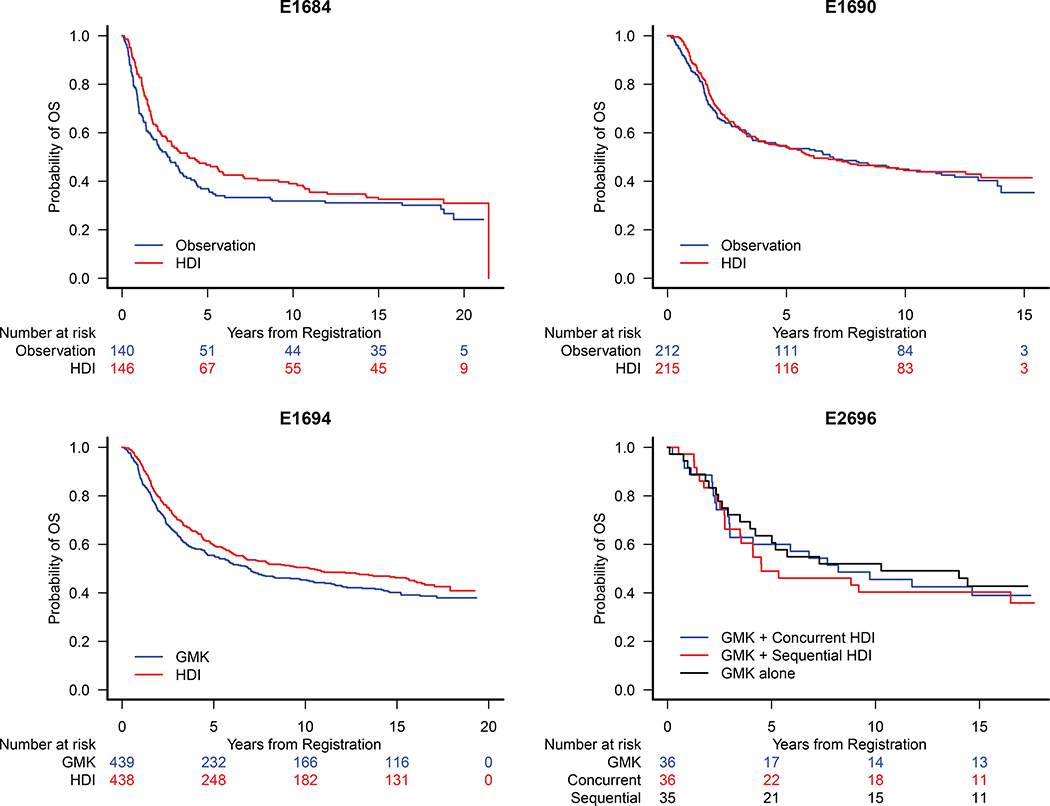
No significant HDI benefit vs. observation was noted in OS analysis: HR=0.82 [0.62, 1.08] (Fig. 3A) on E1684 and HR=0.94 [0.73, 1.21] (Fig 3B) on E1690. The OS benefit of HDI vs. GMK is now marginally significant, HR=0.85 [0.71, 1.01], p=0.068 (Fig 3C) on E1694. No significant benefit of HDI was observed on E2696 (Fig. 3D). In the pooled OS analysis of E1684 and E1690 (n=713), there was no significant benefit of HDI over observation (Fig 2B).
Figure 3.
Kaplan Meier Estimates of Overall Survival (OS) for Patients Treated on A) E1684: HR= 0.82 [95% CI: 0.62, 1.08], B) E1690: HR=0.94 [95% CI: 0.73, 1.21] and C) E1694: HR=0.85 [95% CI: 0.71, 1.01] and D) E1694: Concurrent vs. GMK HR = 1.07 [95% CI: 0.59, 1.95]; Sequential vs. GMK HR = 0.91 [95% CI: 0.49, 1.68].
MSS Analysis
MSS: Main Analysis
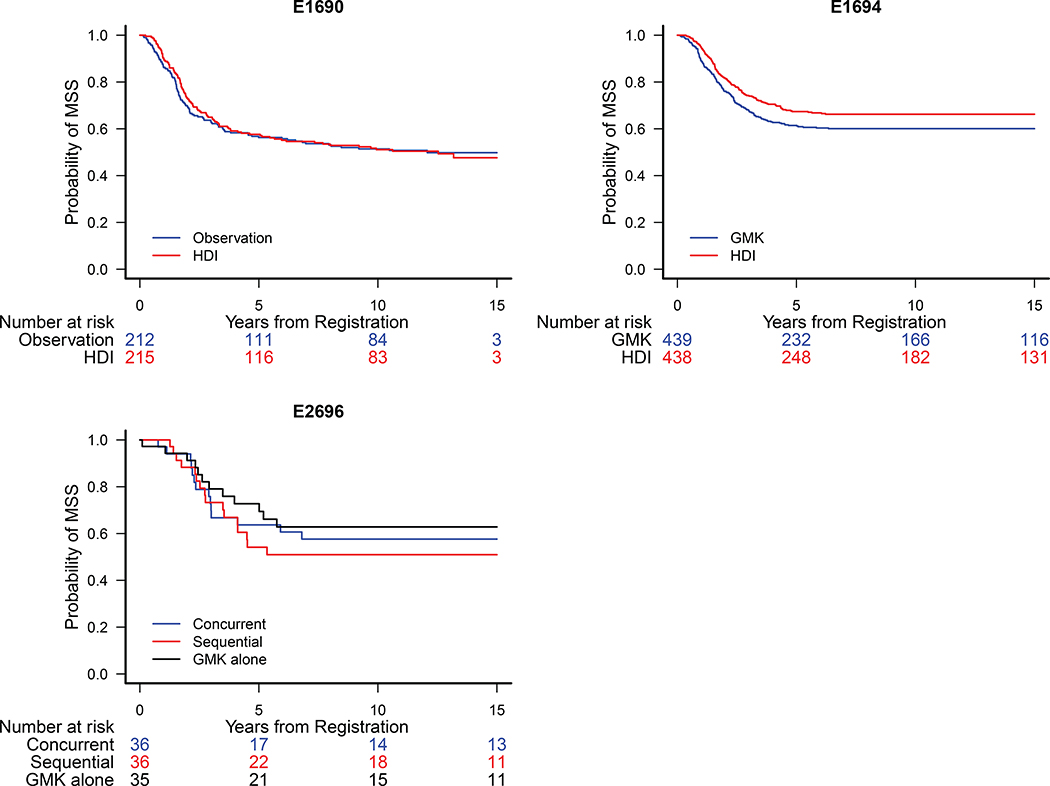
Information on the cause of death (COD) was available for all protocols except E1684. Of the deceased patients, 39 patients on E1690 (10.8%), 174 on E1694 (35.4%) and 19 on E2696 (30.2%) had missing cause of death. Excluding these cases, the MSS analysis included 703 patients from E1694, 400 patients from E1690, and 88 patients from E2696. In the main analysis, MSS for patients who were deceased with unknown COD was censored at the date of death. In assessing the benefit of HDI, there was no significant difference (vs. observation) on E1690 HR = 0.97 [0.74, 1.28], (Fig. 4A), significant difference (vs. GMK) on E1694 HR=0.79 [0.63,0.98], p=0.035 (Fig. 4B) and no significant difference on E2696 HR (concurrent vs. GMK alone) HR = 1.18 [0.57, 2.42], HR (sequential vs. GMK alone) = 0.82 [0.38, 1.78] (Fig. 4C).
Figure 4.
Kaplan Meier Estimates of Melanoma Specific Survival (MSS) for Patients Treated on A) E1690: HR = 0.97 [95% CI: 0.74, 1.28], B) E1694: HR = 0.79 [95% CI: 0.63,0.98] and C) E2696 HR (concurrent vs. GMK alone) = 1.18 [95% CI: 0.57, 2.42], HR (sequential vs. GMK alone) = 0.82 [95% CI: 0.38, 1.78].
MSS: Sensitivity Analyses
Sensitivity analyses were conducted using a complete case approach (including only patients with a known COD) and a presumed melanoma-related death approach (those with unknown COD who died within 1 year of progression were considered MSS events, otherwise they were censored). The results of the sensitivity analyses were consistent with the main analysis. The benefit of HDI vs. GMK was HR= 0.79 [0.63, 0.99], p = 0.039 for the complete case analysis and HR=0.82 [0.67, 1.02], p = 0.074 for the presumed melanoma death analysis in E1694.
Multivariate Analyses of Prognostic Factors
The result of univariate Cox regression model is summarized in the Appendix. For RFS (S Table 1a) and OS (S Table 1b), pooled data from E1684 and E1690 was used, stratified by protocol. For MSS, each study (E1690, S Table 2a, E1694 S Table 2b, E2696 S Table 2c) was evaluated separately. Based on the prognostic significance of factors observed in the univariate analysis, multivariate models were built for RFS (Table 3a), OS (Table 3b) of E1684 and E1690, MSS of E1690 (Table 3c) and MSS of E1694 (Table 3d).
Table 3a.
Multivariate Cox Model for RFS: Pooled E1684 and E1690 Trial Data (HDI Versus Observation)
| HR | 95% CI | Pa | |
|---|---|---|---|
| HDI vs observation | 0.82 | (0.67–0.99) | .044 |
| Ulceration vs no ulceration | 1.34 | (1.10–1.65) | .005 |
| Age ≥49 y vs <49 y | 1.20 | (0.99–1.46) | .064 |
| Recurrent disease vs primary disease | 1.33 | (1.09–1.63) | .005 |
| WBC ≥6.9×109/L vs <6.9×109/L | 1.20 | (0.99–1.46) | .067 |
Abbreviations: HDI, high-dose interferon-α; HR, hazard ratio; RFS, recurrence-free survival; WBC, white blood cell count.
P value was derived from a Cox model.
Table 3b.
Multivariate Cox Model for OS: Pooled E1684 and E1690 Trial Data (HDI Versus Observation)
| HR | 95% CI | Pa | |
|---|---|---|---|
| Ulceration vs no ulceration | 1.34 | (1.09–1.64) | .006 |
| Age ≥49 y vs <49 y | 1.22 | (1.01–1.49) | .041 |
| Recurrent disease vs primary disease | 1.32 | (1.08–1.62) | .006 |
| WBC ≥6.9×109/L vs <6.9×109/L | 1.21 | (1.00–1.47) | .055 |
Abbreviations: HDI, high-dose interferon-α; HR, hazard ratio; OS, overall survival; WBC, white blood cell count.
P value was derived from a Cox model.
Table 3c.
Multivariate Cox Model for MSS: E1690 Trial Data (HDI Versus Observation)
| HR | 95% CI | Pa | |
|---|---|---|---|
| Ulceration vs no ulceration | 1.43 | (1.13–1.81) | .003 |
| Age ≥49 y vs <49 y | 1.22 | (0.96–1.54) | .099 |
| Recurrent disease vs primary disease | 1.18 | (0.94–1.50) | .16 |
Abbreviations: HDI, high-dose interferon-α; HR, hazard ratio; MSS, melanoma-specific survival.
P value was derived from a Cox model.
Table 3d.
Multivariate Cox Model for MSS: E1694 Trial Data (HDI Versus GMK)
| HR | 95% CI | Pa | |
|---|---|---|---|
| HDI vs GMK | 0.79 | (0.62–1.01) | .063 |
| Ulceration vs no ulceration | 2.05 | (1.58–2.66) | <.001 |
| Recurrent disease vs primary disease | 2.13 | (1.64–2.77) | <.001 |
| T1 vs others | 0.61 | (0.39–0.95) | .028 |
Abbreviations: GMK, ganglioside GM2/keyhole limpet hemocyanin vaccine; HDI, high-dose interferon-α; HR, hazard ratio; MSS, melanoma-specific survival.
P value was derived from a Cox model.
The benefit of HDI vs. observation on RFS in 642 patients from E1684 and E1690 was significant, HR=0.82 [0.67, 0.99], p=0.044, while adjusting for ulceration, age, recurrent disease, WBC (Table 3a). Since E1684 did not have COD data, MSS data was not pooled across E1684 and E1690. Multivariate models were developed for E1690 and E1694 separately. The benefit of HDI vs. GMK on MSS in 690 patients from E1694 was marginally significant, HR=0.79 [0.62, 1.01], p=0.063, while adjusting for ulceration, recurrent disease status and T1 vs. other stages.
DISCUSSION
The treatment landscape for patients with resected stage III-IV melanoma has changed drastically in recent years following the evaluation and demonstrated efficacy of several newer agents in the adjuvant setting, including ipilimumab, nivolumab, pembrolizumab and dabrafenib plus trametinib (among the BRAF mutation positive population). In a randomized phase III trial for stage III melanoma, patients received adjuvant ipilimumab 10 mg/kg (n=475) or placebo (n=476) every 3 weeks for four doses, then every 3 months for up to 3 years 7. 3-year RFS was 46·5% (95% CI 41·5–51·3) in the ipilimumab group versus 34·8% (30·1–39·5) in the placebo group 7. In an updated analysis at a median follow-up of 5.3 years, the 5-year RFS was 40.8% versus 30.3%, respectively (HR for recurrence or death 0.76; 95% CI, 0.64–0.89; p<0.001). OS at 5 years was 65.4% in the ipilimumab group and 38.9% in the placebo group (HR for death or distant metastasis =0.76, 95.8% CI, 0.64–0.92; p=0.002). In October 2015, high dose ipilimumab (10 mg/kg) received US FDA approval for adjuvant therapy in patients with resected stage III melanoma. Given the considerable toxicity of high dose ipilimumab, a recent ECOG-ACRIN led intergroup trial E1609 randomized patients with resected high-risk melanoma (stratified by stages IIIB, IIIC, M1a, M1b) to ipilimumab 10 mg/kg or ipilimumab 3 mg/kg versus HDI. A preliminary analysis has shown that toxicity is significantly decreased with low dose compared to high dose ipilimumab, and an unplanned RFS analysis of randomized patients on the two experimental arms showed no difference in RFS 15. S1404 is an ongoing phase III RCT comparing adjuvant pembrolizumab to HDI or ipilimumab. It is important to note that to date, though preliminary data are available for E1609 and S1404 is ongoing, no results are yet available comparing checkpoint blockade immunotherapy to HDI.
CheckMate 238 compared adjuvant nivolumab to high-dose ipilimumab. In a double-blind phase 3 trial, 906 patients with stage IIIB, IIIC or IV melanoma were randomized to undergo complete resection followed by adjuvant ipilimumab at a dose of 10 mg/kg or nivolumab at a dose of 3 mg/kg. The primary endpoint was RFS, and 12 month RFS was significantly improved with nivolumab vs. ipilimumab (70.5% vs. 60.8%, respectively; HR= 0.65, 97.56% CI, 0.51–0.83; p<0.001). Treatment related grade 3 or 4 adverse events were 14.4% in the nivolumab group and 45.9% in the ipilimumab group8. KEYNOTE-054 evaluated the efficacy of pembrolizumab versus placebo in the adjuvant setting in patients with completely resected, stage IIIA, IIIB or IIIC melanoma9. 12- month RFS with pembrolizumab was 75.4% (95% CI, 71.3–78.9), significantly longer than the placebo group at 61.0% (95% CI, 56.5–65.1). OS data from Checkmate 238 and KEYNOTE-054 are not yet available.
The Combi-AD trial evaluated the efficacy of adjuvant dabrafenib plus trametinib vs. placebo. In this double-blind, phase 3 trial, 870 patients with completely resected stage III melanoma with BRAF V600E or V600K tumor mutations received dabrafenib (150 mg twice daily) plus trametinib (2 mg daily) or two matched placebo tablets for 12 months. The primary endpoint was RFS, and at a median follow-up of 2.8 years, the estimated 3-year RFS was 58% in the treatment arm vs. 39% in the placebo group (HR=0.47, 95% CI, 0.42–0.79; p=0.00006) 10. The 3-year OS rate was 86% in the combination-therapy group and 77% in the placebo group (HR for death=0.57; 95% CI, 0.42 –0.79; p=0.0006), which did not cross the prespecified interim analysis boundary of p=0.000019.
Here, we present an updated analysis representing the most mature data that is available on outcomes with adjuvant high dose interferon in the treatment of melanoma. An analysis of the current data in the context of the AJCC 8th edition would have been of great interest as patients enrolled in these studies were staged by AJCC 6th edition. While it was not feasible to restage patients enrolled over two decades ago using the present AJCC 8th edition staging system, a recent analysis of Combi-AD data with updated staging to the AJCC 8th edition revealed that there is no difference in outcome within the stage IIID group. While patients with stage IIID disease had worse outcomes, as expected, the therapeutic impact of dabrafenib/trametinib was the same across the 7th and 8th edition staging system16, suggesting that therapeutic benefit across staging groups may be consistent.
In addition to updating the analyses of the four pivotal trials of adjuvant HDI, we report on new events on each trial since the last update. There have been 137 new relapses and 356 new deaths since the last published update in 2004, and a quarter of the patients enrolled on E1694 have died. To note, E1694 has more events than the other studies because it was followed to November 2016, whereas E1684 and E1690 had follow-up until June 2007, and E2696 had follow up until April 2015. The number of new deaths known to be from melanoma cannot be reported, as melanoma specific survival was not collected in the 2004 update. Moreover, as this is the first trial to report on MSS, there is no comparator.
This updated analysis of the pivotal high-dose IFN trial E1684 confirms the RFS benefit observed in E1684 21 years ago 3, and in 200411 now at a median follow-up of 17.9 years. There remains no significant improvement in OS, as noted at 12.6 years median follow-up 11. This may be related to competing causes of death, with the age of the cohort now in the eighth decade (age >70 years), but data related to deaths from other causes and their influence on OS have not been dissected. E1690 initially revealed improved RFS with IFN compared to observation, but at 6.6 years 11 and now at 12.2 years follow-up, there is no significant difference noted. The dilution of RFS benefit because of cross-over that was documented in the initial analysis at the time of regional recurrence has been previously noted, and associated with the lack of a requirement that patients with stage IIB disease have regional lymphadenectomy. This may also be why no OS benefit was detected, either initially or at follow-up. The RFS benefit of HDI remains evident at median follow-up of 16 years for E1694. The OS benefit no longer reaches statistical significance, compared to the initial findings and the 2.2 year updated analysis, possibly due to the aging study population and competing causes of death. No significant benefit of HDI was noted in terms of RFS or OS at 16.5 years follow-up on E2696.
Outcome of MSS has not been previously reported. Here for the first time, we report the result of an MSS analysis including patients from E1690, E1694 and E2696. Patients from E1684 were excluded because data on the COD was not available. We show a significant difference in MSS between the patients assigned to HDI vs. GMK in E1694. This finding was consistent in sensitivity analyses. There was no significant difference in MSS when assessing the benefit of HDI on E1690 and E2696. While it has been suggested that the ganglioside vaccine may have a negative impact relative to placebo, the EORTC 18961 trial compared the efficacy of GM2-KLH/QS-21 vaccination vs. observation, and found no significant difference in RFS, DMFS or OS. These findings are the our most mature level of evidence for the clinical benefit of HDI with respect to RFS, OS and MSS.
The updated analysis also confirms the RFS benefit of adjuvant HDI vs. observation in E1684 and E1690. These were the only two studies included in the original pooled analysis due to the desire to compare these to an observation arm. At a median follow-up of 13.3 years, there remains a significant difference in RFS when comparing treatment with HDI vs. observation. Not surprisingly, there was no difference in OS between HDI and observation. E1690 included more than double the number of patients than E1684, and did not show OS benefit at the time of initial analysis, likely due to high incidence of cross-over from the observation to the HDI arm (all but one regional relapse patient did so). The significant treatment benefit on RFS was confirmed on multivariate analyses that adjusted for significant covariates such as disease stage at study entry, age, ulceration, and enrollment on E1684. A recent meta-analysis of 15 RCTs, 11 of which had individual patient data, demonstrated a significant improvement in event free survival (HR=0.86, p<0.00001) and OS (HR=0.90, p=0.003) with IFN-α, without benefit of higher doses compared to lower doses17. These data are in line with our own findings, which reveal decreased recurrence risk with adjuvant interferon, in addition to a survival benefit in E1694.
Several characteristics are found to have continued prognostic significance in terms of RFS, OS and MSS. In the pooled analysis of HDI vs. observation (E1684 and E1690), patients treated with HDI had better outcomes than patients in the observation arm. Furthermore, the absence of ulceration and the presence of primary (non-recurrent) disease at study entry were prognostic of improved RFS and OS on multivariate analysis. Multivariate analysis for MSS on E1690 found the absence of ulceration to be associated with a better prognosis. In E1694, which had a significant difference in MSS between the patients assigned to HDI vs. GMK, the absence of ulceration, the presence of primary (non-recurrent) disease and T1 melanoma were all associated with better prognosis.
The treatment landscape for patients with stage III melanoma has changed drastically, and studies of combination immunotherapy and immunotherapy plus targeted therapy are underway. Clinical trials evaluating the efficacy of anti-PD1 in patients with resected IIB and IIC melanoma are ongoing. To date, HDI is the only agent with available data in patients with resected stage IIB/IIC melanoma, and HDI is the only agent approved for adjuvant treatment of patients with stage IIB and IIC melanoma, and thus remains a reasonable treatment option for this population. Furthermore, while completion lymph node dissection was the standard of care for patients with a positive sentinel node, recent data from the MSLT-II trial noted no improvement in MSS following completion lymphadenectomy compared to observation with ultrasounds of the nodal basin 18. Recent adjuvant trials have required that patients undergo completion lymphadenectomy, which is likely to have implications on enrolling patients to ongoing studies, as fewer patients are likely to undergo completion lymph node dissection. Additional studies will be required to define predictive markers, such that we may determine more precisely which patients are likely to benefit most from each adjuvant modality.
Supplementary Material
Acknowledgments
Funding: YGN: Developmental Funds from P30CA047904.
MP, SJL: ECOG Funding
Conflicts of Interest Statement:
YGN: Research Funding, Merck. Consulting: Array BioPharma. JMK: Consulting or Advisory Role, Bristol-Myers Squibb, Novartis, Array BioPharma, Merck, Roche, Amgen, Immunocore. MP: No disclosures. SL: No disclosures.
Contributor Information
Yana G. Najjar, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh, UPMC-Hillman Cancer Center. 5117 Centre Ave, 1.32 E, Pittsburgh, PA 15213..
Maneka Puligandla, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute..
Sandra J. Lee, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard Medical School..
John M. Kirkwood, Department of Medicine, Division of Hematology-Oncology, University of Pittsburgh, UPMC-Hillman Cancer Center..
References
- 1.Society. AC. Cancer Facts & Figures 2018.. Cancer Facts & Figures 2018 Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14(1):7–17. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18(12):2444–2458. [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19(9):2370–2380. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Ibrahim J, Lawson DH, et al. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. J Clin Oncol. 2001;19(5):1430–1436. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 8.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 10.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood JM, Manola J, Ibrahim J, et al. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10(5):1670–1677. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 13.DR C Regression models and life-tables. J Roy Statist Soc Ser B Metho. 1972;34:187–220. [Google Scholar]
- 14.Akaike H A New Look at Statistical Model Identification. IEEE Trans Automatic Control 1974;AC19(6):716–723. [Google Scholar]
- 15.Tarhini A A phase III randomized study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma (U.S. Intergroup E1609): Preliminary safety and efficacy of the ipilimumab arms. Abstract presented at: ASCO 2017. [Google Scholar]
- 16.Larkin J Dabrafenib plus trametinib (D + T) as adjuvant treatment of resected BRAF-mutant stage III melanoma: Findings from the COMBI-AD trial analyzed based on AJCC 8 classification. J Clin Oncol 36 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ives NJ, Suciu S, Eggermont AMM, et al. Adjuvant interferon-alpha for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183. [DOI] [PubMed] [Google Scholar]
- 18.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.