Abstract
Purpose of review:
Although organ transplantation has become the standard life-saving strategy for patients with end-stage organ failure and those with malignancies, effective and safe therapeutic strategies to combat allograft loss remain to be established. With the emerging evidence suggesting the critical role of innate immunity in the mechanism of allograft injury, we summarize the latest understanding of macrophage-neutrophil cross-communication and discuss therapeutic prospects of their targeting in transplant recipients.
Recent findings:
Macrophages and neutrophils contribute to the pathogenesis of early peri-transplant ischemia-reperfusion injury and subsequent allograft rejection immune cascade, primarily by exacerbating inflammatory response and tissue damage. Noteworthy, recent advances enabled to elucidate multifaceted functions of innate immune cells, which are not only deleterious but may also prove graft-protective. Indeed, the efficacy of macrophage polarizing regimens or macrophage targeted migration have been recognized to create graft-protective local environment. Moreover, novel molecular mechanisms in the neutrophil function have been identified, such as neutrophil extracellular traps, tissue-repairing capability, crosstalk with macrophages and T cells as well as reverse migration into the circulation.
Summary:
Since efficient strategies to manage allograft rejection and improve transplant outcomes are lacking, newly discovered and therapeutically attractive innate immune cell functions warrant comprehensive preclinical and clinical attention.
Keywords: Macrophage, Neutrophil, Transplantation, Ischemia-reperfusion Injury, Rejection
Introduction
Organ transplantation (Tx) has become the standard life-saving strategy for patients with end-stage organ failure and those with malignancies (1). Ischemia-reperfusion injury (IRI), in which the tissue damage occurs when host blood supply returns to a donor transplant after a period of ischemia or lack of oxygen, is an inevitable event during the initial phase of Tx leading to primary graft dysfunction. Innate-immune cells such as macrophages and neutrophils are pivotal in the pathogenesis of IRI, while precise mechanisms in which these cells progress or resolve IRI remain to be elucidated and effective macrophage/neutrophil-targeting therapies are warranted. Recent studies have documented that, in addition to adaptive immunity, innate immune cells play an important role in Tx rejection, the major cause of allograft loss. However, despite its clinical importance, management strategies of innate immune cells to combat primary graft dysfunction and later allograft loss are yet to be established not only for improving clinical outcomes but also for successful use of marginal grafts and expansion of donor organ pool available for Tx (2, 3, 4).
In this review, we first summarize progress in our appreciation of the macrophage and neutrophil biology in organ IRI and rejection, especially focusing on liver Tx, and then discuss perspectives of novel innate-immune cell targeting strategies in Tx recipients.
Initial parenchymal cell damage following macrophage activation
Cell death can be broadly categorized into inflammatory (necrosis, necroptosis) and non-inflammatory (apoptosis), based on the presence or absence of danger-associated molecular patterns (DAMPs) release, such as high mobility group box 1 (HMGB1). Necrosis, a passive and unregulated cell death under excessive external stress, is characterized by the disruption of cell and organelle membrane integrity causing massive release of DAMPs. Necroptosis is a programmed necrosis, which involves RIP kinase family members (RIPK1, RIPK3) and mixed-lineage kinase domain-like protein (MLKL). Apoptosis is a process of programmed cell death during which cells retain membrane integrity and do not release DAMPs, while apoptotic cells also actively produce anti-inflammatory signals. Meanwhile, damaged cells unavoidably undergo necrosis if the insult is too severe or the step-by-step apoptosis pathway fails to be achieved in a timely fashion, a process called as a “secondary necrosis”. It seems that initial parenchymal cell death is composed of divergent cell death type mixture.
Macrophages (and their precursors, monocytes) are the ‘big eaters’ within the immune system, strategically located throughout the body tissues, where they ingest/process foreign materials and dead cells/debris. The liver is constituted with heterogeneous macrophage populations, both in homeostatic and pathogenic states, such as IR-stress and injury. Kupffer cells (KCs) represent the resident, highly phagocytotic, non-migratory and self-renewing liver macrophages, which originate from yolk sac-derived specific progenitor cells. Recent progress in the biology of tissue-resident macrophages implicates KCs homeostatic functions. Indeed, hepatic sterile inflammation recruits new populations of extra-hepatic macrophages (emergency repopulation), such as circulating macrophages (bone marrow-derived pro-inflammatory Ly6C-high/CCR2-high/CX3CR1-low subset and spleen-derived anti-inflammatory Ly6C-low/CCR2-lowCX3CR1-high subset) as well as GATA6+ peritoneal cavity-derived macrophages (5).
As “sentinel immune cells”, KCs detect initial hepatic cell damage by sensing early extracellular DAMPs, become activated and lead to the release of chemokines and cytokines, which eventually contribute to tissue IRI. Inflammasome activation is essential for this priming of inflammatory cascade, constituted with transcriptional upregulation of pro-IL1β/pro-IL18, assembly of inflammasome, activation of caspse-1, and release of cleaved/activated IL1β/IL18. Chemokines, such as CCL2, promote the recruitment of Ly6C-high/CCR2-high/ CX3CR1-low monocytes into the damage site, where they develop into pro-inflammatory, angiogenic, and pro-fibrotic macrophage phenotype. KC-derived IL1β also up-regulates intercellular adhesion molecule-1 (ICAM-1), followed by neutrophil recruitment via integrin αMβ2 (Mac1)-dependent adhesion to endothelial ICAM-1. In the latter phase, Ly6C-low/CCR2-lowCX3CR1-high restorative macrophages appear at the damage site. The highly plastic CCR2-high macrophage subset eventually converts to CCR2-low phenotype in CSF1-dependent manner (5). Damaged hepatocytes release ATP, attracting GATA6+ peritoneal macrophages to the sites of liver injury via trans-capsular route (not via blood vessels), where they contribute to necrotic cells clearance as well as revascularization of damaged tissue (6), while relatively little is known on the role of peritoneal cavity macrophages in organ Tx.
In addition to early innate immune-driven tissue IRI, an emerging body of work supports the important role for macrophages in adaptive immune-dependent Tx rejection. Macrophages launch allo-immune response against the graft through antigen processing and presentation, as well as providing co-stimulation signaling to enhance allo-immunity. In addition, crosstalk with other immune cells and graft endothelial cells causes tissue damage or fibrosis in transplanted organs, ultimately leading to Tx loss. In this context, clinical studies identified a positive correlation between frequency of infiltrating macrophages and allo-Tx rejection response (7). On the other hand, some macrophages may function as regulatory cells to suppress allogeneic T cells, induce Treg differentiation and promote transplant tolerance. The functional diversity of macrophages in organ transplantation is consistent with their plasticity and heterogeneity, as detailed below.
Macrophage heterogeneity
Macrophages are highly dynamic and plastic, as a variety of environmental stimuli may alter their activation status and function. Thus, M1 macrophage (classically activated macrophage) is the pro-inflammatory and tissue distractive subset, characterized by increased expression of CD86, iNOS, TNF-α, IL-1 and IL-6. In vitro, stimulation with IFN-γ and LPS polarizes macrophages towards M1 phenotype. Contrary, the M2 macrophage (alternatively activated macrophage) is the anti-inflammatory and tissue-repairing subset, characterized by high expression of CD163, CD206, Arg1, and IL-10. In vitro, stimulation with IL-4 and IL-13 promotes M2 macrophage generation. Macrophages over-expressing heme-oxygenase-1 (HO-1; hsp32) were squeezed towards the M2 phenotype (inhibited M1 characteristics), while adoptive transfer of HO-1 over-expressing macrophages alleviated IRI in mouse liver Tx (8). In addition, increased post-Tx hepatic HO-1 expression dictated superior liver transplant patient outcomes (8) (9) (10). Neutralization of lipocalin2 (Lcn2), a defense mediator expressed in response to TLR activation, suppressed macrophage M1 but enhanced M2 phenotype mitigating cardiac IRI (11).
Regulatory macrophages (Mregs), a less well-characterized macrophage subtype, can be induced in vitro following stimulation with M-CSF and IFN-γ, express iNOS, MHC class II, and PD-L1, though little CD40 or CD86; and were shown to suppress T cell function. Devoid of most surface M1 or M2 markers, Mregs were shown to mitigate acute and chronic inflammation in different disease models (12). Adoptive transfer of Mregs improved cardiac allograft survival in non-immunosuppressed fully MHC-incompatible mouse recipients. Interestingly, transfer of iNOS-deficient Mregs failed to prolong graft survival, indicating an iNOS-dependent mechanism of the tolerogenic effect (13). Human Mregs, distinctly characterized by robust expression of DHRS9 (14), are similarly capable of suppressing the proliferation/activation and depleting allogeneic T cells (15).
Macrophage-targeted therapies
Although increased frequency of hepatic macrophages is one of the established indicators of deteriorated IRI or Tx rejection pathology, global macrophage depletion remains a questionable therapeutic approach because of macrophage plasticity, while tissue destructive pro-inflammatory macrophage may turn out essential in the inflammatory resolution and tissue remodeling. First, strategies to modulate pathogenic KCs activation have been reported, such as microbiota modification, blockade of inflammasome signaling, reduction of extracellular DAMPs, or inhibition of DAMPs sensing receptors on macrophages. In this context, by abrogating gut microbiome, a broad-spectrum antibiotics treatment in the Tx donor, reduced the number of KCs, shifted KCs to highly phagocytic phenotype, depressed MHC II expression and alleviated IRI in liver Tx (16); as did treatment with HMGB1 neutralizing antibody or recombinant thrombomodulin (HMGB1 inactivator) (17). Second, interruption of chemokine-chemokine receptor signaling can suppress pro-inflammatory Ly6C-high monocyte recruitment into the damage site. Indeed, targeting CCR2, a receptor for monocyte chemo-attractant protein 1, protected mice from kidney IRI (18). Third, skewing macrophage differentiation to anti-inflammatory and restorative phenotypes can decrease inflammatory injury and promote tissue repair (8). Braza et al. has recently reported the efficacy of macrophage-targeting nano-immunotherapy to inhibit inflammatory cytokine production, promote Treg expansion, and ultimately improving mouse cardiac Tx survival (19). This group has also identified a novel pathway of allograft rejection associated with macrophage activation via long-term functional reprogramming of myeloid cells, termed “trained immunity”, i.e., the ability of innate immune cells to switch/maintain their functional, transcriptional, epigenetic and metabolic programs after the engagement of pattern recognition receptors. Fourth, cell-based macrophage therapies may be advantageous to combat IRI by transferring ex-vivo polarized organ-protective macrophage subsets (8) (13). A recent clinical study has shown early promise of Mreg as a cell-based adjunctive immunosuppression. Human Mregs converted allogeneic CD4+ T cells into IL-10-producing, TIGIT+ FoxP3+-induced Tregs that non-specifically suppressed bystander T cells and inhibited dendritic cell maturation. Simultaneously, preoperative administration of donor-derived Mregs resulted in a rapid increase of circulating TIGIT+ Tregs in living-donor kidney transplant recipients (20).
Neutrophil activation in IRI
Neutrophils, the largest circulating fraction of leukocytes, are continuously generated from myeloid precursors in the bone marrow in a process of “granulopoiesis”. Neutrophils exert tissue damage primarily via elaborated ROS and proteases. In addition, activated neutrophils can enhance inflammatory tissue damage by releasing neutrophil extracellular traps (NETs), extracellular scaffolds of DNA fibers decorated with histone, granule-derived antimicrobial peptides and enzymes, such as neutrophil erastase, cathepsin G, and MPO. Generation of ROS by NADPH oxidase and activation of protein-arginine deiminase 4 (PAD4), an enzyme that converts arginine to citrulline on histones, are essential steps for chromatin decondensation in the NET formation (21). NETs function was originally described as the efficient means to immobilize, catch and eliminate pathogens, whereas recent studies have recognized its critical involvement in non-infectious inflammatory states, including IR-stress. Indeed, biopsies from human kidney transplant recipients with acute tubular necrosis exhibited increased NETs formation (22), while bronchoalveolar lavage fluid collected from human lung transplant recipients with primary graft dysfunction contained extensive NETs (23). Furthermore, several NET-targeting agents successfully attenuated IRI in murine models, including PAD4 inhibitors (YW3–56, YW4–03), which reduced NETs formation and decreased severity of liver IRI (24); PAD inhibitor (Cl-amidine), which suppressed NETs and alleviated renal IRI (22); or DNase I combined with recombinant tissue-type plasminogen activator, which inhibited NETs and alleviated myocardial IRI (25).
Anti-Inflammatory and tissue-repairing neutrophil functions
In addition to pro-inflammatory and tissue-destructive functions, recent studies have identified previously unappreciated role of neutrophils to control inflammation and resolve tissue damage. Several lines of evidence indicate that certain microenvironments may promote neutrophil polarization into two functionally distinct phenotypes (26). Thus, N1 neutrophils are anti-tumoral/pro-inflammatory with increased TNFα expression and reduced Arg1/CXCR4/MMP-9/VEGF levels, whereas N2 neutrophils are pro-tumoral/anti-inflammatory, and characterized by decreased TNFα yet increased Arg1/CXCR4/MMP-9/VEGF expression levels. Some cytokines may contribute to N1/N2 polarization, with TGFβ promoting neutrophils to acquire the N2 phenotype by inhibiting generation of N1 neutrophils (26). Endogenous IFNβ imprints neutrophils to N1 phenotype, which restrict tumor angiogenesis and enhance inflammatory cytotoxicity (27). Noteworthy, rosiglitazone (peroxisome proliferator-activated receptor-γ agonist) treatment in a mouse brain stroke model shifted neutrophil population toward N2 phenotype, suppressed inflammation, promoted neutrophil clearance and alleviated brain damage (28). Although macrophage M1/M2 plasticity is broadly accepted, neutrophils generally have short lifespan (6–8 h in the blood stream), and their plasticity remains largely unknown. Future studies are needed to elucidate whether N1/N2 neutrophil polarization is dominated by re-programming of existing cells or alternatively by environmental influence on de novo neutrophil populations.
Neutrophils may also contribute to tissue repairing processes following infectious or sterile inflammatory organ damage (29). Clinical observations support this concept, as patients with leukocyte adhesion type 1 deficiency (an autosomal recessive disorder characterized as defects of neutrophil adhesion ability) experience delayed wound healing (30). In the beginning of tissue repair process, neutrophils acting as professional phagocytes remove tissue debris. Indeed, in a thermal hepatic injury model, neutrophil depletion resulted in far more debris remaining at the injury site (31). Next, neutrophil apoptosis shifts the phagocytosing macrophages to the resolution-phase (M2) to govern tissue-repairing by M2 macrophages, secreting IL-1Rα, IL10, TGF-β and VEGF, leading to fibroblast differentiation into myofibroblasts, synthesis of interstitial fibrillar collagens by myofibroblasts and expression of MMPs/TIMPs that control ECM turnover (32). Christoffersson et al. identified a CD11b+/Gr1+/CXCR4high neutrophil subset in a syngeneic mouse pancreatic islet transplant model, which was recruited via VEGF-A dependent pathway. It contained higher amount of MMP9 than those recruited to an inflammatory stimulus, and contributed to revascularization via MMP9 dependent mechanism (33). In a liver thermal injury model, limited period of neutrophil depletion resulted in delayed revascularization at 7 days and the presence of non-healing injury area at 4 weeks, suggesting the requirement for neutrophil in the hepatic homeostatic recovery (31). Likewise, in a transplant model of bioengineered U-graft, which contained an unassembled suspension of vascular cells embedded in a hydrogel, host-derived alternatively polarized neutrophils (N2) contributed to graft revascularization (34).
Termination of neutrophil effector functions
In a successful response to an acute injury, it is crucial to prevent tissue damage by promoting local resolution of the inflammation through the removal of neutrophils from the injury site (35). Apoptosis is considered as a favorable neutrophil death mechanism because organized cell elimination can limit uncontrolled release of DAMPs and shifts engulfing macrophages into an anti-inflammatory transcriptional program (32). On the other hand, recent evidence suggests that neutrophils do not necessarily die at an inflamed site, and instead can leave the site of tissue damage in a process termed as a “reverse migration”. In a mouse thermal hepatic injury model, neutrophils recruited to the stressed tissue neither underwent apoptosis, necrosis, nor phagocytosis by monocytes, but instead reversely migrated to the circulation, became arrested within the lung vasculature without causing local tissue injury, and then homed to the bone marrow where they ultimately died by apoptosis (31). In contrast, in hepatic IRI or acute pancreatitis model, activated neutrophils were redistributed to other locations to produce remote organ injury (36) (37) (38). It is noteworthy that neutrophils undergoing trans-endothelial migration in vitro expressed specific marker, ICAM1high/ CXCR1low, which was resistant to apoptosis and produced more ROS (36), whereas patients with acute pancreatitis who developed acute lung injury had more ICAM1high/CXCR1low expressing neutrophils in their circulation (38). This implies the potential for neutrophil reprograming or existence of active/inactive neutrophil subsets after reverse migration. However, there is no rigorous evidence as to why and how reversely-migrating neutrophils can become toxic in some cases but inactive in other pathology states.
Neutrophils in organ Tx rejection
By retaining the potential to inhibit T cell cytotoxicity, neutrophils may protect transplanted tissue from the host rejection response. Activated neutrophils release Arginase-1 (Arg1) during degranulation process, which inhibit T cell proliferation/expansion (39, 40); while neutrophil elastase cleaves CD2, CD4, and CD8 on peripheral blood T lymphocytes, leading to reduced IL-2 production and suppressed T-cell cytotoxicity (41). Neutrophil-derived ROS may also suppress T cells via inactivation of coffilin, an actin-remodeling protein, resulting in impaired formation of the immune synapse and cell activation (42). Moreover, as Treg are less sensitive to inhibition by ROS (43), production of ROS by neutrophils effectively creates a Treg dominant local environment (44). In addition, studies by Tirouvanziam et al. identified a CD63+/MHC-II+/CD80+/CD294+ human neutrophil subset in mature cystic fibrosis airway, which retained anabolic/pro-survival phenotype and suppressed T-cell functions (39) (45). In addition, Pillay et al. found a subset of mature human CD16bright/CD62Ldim neutrophils in endotoxin-challenged blood, which suppressed T cell proliferation via neutrophil MAC-1 dependent mechanism (46). Noteworthy, a recent study documented that neutrophil-derived CSF1 preferentially induced tolerogenic macrophages to acquire Tx tolerance in a mouse cardiac Tx model (47).
Neutrophil-targeted therapies
It is important to keep in mind that Tx recipients remain immunocompromised while neutrophils constitute the forefront of pathogen host defense. Therefore, therapies limiting neutrophil trafficking and/or effector functions may in turn increase the risk of infections. Inhibition of NETs formation by specifically targeting highly destructive neutrophil function, seems biologically beneficial. However, future studies need to evaluate potential risks, as this maneuver may spread abundant DAMPs or pathogens, which otherwise should be isolated inside the NETs areas. Since DNase has been successfully applied for the treatment of cystic fibrosis patients and the NETs digestion is considered to be a part of its beneficial effects (48), these investigations are warranted. On the other hand, a recent study reported the efficacy of neutrophil recruiting formyl peptide receptor 1 antagonist to decrease the number and crawling velocity of neutrophils and alleviate liver IRI (49).
Apoptosis is a favorable way of neutrophil termination, leading to a shift of phagocytosing Kupffer cells and prevention of the DAMPs burst, while excessive neutrophil apoptosis may cause unnecessary immune suppression. In this context, ectoine improved inflammatory resolution in a sterile inflammation lung model by preventing anti-apoptotic mechanism, extending life-span of activated but not inactivated neutrophils, and reducing neutrophil numbers (50). Furthermore, a randomized clinical trial in elderly patients highlights the efficacy of inhaled ectoine on neutrophilic lung inflammation (51). A recent study has also shown that extracorporeal photophoresis (ECP) predominantly triggered neutrophil apoptosis and release Arg1 (52), whereas ECP was reported beneficial against chronic rejection of liver, lung, heart and kidney Tx (53).
Studies by Christoffersson et al. have identified a unique VEGF-A-induced CXCR4high neutrophil subset with the revascularization ability in a pancreatic islet transplant model (33), while a study by Wang et al. (31) implied CXCR4 expressing non-inflammatory neutrophil subset was capable of homing back to bone marrow in the “reverse migration” mechanism (54). Although the neutrophil plasticity has been neither rigorously studied nor proven, one may envision great potential for the future neutrophil shifting strategies towards angiogenetic, reverse-migratory, CSF1-proficient, or N2 polarized phenotypes.
Conclusion
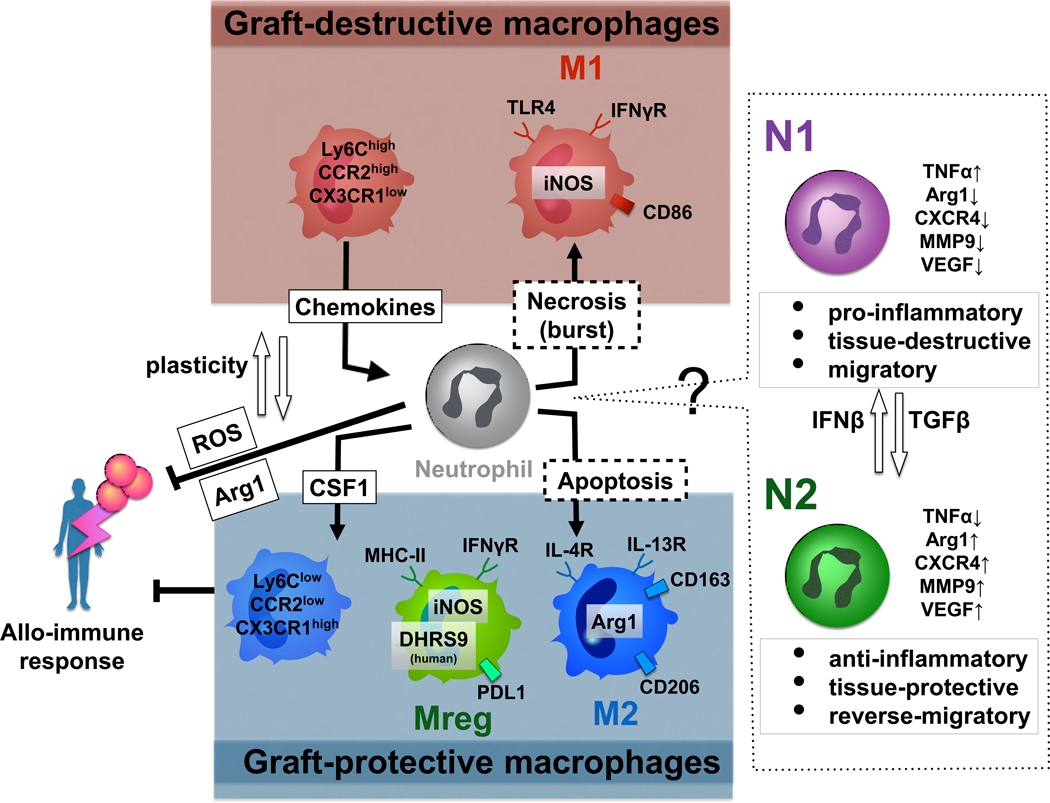
Recent technological advances contributed to a remarkable progress in our appreciation of the macrophage and neutrophil biology, especially their immune regulatory networks and tissue repairing capabilities (Figure). Since efficient and safe strategies to prevent allograft rejection and improve clinical outcomes have not been yet established, newly discovered and therapeutically attractive innate immune cell functions, as discussed in this review, warrant future comprehensive preclinical and clinical attention in Tx recipients.
From bench-to-bedside: A simplified scheme of differential cell surface characteristics, putative multifaceted molecular interactions, and divergent functions of polarized macrophage (M11, M2, Mreg) and neutrophil (N1, N2) subsets in allograft recipients (see text for details).
Key points:
Macrophages and neutrophils retain multifaceted functions, which are not only deleterious but may also prove cytoprotective in transplant recipients.
Macrophage polarizing regimens and targeted migration of cytoprotective macrophage subsets have emerged as attractive new therapeutic options in organ transplantation.
Previously unrecognized neutrophil functions and molecular signaling pathways provide new insights to the future investigational and therapeutic means in organ transplantation.
Acknowledgement
Partially supported by NIH Grants P01 AI120944; R01 DK062357, DK107533, DK102110.
Abbreviations:
- Arg1
arginase-1
- DAMPs
danger-associated molecular patterns
- ECP
extracorporeal photophoresis
- HMGB1
high mobility group box 1
- HO-1
heme oxygenase-1
- IRI
ischemia-reperfusion injury
- KC
Kupffer cells
- MLKL
mixed-lineage kinase domain-like protein
- Mreg
regulatory macrophages
- NET
neutrophil extracellular trap
- PAD4
protein-arginine deiminase 4
- ROS
reactive oxygen species
- Treg
regulatory T cells
- Tx
transplant
Footnotes
Conflicts of interest
There are no conflicts of interest.
Reference and recommended reading
* of special interest
- 1.Dutkowski P, Linecker M, DeOliveira ML, Mullhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148(2):307–23. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Annals of surgery. 2005;241(6):905–16; discussion 16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature reviews Gastroenterology & hepatology. 2013;10(2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura K, Kageyama S, Kupiec-Weglinski JW. The Evolving Role of Neutrophils in Liver Transplant Ischemia-Reperfusion Injury. Current Transplantation Reports. 2019;6(1):78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacke F Targeting hepatic macrophages to treat liver diseases. Journal of hepatology. 2017;66(6):1300–12. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165(3):668–78. [DOI] [PubMed] [Google Scholar]
- 7.Brasen JH, Khalifa A, Schmitz J, Dai W, Einecke G, Schwarz A, et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney international. 2017;92(2):479–89. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K, Kageyama S, Yue S, Huang J, Fujii T, Ke B, et al. Heme oxygenase-1 regulates sirtuin-1-autophagy pathway in liver transplantation: From mouse to human. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(5):1110–21.* This translational study highlights the efficacy of M2-polarized HO-1-overexpressed macrophage therapy in a mouse liver Tx model.
- 9.Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa RA, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. Journal of hepatology. 2017;67(6):1232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kageyama S, Hirao H, Nakamura K, Ke B, Zhang M, Ito T, et al. Recipient HO-1 inducibility is essential for posttransplant hepatic HO-1 expression and graft protection: From bench-to-bedside. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19(2):356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Xing H, Mao X, Li L, Li X, Li Q. Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model. Scandinavian journal of immunology. 2015;81(1):31–8. [DOI] [PubMed] [Google Scholar]
- 12.Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Current opinion in organ transplantation. 2015;20(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riquelme P, Tomiuk S, Kammler A, Fandrich F, Schlitt HJ, Geissler EK, et al. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(2):409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riquelme P, Amodio G, Macedo C, Moreau A, Obermajer N, Brochhausen C, et al. DHRS9 Is a Stable Marker of Human Regulatory Macrophages. Transplantation. 2017;101(11):2731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. Journal of immunology (Baltimore, Md : 1950). 2011;187(5):2072–8. [DOI] [PubMed] [Google Scholar]
- 16.Corbitt N, Kimura S, Isse K, Specht S, Chedwick L, Rosborough BR, et al. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. The American journal of pathology. 2013;182(1):180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadono K, Uchida Y, Hirao H, Miyauchi T, Watanabe T, Iida T, et al. Thrombomodulin Attenuates Inflammatory Damage Due to Liver Ischemia and Reperfusion Injury in Mice in Toll-Like Receptor 4-Dependent Manner. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(1):69–80. [DOI] [PubMed] [Google Scholar]
- 18.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. Journal of the American Society of Nephrology : JASN. 2003;14(10):2503–15. [DOI] [PubMed] [Google Scholar]
- 19.Braza MS, van Leent MMT, Lameijer M, Sanchez-Gaytan BL, Arts RJW, Perez-Medina C, et al. Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity. 2018;49(5):819–28.e6.* This study repored a novel macrophage-targeting synergistic nanoimmunotherapy to inhibit inflammatory cytokine production, promote Treg expansion, and leading to improved heart graft survival in a mouse model.
- 20.Riquelme P, Haarer J, Kammler A, Walter L, Tomiuk S, Ahrens N, et al. TIGIT(+) iTregs elicited by human regulatory macrophages control T cell immunity. Nature communications. 2018;9(1):2858.* A seminal study on a promising clinical use of donor-derived Mregs to markedly enhance circulating TIGIT+ Tregs in living-donor kidney transplant recipients.
- 21.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature medicine. 2017;23(3):279–87. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, et al. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. Journal of the American Society of Nephrology : JASN. 2017;28(6):1753–68.* This study documented the efficacy of PAD inhibitor to suppress NETs and alleviate renal IRI in a mouse model.
- 23.Sayah DM, Mallavia B, Liu F, Ortiz-Munoz G, Caudrillier A, DerHovanessian A, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine. 2015;191(4):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology (Baltimore, Md). 2015;62(2):600–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L, Zhou X, Ji WJ, Lu RY, Zhang Y, Zhang YD, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. American journal of physiology Heart and circulatory physiology. 2015;308(5):H500–9. [DOI] [PubMed] [Google Scholar]
- 26.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16(3):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of clinical investigation. 2010;120(4):1151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke. 2013;44(12):3498–508. [DOI] [PubMed] [Google Scholar]
- 29.Wang J Neutrophils in tissue injury and repair. Cell and tissue research. 2018;371(3):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. The New England journal of medicine. 2000;343(23):1703–14. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science (New York, NY). 2017;358(6359):111–6.* Important study visualizing the non-inflammatory reverse migration of neutrophils and their homing back to the bone marrow.
- 32.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO molecular medicine. 2013;5(5):661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin RZ, Lee CN, Moreno-Luna R, Neumeyer J, Piekarski B, Zhou P, et al. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nature biomedical engineering. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nature reviews Immunology. 2010;10(6):427–39. [DOI] [PubMed] [Google Scholar]
- 36.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature immunology. 2011;12(8):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, et al. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity. 2015;42(6):1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D, Zeng Y, Fan Y, Wu J, Mulatibieke T, Ni J, et al. Reverse-migrated neutrophils regulated by JAM-C are involved in acute pancreatitis-associated lung injury. Scientific reports. 2016;6:20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingersoll SA, Laval J, Forrest OA, Preininger M, Brown MR, Arafat D, et al. Mature cystic fibrosis airway neutrophils suppress T cell function: evidence for a role of arginase 1 but not programmed death-ligand 1. Journal of immunology (Baltimore, Md : 1950). 2015;194(11):5520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fletcher M, Ramirez ME, Sierra RA, Raber P, Thevenot P, Al-Khami AA, et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer research. 2015;75(2):275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doring G, Frank F, Boudier C, Herbert S, Fleischer B, Bellon G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. Journal of immunology (Baltimore, Md : 1950). 1995;154(9):4842–50. [PubMed] [Google Scholar]
- 42.Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29(3):404–13. [DOI] [PubMed] [Google Scholar]
- 43.Mougiakakos D, Johansson CC, Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113(15):3542–5. [DOI] [PubMed] [Google Scholar]
- 44.Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity. 2017;46(1):15–28. [DOI] [PubMed] [Google Scholar]
- 45.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, et al. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of clinical investigation. 2012;122(1):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braza MS, Conde P, Garcia M, Cortegano I, Brahmachary M, Pothula V, et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(5):1247–55.* Important study showing neutrophil-derived CSF1 predominantly induced tolerogenic macrophages to confer immune tolerance in a mouse heart Tx model.
- 48.Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nature communications. 2015;6:6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda M, Takeichi T, Hashimoto S, Yoshii D, Isono K, Hayashida S, et al. Intravital Imaging of Neutrophil Recruitment Reveals the Efficacy of FPR1 Blockade in Hepatic Ischemia-Reperfusion Injury. Journal of immunology (Baltimore, Md : 1950). 2017;198(4):1718–28. [DOI] [PubMed] [Google Scholar]
- 50.Sydlik U, Peuschel H, Paunel-Gorgulu A, Keymel S, Kramer U, Weissenberg A, et al. Recovery of neutrophil apoptosis by ectoine: a new strategy against lung inflammation. The European respiratory journal. 2013;41(2):433–42. [DOI] [PubMed] [Google Scholar]
- 51.Unfried K, Kramer U, Sydlik U, Autengruber A, Bilstein A, Stolz S, et al. Reduction of neutrophilic lung inflammation by inhalation of the compatible solute ectoine: a randomized trial with elderly individuals. International journal of chronic obstructive pulmonary disease. 2016;11:2573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin C, Cesko E, Hillen U, Schilling B, Brandau S. Modulation and Apoptosis of Neutrophil Granulocytes by Extracorporeal Photopheresis in the Treatment of Chronic Graft-Versus-Host Disease. PloS one. 2015;10(8):e0134518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knobler R, Berlin G, Calzavara-Pinton P, Greinix H, Jaksch P, Laroche L, et al. Guidelines on the use of extracorporeal photopheresis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28 Suppl 1:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125(3):281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]